1,3-Dimethylimidazolium-2-carboxylate: the unexpected synthesis of an ionic liquid precursor and carbene-CO2 adduct†
John D.
Holbrey
a,
W. Matthew
Reichert
a,
Igor
Tkatchenko
*b,
Ezzedine
Bouajila
b,
Olaf
Walter
c,
Immacolata
Tommasi
d and
Robin D.
Rogers
*a
aCenter for Green Manufacturing and Department of Chemistry, The University of Alabama, Tuscaloosa, AL 35487, USA. E-mail: rdrogers@bama.ua.edu; Fax: 205 348 0823; Tel: 205 348 4323
bLaboratoire de Synthèse et Electrosynthèse Organométallique, UMR 5632 CNRS-Université de Bourgogne, Faculté des Sciences Mirande, 9 ave A. Savary, BP 47870, F-21078, Dijon Cedex, France. E-mail: tkatchen@u-bourgogne.fr
cInstitut für Technische Chemie-CPV, Forschungszentrum Karlsruhe, PO Box 3640, 76021, Karlsruhe, Germany
dDepartment of Chemistry, University of Bari, Campus Universitario, Bari, 70126, Italy
First published on 5th December 2002
Abstract
1,3-Dimethylimidazolium-2-carboxylate is formed in good yield, rather than the anticipated organic salt, 1,3-dimethylimidazolium methyl carbonate, as the reaction product resulting from both N-alkylation and C-carboxylation of 1-methylimidazole with dimethyl carbonate; the crystal structure of the zwitterion exhibits π-stacked rings and two-dimensional sheets constructed by hydrogen-bonds from imidazolium-ring hydrogens to the carboxylate group.
A significant focus of ionic liquids research is on their applications as solvents for green chemical processes,1 particularly in the context of separations and extraction technologies.2,3 In order to increase the integration of ionic liquids processes into the green chemistry portfolio, cleaner more efficient, and less waste producing methods of manufacture of the ionic liquids themselves are needed. One particularly appealing reagent is dimethyl carbonate (DMC), which can be used as an environmentally benign methylating agent.4,5 The question arose, could DMC be used for the production of ionic liquids in an environmentally compatible chemical process without generating undesirable acidic, or salt waste.
Quaternary ammonium methyl carbonate salts have been prepared from the reaction of the corresponding trialkylamines with DMC on heating in a sealed ampoule at 80 °C for 3 days.5 Mori et al.6 describe a general procedure for the formation of quaternary ammonium and phosphonium methyl carbonate salts in the patent literature7 and recently reported the synthesis of ionic liquids using this process, in which 1-ethylimidazole is reacted with DMC in methanol at 145 °C, 5 atm pressure. The resulting methyl carbonate salt solutions were converted to ionic liquids by hydrolysis of the anion with acid and removal of the solvent, the only by-products being methanol and CO2. In contrast, Fischer et al.8 disclose that the reaction of DMC with 1-methylimidazole at high temperature (100–150 °C) yields 1,3-dimethylimidazolium-4-carboxylate, an antimicrobial agent.8
We set out to explore the synthesis of 1,3-dialkylimidazolium salts using these procedures and report here, the preparation and X-ray crystal structure of the zwitterion, 1,3-dimethylimidazolium-2-carboxylate,‡ which was obtained in high yield from the reaction of DMC with 1-methylimidazole (Fig. 1), rather than either 1,3-dimethylimidazolium methyl carbonate or 1,3-dimethylimidazolium-4-carboxylate, anticipated.6,8.
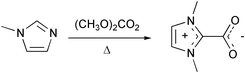 | ||
| Fig. 1 Synthesis of 1,3-dimethylimidazolium-2-carboxylate. | ||
DMC can act as either a carboxymethylating agent (BAC2 mechanism) or as an alkylating agent (BAL2 mechanism).
Formation of the zwitterion product is directly linked to initial alkylation of imidazole followed by further reaction of the methyl carbonate anion with the imidazolium cation.9 A possible mechanism is an initial BAL2 step in which the imidazole is N-alkylated by DMC. The acidic C2-hydrogen of the resulting 1,3-dimethylimidazolium cation is abstracted by the methyl carbonate anion, leading to the heterocarbene and HOC(O)OMe which is unstable and gives rise to MeOH and CO2. Nucleophilic attack on CO2 by the carbene is the only favored process and leads to the observed zwitterion. In addition, support for this mechanism is also given by the observed formation of 1,2,3-trimethylimidazolium methyl carbonate on reaction of DMC with 1,2-dimethylimidazole, which contains no abstractable C2-hydrogen.
