Ni(II) Complexes containing chiral tridentate phosphines as new catalysts for the hydroamination of activated olefins
Luca
Fadini
and
Antonio
Togni
*
Department of Chemistry, Swiss Federal Institute of Technology, ETH Hönggerberg, CH-8093 Zürich, Switzerland. E-mail: togni@inorg.chem.ethz.ch; Fax: +41 1 6321310
First published on 25th November 2002
Abstract
Ni(II) complexes containing chiral tridentate ferrocenyl phosphines (Ni(PPP)) have been found to efficiently catalyse the hydroamination of activated olefins with both anilines and aliphatic amines at r.t. (TON up to 71, TOF up to ca. 3 h−1, and enantioselectivities up to 69% ee).
Amines constitute an industrially very important class of products and very valuable intermediates.1 The direct addition of a secondary amine to an olefin, leading to a new tertiary amine, is particularly attractive. However, this transformation is generally only weakly exergonic and its reaction entropy is highly negative.2 These thermodynamic characteristic have made hydroamination of olefins a reaction particularly difficult to catalyse and in fact no generally applicable system has been reported.3 After a predominance of work concerning catalysts containing group 9 metals,4,5 early transition metals,6 or lanthanides,7 Hartwig and co-workers recently disclosed systems based on group 10 metals, in particular Pd(II)8 and Ni(II)9 complexes containing bidentate ligands, as active catalysts for the hydroamination of vinylarenes and 1,3-dienes, respectively. We, on the other hand, reported first-principle computational studies indicating that Ni(II) derivatives should constitute the potentially most active catalysts for hydroamination reactions occurring via olefin activation and nucleophilic attack of the amine, by virtue of the lowest activation energy for the rate-determining step, i.e. the protonolysis of the intermediate 2-aminoalkyl complex.10 It was therefore obvious for us to undertake an experimental study of Ni catalysts, addressing both activity and stereoselectivity for model reactions involving the addition of both arylic and aliphatic amines to activated olefins.11
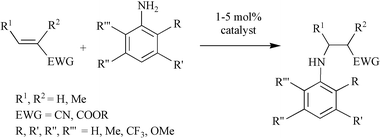
We found that all three group 10 metals bearing either bi- or tridentate ligands gave species that were able to catalyse the known addition of an amine to acrylonitrile, forming exclusively the anti-Markovnikoff product. We therefore focussed our attention on the addition of derivatives of aniline to crotonitrile and methacrylonitrile using Ni(II) catalysts (Scheme 1). In the absence of catalyst such reactions require very high temperatures, and for the group 10 metals only the combination of Pd(OAc)2 or Pd(TFA)2 with PCP or PNP ligands gave complete conversion at 100 °C.11Scheme 2 shows the chiral bi- and tridentate ligands, containing one or two ferrocenyl units, respectively, that were exploited in our study. The reactions were carried out at room temperature using catalysts formed in situ,† however isolated complexes showed similar activities and selectivities under the reported conditions. Table 1 collects the results obtained for the addition of aniline derivatives to cyanoolefins using a catalyst containing the tridentate ligand Pigiphos.12 Surprisingly, the bidentate ferrocenyl phosphine Josiphos did not afford active catalysts. The new Ni catalyst showed relatively high activities with TON reaching at least 70 for the addition of aniline to crotonitrile, and the isolated yield of hydroamination product were moderate to high.
 | ||
| Scheme 1 Catalytic addition of aromatic amines to activated olefins. | ||
![PP and PPP ligands.Josiphos = (R)-1-[(S)-2-(diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine and Pigiphos12 = (bis((S)-1-[(R)-2-(diphenylphosphino)ferrocenyl]ethyl)cyclohexylphosphine.](/image/article/2003/CC/b210680a/b210680a-s2.gif) | ||
| Scheme 2 PP and PPP ligands.‡ | ||
| Entry | Amine | Olefin | Conditionsa | Yieldb | eec |
|---|---|---|---|---|---|
| a Reactions in THF, under inert conditions, catalyst: in situ generated [Ni(PPP)(THF)](ClO4)2. b Yields are for isolated material after FC. c Enantioselectivity determined by GC or HPLC. d No separation conditions were found. | |||||
| 1 |

|
|
r.t., 24 h, 5% cat.
r.t., 24 h, 1% cat. r.t., 24 h, 5% cat. in CH3CN |
91%
71% 50% |
22%
15% 21% |
| 2 |
|

|
r.t., 24 h, 5% cat.
r.t., 24 h, 1% cat. |
85%
35% |
18%
22% |
| 3 |

|

|
r.t., 24 h, 5% cat. | 35% | 18% |
| 4 |
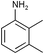
|

