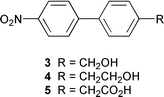
Enhanced photoreactivity of the nitrobiphenyl chromophore
Received
26th September 2001
, Accepted 1st October 2001
First published on 2nd January 2002
Abstract
Several p-nitrobiphenyl derivatives show significantly higher photoreactivity compared to their p-nitrophenyl analogs, in addition to displaying a new acid-catalyzed photodecarboxylation mechanism in p-(p′-nitrophenyl)phenylacetic acid.
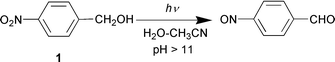
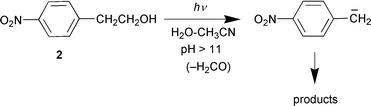
Electronically excited states of aromatic compounds with appropriate substituents (particularly amino, cyano, methoxy, and nitro) are known to be highly polarized as a result of their charge-transfer character.1,2 A number of nucleophilic and electrophilic aromatic photosubstitution reactions and other types of reactions have been attributed to these enhanced effects.1 Some time ago, we discovered that the enhanced electron-withdrawing effect of both m- and p-nitro groups in the excited triplet state induces intramolecular redox chemistry observed for m- and p-nitrobenzyl alcohols (eqn. (1)) and photo-retro-Aldol-type (eqn. (2)) and related reactions for other nitroaromatic compounds.3 For the first two types of reactions, the primary photochemical step has been proposed to be benzylic C–H
or C–C bond heterolysis, respectively, to generate the corresponding nitrobenzyl carbanion intermediate. This is facilitated by a highly polarized triplet excited state induced by the nitro group. There is now ample evidence that appropriately substituted biaryls and terphenyls are even more polarized than their single ring counterpart, through a more planar geometry available on the excited state surface.2,4 In this report, we show that of the reactions investigated, nitrobiphenyls are significantly more reactive than their single ring counterpart.
| |
| (1) |
| |
| (2) |
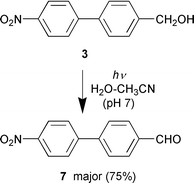
The exploratory study involved three readily available p-nitrobiphenyls 3–5, each designed to test a type of reaction. They were all readily synthesized from p-methyl-p′-nitrobiphenyl (6). Nitrobiphenyl 3 is the biphenyl version of 1 and was designed to test for photoredox chemistry. Nitrobiphenyls 4 and 5 were designed to test for the photo-retro-Aldol type reaction (eqn. (2)) and photodecarboxylation, respectively. Photolysis (≈10−3 M, 1 ∶ 1 H2O–CH3CN; pH 7 unless otherwise noted; Rayonet RPR 100 photochemical reactor, 300 or 350 nm lamps, argon purged continuously; ≈12 °C; photolysis times 5–60 min; NMR tubes or 100 mL quartz tubes) of 3 under conditions in which 1 was completely unreactive gave p-(p′-nitrophenyl)benzaldehyde (7) as the
major product (75%), with Φ
≈ 0.1† (eqn. (3)). A minor product (25%) tentatively assigned to the azo compound derived from partial reduction of 7 was also observed. Although the photoredox chemistry observed for 3 is not entirely analogous to that reported for 1 (i.e., lack of a nitroso group on the product), the reaction exhibits many of the characteristics associated with the reported photoredox chemistry of nitrobenzenes.3a For example, the reaction efficiency of 3 shows no concentration dependence and requires water in the solvent. In addition, if the readily oxidizable
CH2OH moiety was replaced by a methyl group (i.e., compound 6), no reaction was observed.