1,3-Dialkylimidazolium-2-carboxylate esters have been previously reported, synthesized by reaction of 2-lithio-N-methylimidazole with trimethylsilyl chloride and alkylchloroformate followed by N-alkylation,10 and are of interest as alkoxycarbonyl transfer reagents that mimic biological processes (for example, the action of thiamine in vitamin B1). Only one example of a free zwitterionic carboxylate salt, 1,3-diisopropyl-4,5-dimethylimidazolium-2-carboxylate11 and four dithiocarboxylate analogs12,13 including 1,3-dimethylimidazolium-2-dithiocarboxylate13 are known. In each case, the salts, were obtained as stable adducts from the condensation of the corresponding imidazolyl carbene with CO2or CS2respectively.
The structure, and atom numbering of the zwitterion§ is shown in Fig. 2, where intermolecular hydrogen-bonded sheets containing head-to-tail hydrogen-bonding from the two aromatic imidazolium-ring hydrogens to carboxylate groups are observed. The molecule resides on a crystallographic two-fold axis and consists of a planar imidazolium ring with all the C atoms of the molecule in the plane, and the carboxylate O atoms twisted out of this plane (δ = 29.03°). The C–COO bond distance (1.523(3) Å) corresponds to that of a single bond, and the C–O distances are equivalent by symmetry (1.2398(15) Å), indicating that the negative charge is equally distributed on the fully ionized carboxylate group with no electronic delocalization between the ring and carboxylate group. The ring bond distances, C–C (1.347(3) Å), N–CCOO (1.3452(16) Å), and N–C (1.3800(18) Å) are comparable with the mean bond-lengths of imidazolium salts (1.342, 1.345, 1.375 Å respectively).14,15
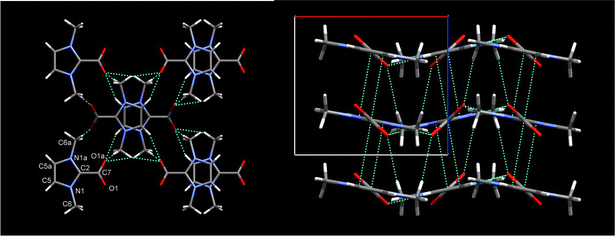 | ||
| Fig. 2 Packing and hydrogen-bonding in the crystal structure of 1,3-dimethylimidazolium-2-carboxylate showing the columnar ±b π–π stacking of imidazolium rings along the c axis and C–O⋯H hydrogen bonding in the ab plane (left), and the undulating sheet structure in the ab axis (right). | ||
The carboxylate zwitterion, in comparison to 1,3-dimethylimidazolium-2-dithiocarboxylate,13 has a similar molecular structure (the relative positions of all C and N atoms are equivalent), but a significantly different crystal structure which results from the different tilt angle of the carboxylate group relative to the heterocyclic ring. For the dithiocarboxylate, the CS2 group is tilted almost perpendicularly relative to the ring; (δ = 77°), which precludes π–π stacking and instead, the methyl groups point towards adjacent imidazolium rings.
The zwitterions stack into columns along the crystallographic c axis, the direction of the carboxylate groups alternating ± b along the columns, reminiscent of the packing of imidazolium cations in emimBr/I.15 Each columnar stack, contains imidazolium rings close-packed via π–π interactions with inter-ring atom close contacts of C2–C5 = 3.378 Å, and N1–C5 =3.300 Å. The heterocyclic rings are tilted 10.61° from perpendicular and the carboxylate group is 39.64° from the perpendicular. Within the ab plane, alternating hydrogen-bonded sheets are formed, aligned along the b axis in which each carboxylate group participates in three intermolecular hydrogen-bonds (O1–H5C, O1–H6C, and O1–H6A) and one intra-molecular hydrogen-bond (O1–H6B). The shortest hydrogen-bonds (O1–H5C = 2.261 Å) to the two most acidic hydrogens in the molecule (C5–H and C5A–H) propagate the sheet while the O1–H6A hydrogen-bond is a ‘between sheet’ bond to the π-stacked neighbors which maintains the packed structure.
In conclusion, the initial synthesis and crystal structure of an unusual zwitterion which was obtained unexpectedly, and in excellent yield, as the sole product from the attempted synthesis of a methylcarbonate-containing ionic liquid, by the reaction of DMC with 1-methylimidazole at 120–130 °C are reported. The synthesis, in which the acidic C2-hydrogen of the imidazolium cation initially formed is abstracted by the anion producing, via a carbene, the C2-carboxylate zwitterion, is of interest as a potentially clean, minimal waste, route to prepare ionic liquids, and also for the preparation of heterocyclic carbene adducts without using traditional strong bases.
U. A. acknowledges financial support from the NASA, Kennedy Space Center. LSEO thank UB, CNRS, and Région Bourgogne for support (EB, IT).