|
r.t., 24 h, 5% cat. | 26% | 24% |
| 5 |

|

|
r.t., 24 h, 5% cat. | 69% | 18% |
| 6 |

|
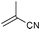
|
r.t., 24 h, 5% cat. | 52% | 8% |
| 7 |

|
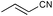
|
r.t., 24 h, 5% cat. | 77% | n.d.c |
| 8 |

|

|
r.t., 24 h, 5% cat. | 62% | n.d.d |
However, the substitution degree and pattern of the aromatic amine clearly influence the outcome of the catalytic reaction. In particular, ortho-substituents are detrimental to high catalytic activity (Table 1, entries 3–4), obviously for steric reasons. Strongly electron-releasing substituents, such as in 3,5-dimethoxyaniline (Table 1, entries 7–8), led to slightly increased yields, as compared to methyl groups in the same position (entries 5–6). On the other hand, 3,5-bis(trifluoromethyl)aniline could not be added to any cyanoolefin under the same conditions. A strongly coordinating solvent such as acetonitrile has a negative effect on the catalytic activity. Acetonitrile is probably competing with the cyanoolefin as ligand for Ni.
The activities observed for our Ni catalyst are comparable to or better than those previously reported for Pd catalysts,11 for which generally higher temperatures and longer reaction times are required. However, the enantioselectivities obtained are generally low and for the substrate combinations of Table 1 reach only ca. 25% ee. Whereas the presence of a source of H+ was necessary for previously reported systems,13 in the case of the Ni-catalysed reaction the addition of TfOH led to complete catalyst deactivation and no reaction was observed.
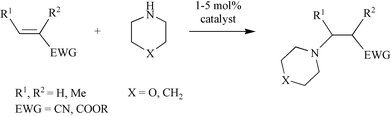
Encouraged by the results obtained with anilines, we turned our attention to aliphatic amines, in particular morpholine and piperidine, and investigated their catalytic addition to activated olefins (Scheme 3). As expected, the catalytic activities observed in this case were higher than with anilines. Table 2 summarises the results obtained for reactions of aliphatic amines with acrylic acid derivatives. Using 5 mol% catalyst the addition of the selected aliphatic amines to crotonitrile, methacrylonitrile, methyl acrylate, and methyl or ethyl crotonate afforded good to excellent isolated yields of the corresponding product, again working at room temperature and with a reaction time of not more than 24 h. The most remarkable example (Table 2, entry 2) concerns the hydroamination of methacrylonitrile with morpholine that gave the product in quantitative yield and 69% ee. This is one of the still rare example of an asymmetric hydroamination reaction characterised by both relatively high catalytic activity and enantioselectivity.14 Previous examples were additions of anilines reported either by Hartwig using Pd catalysts or by us with the Ir/fluoride system (80% yield and 81% ee for p-trifluoromethylstyrene,8 83% yield and 95% ee for cyclohexadiene,9 and 22% yield and 95% ee for norbornene5). Crotonates add morpholine quite effectively (Table 2, entries 6–7), however, no chiral induction was observed.
| Entry | Amine | Olefin | Conditionsa | Yieldb | eec |
|---|---|---|---|---|---|
| a Reactions in THF, under inert conditions, catalyst: in situ generated [Ni(PPP)(THF)](ClO4)2. b Yields are for isolated material after FC. c Enantioselectivity determined by GC or HPLC analysis. d The results are an average of 3 runs. | |||||
| 1 |

|

|
r.t., 24 h, 5% cat.
r.t., 24 h, 1% cat. |
99%
62% |
rac
rac |
| 2 |

|

|
r.t., 24 h, 5% cat. | 99% | 69%d |
| 3 |

|
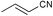
|
r.t., 24 h, 5% cat. | 85% | 7% |
| 4 |
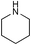
|
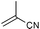
|
r.t., 24 h, 5% cat. | 99% | 20% |
| 5 |

|

|
r.t., 24 h, 5% cat. | 70% | |
| 6 |

|
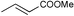
|
r.t., 24 h, 5% cat. | 63% | rac |
| 7 |
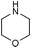
|

|
r.t., 24 h, 5% cat. | 77% | rac |
The results obtained with our Ni catalyst are very encouraging because they concerns olefins that have not yet been investigated from the point of view of asymmetric hydroamination and because they are substrates for which an η3-allylic intermediate is not accessible.8,9 We are currently pursuing modifications of the Pigiphos ligand framework with a view to improving the enantioselectivity of the hydroamination reactions reported here.
This work was supported by the Swiss National Science Foundation.
Notes and references
- J. J. Brunet and D. Neibecke, in Catalytic Heterofunctionalization, ed. A. Togni and H. Grützmacher, VCH, Weinheim, 2001, pp. 91–141 Search PubMed.
- D. Steinborn and R. Z. Taube, J. Mol. Catal., 1989, 49, 235 CrossRef.
- T. Müller and M. Beller, Chem. Rev., 1998, 98, 675 CrossRef and references therein.
- J. J. Brunet, G. Commenges, D. Neibecker and K. Philippot, J. Organomet. Chem., 1994, 469, 221 CrossRef CAS; M. Beller, H. Trauthwein, M. Eichberger, C. Breindl and T. Müller, Eur. J. Inorg. Chem., 1999, 1121 CrossRef CAS; A. L: Casalnuovo, J. C. Calabrese and D. Milstein, J. Am. Chem. Soc., 1988, 110, 6738 CrossRef CAS.
- Dorta, P. Egli, F. Zürcher and A. Togni, J. Am. Chem. Soc., 1997, 119, 10857 CrossRef CAS.
- C. Cao, J. T. Ciszewski and A. L. Odom, Organometallics, 2001, 20, 5011 CrossRef CAS; L. Ackermann and R. G. Bergman, Org. Lett., 2002, 4, 1475 CrossRef CAS; E. Haak, H. Siebenreicher and S. Doye, Org. Lett., 2000, 2, 1935 CrossRef CAS and references therein.
- S. Tian, V. M. Arredondo, C. L. Stern and T. J. Marks, Organometallics, 1999, 18, 2568 CrossRef CAS.
- M. Kawatsura and J. F. Hartwig, J. Am. Chem. Soc., 2000, 122, 9546 CrossRef CAS; U. Nettekoven and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 1166 CrossRef CAS.
- J. Pawlas, Y. Nakao, M. Kawatsura and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 3670 CrossRef; O. Löber, M. Kawatsura and J. F. Hartwig, J. Am. Chem. Soc., 2001, 123, 4366 CrossRef CAS.
- H. M. Senn, P. Blöchl and A. Togni, J. Am. Chem. Soc., 2000, 122, 4098 CrossRef CAS.
- M. Kawatsura and J. F. Hartwig, Organometallics, 2001, 20, 1960 CrossRef CAS.
- P. Barbaro and A. Togni, Organometallics, 1995, 14, 3570 CrossRef CAS.
- A. L. Seligson and W. C. Trogler, Organometallics, 1993, 12, 744 CrossRef CAS.
- M. Nobis and B. Driessen-Hölscher, Angew. Chem., Int. Ed., 2001, 40, 3983 CrossRef CAS.
Footnotes |
| † General procedure for the nickel-catalysed addition of aniline to activated olefins: Ni(ClO4)2·6H2O (22.5 mg, 0.06 mmol), Pigiphos (0.54 ml of a 0.1105 M standard solution in THF, 0.06 mmol), aniline (112 μl, 1.23 mmol) and crotonitrile (0.2 ml, 2.46 mmol) were dissolved in 2 ml THF. The reaction mixture was stirred at r.t. for 24 h. The product was isolated by silica gel column chromatography (5∶1 hexane∶ethyl acetate) to give 179.2 mg (91%) of 2-anilinopropyl cyanide. The enantioselectivity was determined by GC on a chiral stationary phase (α-cyclodextrin, 120 °C iso, tR = 116.3 min and 118.5 min), [α]D20 = +18.88 (c = 1 in CHCl3).General procedure for the nickel-catalysed addition of morpholine to activated olefins: Ni(ClO4)2)·6H2O (22.5 mg, 0.06 mmol), Pigiphos (0.906 ml of a 0.0662 M standard solution in THF, 0.06 mmol), morpholine (107 μl, 1.23 mmol) and methacrylonitrile (0.2 ml, 2.46 mmol) were dissolved in 2 ml THF. The reaction mixture was stirred at r.t. for 24 h. The product was isolated by silica gel column chromatography (1∶1 hexane∶ethyl acetate + 5% NEt3) to give 189 mg (99%) of 1-methyl-2-morpholinoethyl cyanide. The enantioselectivity was determined by GC on a chiral stationary phase (β-cyclodextrin, 92 °C iso, tR = 139.2 min and 142.7 min), [α]D20 = −23.78 (c = 1 in CHCl3). |
| ‡ Josiphos = (R)-1-[(S)-2-(diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine and Pigiphos12 = (bis((S)-1-[(R)-2-(diphenylphosphino)ferrocenyl]ethyl)cyclohexylphosphine. |
| This journal is © The Royal Society of Chemistry 2003 |