| |
| (3) |
We next examined the photochemistry of 4. Although no photochemistry was observed on photolysis of 4 at pH 7, very efficient photo-retro-Aldol chemistry was observed at pH > 11 (the product was 6), with quantum efficiencies about an order of magnitude higher than those measured for 2 at the same pH (Φ
≈ 0.5, pH 13 for 4).‡ The enhanced reactivity of the nitrobiphenyl moiety was further demonstrated by the photodecarboxylation of 5. p-Nitrophenylacetic acid is the parent system in this case, which in itself is known to be highly reactive to photodecarboxylation (pH > 3), with Φ
= 0.603b (pH 7). However, below about pH 3, the compound is photoinert indicating that the reaction requires the carboxylate form of the acid.3b Significant differences in the photodecarboxylation of 5 compared to the parent p-nitrophenylacetic acid were observed. Firstly, in the range pH 6–12, the compound photodecarboxylates
with Φ
≈ 1, the major product (>95%) being 6, obtained via protonation of the corresponding carbanion intermediate. The higher quantum yield is consistent with a more reactive chromophore, already suggested by the reactivity of 3 and 4. Between pH 3–6 (Fig. 1), there is a drop in reaction efficiency indicating that the acid form of the compound is less reactive at these pHs, as observed for p-nitrophenylacetic acid. However, below pH 3, instead of decreasing smoothly to the baseline (as observed for p-nitrophenylacetic acid),3b the plot of quantum yield vs. pH (Fig. 1) takes on a more complex behavior where increasing the acidity actually results in an increase in reactivity. Moreover, a new pathway was observed in acid, as evidenced by the formation of a new photoproduct, p-(p′-nitrosophenyl)benzyl
alcohol (9) (Fig. 1, Scheme 1). UV-Vis traces of the photoreaction at pH 2 clearly show the operation of a new photochemical pathway in acid (Fig. 2). A logical intermediate in the formation of 9 is the protonated aci-nitro species 8, formed via loss of CO2 concerted with protonation at the oxygen of the nitro group. This new mechanism is made possible via an excited state that has significantly more charge-transfer character than the parent system. This would allow protonation at the nitro group before or concerted with decarboxylation of the acid form of 5, giving rise to 8 that is sufficiently long-lived in acid to be completely trapped (in a nucleophilic manner) by water to give 9.
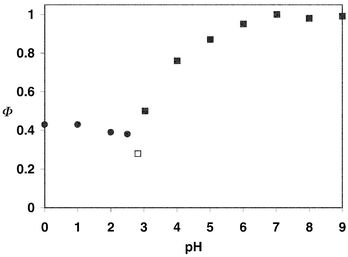 |
| | Fig. 1 Plot of quantum yield for photodecarboxylation of 5vs. pH (in 1 ∶ 1 H2O–CH3CN, pH is of the water portion). The solid squares are for the normal decarboxylation pathway, to give p-methyl-p′-nitrobiphenyl (6). The solid circles are for the acid-catalyzed pathway, to give p-(p′-nitrosophenyl)benzyl alcohol (9). A mixture of 6 and 9 was observed at pH 2.8 (open square), where both mechanisms are operative. | |
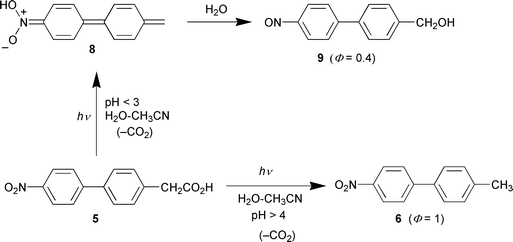 |
| | Scheme 1 | |
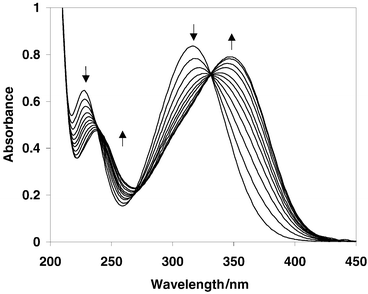 |
| | Fig. 2 UV-Vis traces of the acid-catalyzed photodecarboxylation of 5 in 1 ∶ 1 H2O–CH3CN, pH 2 (λex
= 300 nm; deaerated). Each trace represents 5 s of photolysis. The longer wavelength band that increases in absorbance at 350 nm is that of p-(p′-nitrosophenyl)benzyl alcohol (9). These UV-Vis changes were not observed at pH > 3 where the normal photodecarboxylation route takes place (where the change in chromophore on decarboxylation is minimal). | |
In summary, we have shown that the charge-transfer induced photochemistry observed for nitrophenyl systems are also observed in biphenyl systems with overall higher quantum efficiency. Moreover, new reactivity is possible, as shown in the acid-catalyzed photodecarboxylation of p-(p′-nitrophenyl)phenylacetic acid (5). Extension to analogous terphenyl and oligophenylene systems is envisaged.
Acknowledgements
We acknowledge the continued financial support of the Natural Sciences and Engineering Research Council (NSERC) of Canada and the University of Victoria.
Notes and references
-
(a) E. Havinga and J. Cornelisse, Chem. Rev., 1975, 75, 353 CrossRef;
(b)
J. Cornelisse, in CRC Handbook of Organic Photochemistry and Photobiology, W. M. Horspool and P.-S. Song, eds., CRC Press, Boca Raton, Florida, 1995, ch. 20 Search PubMed;
(c)
D. Döpp, in CRC Handbook of Organic Photochemistry and Photobiology, W. M. Horspool and P.-S. Song, eds., CRC Press, Boca
Raton, Florida, 1995, ch. 81 Search PubMed;
(d)
N. J. Turro, Modern Molecular Photochemistry, Benjamin/Cummings Publishing Co., Menlo Park, California, 1978 Search PubMed;
(e)
A. Gilbert and J. Baggott, Essentials of Molecular Photochemistry, CRC Press, Boca Raton, Florida, 1991 Search PubMed;
(f) H. Shizuka, Acc. Chem. Res., 1985, 18, 141 CrossRef CAS;
(g) P. Wan and G. Zhang, Res. Chem. Intermed., 1993, 19, 119 Search PubMed;
(h) P. Wan, D. W. Brousmiche, C. Z. Chen, J. Cole, M. Lukeman and M. Xu, Pure Appl. Chem., 2001, 73, 529 Search PubMed.
-
(a) M. Maus, W. Rettig, D. Bonafoux and R. Lapouyade, J. Phys. Chem. A, 1999, 103, 3388 CrossRef CAS;
(b) S. Delmond, J.-F. Létard, R. Lapouyade and W. Rettig, J. Photochem. Photobiol. A, 1997, 105, 135 Search PubMed.
-
(a) P. Wan and K. Yates, Can. J. Chem., 1986, 64, 2076 CAS;
(b) P. Wan and S. Muralidharan, J. Am. Chem. Soc., 1988, 110, 4336 CrossRef CAS;
(c) P. Wan, M. J. Davis and M.-A. Teo, J. Org. Chem., 1989, 54, 1354 CrossRef CAS.
-
(a) C.-G. Huang, K. A. Beveridge and P. Wan, J. Am. Chem. Soc., 1991, 113, 7676 CrossRef CAS;
(b) Y. Shi and P. Wan, J. Chem. Soc., Chem. Commun., 1995, 1217 RSC;
(c) M. Lukeman and P. Wan, Chem. Commun., 2001, 1004 RSC.
Footnotes |
| † Quantum yields measured with reference to the known quantum yield for photodecarboxylation for p-nitrophenylacetic acid (Φ
= 0.60, pH 7).3b |
| ‡ The products of photo-retro-Aldol reaction and photodecarboxylation of p-nitrobenzyl derivatives are known to be pH dependent due to competing simple protonation and “disproportionation” pathways of the initially formed p-nitrobenzyl carbanion.3b For the p-nitrobenzyl carbanion, simple protonation gives p-nitrotoluene whereas “disproportionation” gives p,p′-dinitrobibenzyl. At pH 13, the major product (>95%) for 2
is p,p′-dinitrobibenzyl.3b The major product (>98%) observed for 4 at pH 13 was the protonation product, p-methyl-p′-nitrobiphenyl (6). |
|
| This journal is © The Royal Society of Chemistry and Owner Societies 2002 |
Click here to see how this site uses Cookies. View our privacy policy here.