Notes and references
- See for example Ionic Liquids: Industrial Applications for Green Chemistry, eds. R. D. Rogers and K. R. Seddon, ACS Symposium Series 818, American Chemical Society, Washington DC, 2002 Search PubMed.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC; R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974 CrossRef CAS.
- J. Zimmermann, P. Wasserscheid, I. Tkatchenko and S. Stutzmann, Chem. Commun., 2002, 760 RSC.
- A.-A. Shaik and S. Siravam, Chem. Rev., 1996, 96, 951 CrossRef CAS; P. Tundo, Pure Appl. Chem., 2001, 73, 1117 Search PubMed; M. Selva, P. Tundo and A. Perosa, J. Org. Chem., 2001, 66, 677 CrossRef CAS; M. Lissel, Liebigs Ann. Chem., 1987, 77 Search PubMed; M. Lissel, S. Schmidt and B. Neumann, Synthesis, 1986, 382 CrossRef CAS.
- B. Albert and M. Jansen, Z. Anorg. Allg. Chem., 1995, 621, 1735 CrossRef CAS.
- S. Mori, K. Ida and M. Ue, US Pat., 4,892,944, 1990.
- M. Ue, M. Tekeda, T. Takahashi and M. Takehara, Electrochem. Solid-State Lett., 2002, 5, 119A CrossRef.
- J. Fischer, W. Siegel, V. Bomm, M. Fischer and K. Mundinger, US Pat., 6,175,019, 2001.
- E. Bouajila, H. Chermette, D. Poinsot, I. Tommasi, I. Tkatchenko and O. Walter, Angew. Chem., Int. Ed., submitted Search PubMed.
- C. Bakhtiar and E. H. Smith, J. Chem. Soc., Perkin Trans. 1, 1994, 234 RSC.
- N. Kuhn, M. Steimann and G. Weyers, Z. Naturforsch., Teil B, 1999, 54, 427 Search PubMed.
- N. Kuhn, E. Niquet, M. Steimann and I. Walker, Z. Naturforsch., Teil B, 1999, 54, 1181 Search PubMed; N. Kuhn, H. Bohnen and G. Henkel, Z. Naturforsch., Teil B, 1994, 49, 1473 Search PubMed.
- L. L. Borer, J. V. Kong and D. E. Oram, Acta Crystallogr., Sect. C, 1989, 45, 1169 CrossRef.
- A. K. Abdul-Sada, A. M. Greenway, P. B. Hitchcock, T. J. Mohammed, K. R. Seddon and J. A. Zora, J. Chem. Soc., Chem. Commun., 1986, 1253 RSC; J. S. Wilkes and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1994, 965 RSC; J. Fuller, R. T. Carlin, H. C. De Long and D. Haworth, J. Chem. Soc., Chem. Commun., 1994, 299 RSC; J. J. Golding, D. R. MacFarlane, L. Spiccia, M. Forsyth, B. W. Skelton and A. H. White, Chem. Commun., 1998, 1593 RSC.
- A. Elaiwi, P. B. Hitchcock, K. R. Seddon, N. Srinivasan, Y. M. Tan, T. Welton and J. A. Zora, J. Chem. Soc., Dalton Trans., 1995, 3467 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental data for 1,3-dimethylimidazolium-2-carboxylate. Supplemental crystal structure data. ORTEP, hydrogen bonding and packing diagrams. See http://www.rsc.org./suppdata/cc/b2/b211519k/ |
| ‡ Dimethyl carbonate (1.5 mL) and 1-methylimidazole (1 mL) were combined and heated in a sealed screw-top pressure tube (Ace Glass) for 24 h at 120 °C. After ca. 1 h, the initially clear solution started to cloud, and yielded colorless crystals formed in a pale yellow supernatant liquid. The reaction has also been scaled-up in MeOH solution, to yield batches between 15–35 g of the zwitterion salt. |
| § X-Ray structure of 1,3-dimethylimidazolium-2-carboxylate. Identical crystals were collected (i) directly from the reaction mixture and (ii) via crystal growth on a cold finger from a suspension of the zwitterion in toluene under reflux. In either case, the crystals obtained were equivalent; both structures are included in the supplemental data.Data were collected on a Siemens CCD area detector-equipped diffractometer with Mo-Kα (λ = 0.71073 Å) radiation and solved using the SHELXTL software package. All non-hydrogen atoms were anisotropically refined and were isotropically refined. Crystal data: formula C6H8N2O2, M = 140.14, orthorhombic, a = 8.334(4), b = 11.605(5), c = 6.706(3) Å, V = 648.6(5) Å3, T = 173 K, space group Pbcn (#60), Z = 4, μ(Mo-Kα) = 0.110 mm−1, R1 = 0.0288, wR2 = 0.0824 (I > 2σ(I)). CCDC 198167. See http://www.rsc.org/suppdata/cc/b2/b211519k/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |
