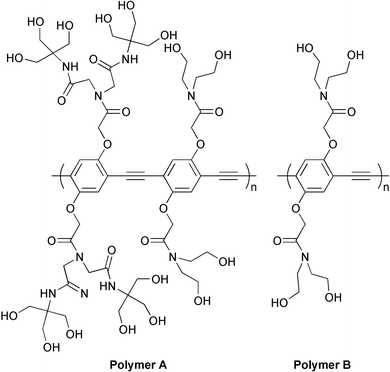
Synthesis of a nonionic water soluble semiconductive polymer
Kenichi
Kuroda
and
Timothy M.
Swager
*
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA, USA 02139
First published on 28th November 2002
Abstract
A new nonionic water-soluble fluorescent conjugated polymer is reported with hydroxyl and amide side chains surrounding an aromatic polymer backbone.
Conjugated polymer biosensory materials are of great interest because of the high sensitivity of their optical and conducting properties in the presence of analytes.1,2 In particular, water-soluble conjugated polymers offer powerful new methods for the trace detection of analytes in aqueous environments. However, applications to aqueous sensing have been limited as a result of the challenges associated with strong interactions between hydrophobic backbones and aromatic π–π stacking that severely restricts their water solubility. The conventional approach to water-soluble conjugated polymers employs ionic (often in the side chains) sulfonic,3 carboxylic4 or ammonium groups.5 These groups give strong enthalpic interactions with water and electrostatic repulsions between the polymer chains, both of which promote solubility. Ionic conjugated polymers have been applied in biosensor schemes,5–7 however, these systems have several disadvantages: the solution’s pH and ionic strength have to be adjusted to prevent aggregation of conjugated polymers and non-specific electrostatic interactions between ionic polymers and biomolecules such as proteins and DNA will reduce target specificity. Considering the demerits of ionic sensory polymers, we have chosen to develop nonionic (neutral) water-soluble semiconductive polymers as a platform for high specificity biosensory polymers.
To achieve high water-solubility without ionic groups, we have investigated a number poly(phenylene ethynylene)s (PPEs) with hydrophilic groups in the side chains. The extremely hydrophobic nature of the PPE backbone made it necessary to place many hydroxyls proximate to the polymer backbone and thereby shield it from water. Based upon this design principle, we synthesized nonionic and completely water-soluble PPE A with hydroxyl and amide groups surrounding the polymer chain. Polymer A was synthesized by a Sonogashira cross-coupling reaction of diiodobenzene (9) and diethynylene benzene derivatives (6) prepared according to Schemes 1 and 2. As shown in Scheme 1 diiodohydroquinone (1) was reacted with ethyl bromacetate to give ester (2) which after reaction with diethanolamine was treated with triisopropylsilyl chloride in THF to give organic soluble 3, which is easily purified by re-crystallization from CH2Cl2/methanol. Compound 5 is obtained from 3 by standard Sonogashira cross-coupling procedures. The TIPS groups were removed by treatment with tributylammonium fluoride (TBAF) in THF and compound 6 precipitates from MeOH/CH2Cl2.† Diiodobenzene derivative 4 was made by cleavage of TIPS groups from 3 in the same procedure for 6.
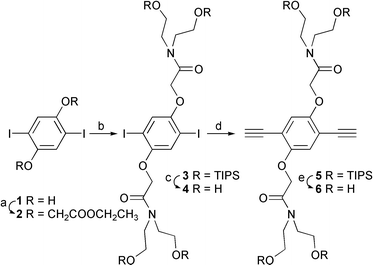 | ||
| Scheme 1 (a) Ethyl bromacetate, K2CO3, 2-butanone, reflux; (b) (i) diethanolamine, ethanol, reflux, (ii) triisopropylsilyl chloride, imidazole, THF; (c), (e) TBAF, THF; (d) (i) (trimethylsilyl)acetylene, CuI, (PPh3)2PdCl2, benzene/triethylamine, 60°C, (ii) K2CO3, MeOH/THF. | ||
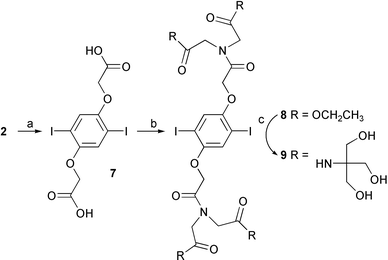 | ||
| Scheme 2 (a) NaOH/methanol, reflux; (b) (i) oxalyl chloride, reflux, (ii) diethyl iminodiacetate, CH2Cl2, Et3N; (c) Tris, DMSO, K2CO3. | ||
The most hydrophilic monomer 9† was prepared in three steps from 2 (Scheme 2). The ester group of 2 is hydrolyzed, converted to the acid chloride, and reacted with diethyl iminodiacetate to give 8. The target compound 9 was afforded by adaptation of Newkome’s synthesis of arborols.8 Compound 8 is treated with tris(hydroxylmethyl)aminomethane (Tris) in DMSO in the presence of K2CO3 at room temperature.8,9 After workup the oily residue was rinsed with CH2Cl2 to give 9 as a precipitate. In this synthetic approach, 9 was obtained without protection of the hydroxyl group in contrast to the synthesis of 6. This monomer is completely soluble in DMF, DMSO, and water.
Monomers 6 and 9 (54 mM) were polymerized by Sonogashira cross-coupling reaction in the presence of 5% (PPh3)4Pd and 5% CuI in morpholine at 50 °C overnight. The polymer was precipitated in ethyl acetate and dried under vacuum. The obtained polymer A is readily soluble in water dissolving in essentially all proportions instantaneously without heating. Gel permeation chromatography analysis (GPC) (eluent: DMF, PMMA standards) indicated that Mw and Mn of the obtained polymer are 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 32
000 and 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. We also synthesized a homopolymer, polymer B, by coupling 4 and 6 in the same polymerization procedure. (Mn = 12
000, respectively. We also synthesized a homopolymer, polymer B, by coupling 4 and 6 in the same polymerization procedure. (Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487, Mw = 24
487, Mw = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 122). However this material is only slightly soluble (est. 0.1 mg ml−1) in water after heating at 60°C overnight.
122). However this material is only slightly soluble (est. 0.1 mg ml−1) in water after heating at 60°C overnight.
UV-vis and fluorescence spectra of aqueous and DMF solutions of polymer A are shown in Fig. 1. The aqueous solution prepared by dissolving polymer A in water at room temperature showed small peaks in the higher wavelength region of the absorption and fluorescence. These features disappeared after heating at 60 °C overnight. Aggregation of conjugated polymers generally produces such new low energy absorption and emission features. Therefore, the spectral change indicates that polymer A is initially aggregated under the conditions of the fluorescence experiments but dissociates after heating. Quantum yields of polymer A were 0.07 in water and 0.27 in DMF determined with quinine sulfate as a standard. The origin of the reduced quantum yield in water is unclear. It is still possible that the strong hydrogen bonding tendency of the side chains produces an unfavorable organization about the polymers. Although not apparent from the spectra after heating, a very weak aggregation can not be ruled out. Newkome reported aggregation and gelation in water of arborols, which consist of two hydrophilic Tris-based dendron spheres connected with a lipophilic bridge such as an alkyl chain or phenyl groups.8 Further spectroscopic characterizations of the polymer solution are in progress in our laboratory.
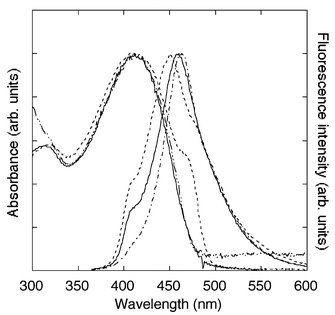 | ||
| Fig. 1 UV-vis absorption spectra and fluorescence spectra of polymer A: aqueous solution before heating (dashed line), after heating (solid line) and DMF solution (broken-dot line). | ||
This work was supported by the National Science Foundation through the Center for Materials Science and Engineering at MIT and NASA.
Notes and references
- D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS.
- T. M. Swager, Acc. Chem. Res., 1998, 31, 201 CrossRef CAS.
- S. Shi and F. Wudl, Macromolecules, 1990, 23, 2119 CrossRef CAS.
- H. Häger and W. Heitz, Macromol. Chem. Phys., 1998, 199, 1821 CrossRef.
- M. Stork, B. S. Gaylord, A. J. Heeger and G. C. Bazan, Adv. Mater., 2002, 14, 361 CrossRef CAS.
- C. Fan, K. W. Plaxco and A. J. Heeger, J. Am. Chem. Soc., 2002, 124, 5642 CrossRef CAS.
- R. M. Jones, T. S. Bergstedt, D. W. McBranch and D. G. Whitten, J. Am. Chem. Soc., 2001, 123, 6726 CrossRef CAS.
- G. R. Newkome, X. Lin, C. Yaxiong and G. H. Escamilla, J. Org. Chem., 1993, 58, 3123 CrossRef CAS.
- L. J. Twyman, A. E. Beezer, R. Esfand, M. J. Hardy and J. C. Mitchell, Tetrahedron Lett., 1999, 40, 1743 CrossRef CAS.
Footnote |
| † Compound 6: 1H NMR (300 MHz, DMSO-d6): δ 6.91 (s, 2H), 5.00 (t, 2H), 4.94 (s, 2H), 4.71 (t, 2H), 4.34 (s, 2H), 3.59-3.33 (m, 16H); 13C NMR (75 MHz, DMSO-d6): 167.15, 152.89, 116.88, 111.84, 86.06, 79.98, 66.21, 58.61, 49.23, 47.73. HR-MS (ESI) calcd. for C22H28N2O8: 449.1918 (M+ + H), found: 449.1903 (M+ + H), 471.1725 (M+ + Na). Compound 9: 1H NMR (300 MHz, DMSO-d6): δ 7.73 (bs, 2H), 7.42 (bs, 2H), 7.23 (bs, 2H), 4.85 (bs, 4H), 4.62 (bs, 12H), 4.06 (bs, 8H), 3.95 (bs 4H), 3.56 (bd, 24H). HR-MS (ESI) calcd. for C34H54I2N6O20: 1143.1374 (M+ + Na), found: 1143.1377 (M+ + Na). |
| This journal is © The Royal Society of Chemistry 2003 |