Selective transport of potassium ions across a planar phospholipid bilayer by a calix[4]arene-crown-5 as a synthetic carrier
Takashi
Jin
Section of Intelligent Materials and Devices, Research Institute for Electronic Science, Hokkaido University, Sapporo 060-0812, Japan. E-mail: jin@imd.es.hokudai.ac.jp
First published on 21st November 2001
Abstract
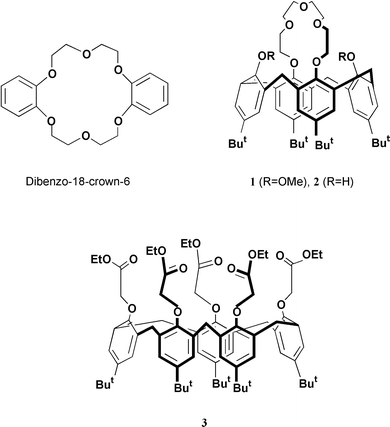
The ion transport ability in a planar phospholipid bilayer, of two p-tert-butylcalix[4]arene-crown-5 derivatives (1, 2), a p-tert-butylcalix[5]arene ester 3, dibenzo-18-crown-6, and valinomycin have been investigated using a voltage clamp method. Membrane current measurements showed that the synthetic calixarene ionophores except for dibenzo-18-crown-6, show ion transport activities for K+ in the bilayer membranes. The order of K+ transport activities of the compounds was valinomycin >1 > 3 > 2. From the measurements of reversal potentials, the relative ion permeability across the bilayer was determined for 1, 3 and valinomycin. Both 1 and 3 showed ion transport selectivity for K+, while valinomycin showed ion transport selectivity for Rb+. Among the calixarene ionophores, compound 1 showed the highest K+ conductivity and K+/Na+ selectivity. Although the K+/Na+ selectivity of 1 is less than that of valinomycin by a factor of ca. 2, calix[4]arene-based ionophore 1 has the potential for use as a synthetic K+ carrier in phospholipid bilayer membranes.
Introduction
Selective ion transport across biological membranes is of crucial importance for all living cells and organisms.1 To understand the mechanism of ion transport in the living systems, artificial phospholipid bilayer membranes have been widely used as models of biological membranes.1,2 Phospholipid bilayer membranes have a thickness of about 30 Å (the region of hydrocarbon chains), and they are highly impermeable to ions, because of the low relative permittivity of hydrocarbon chains of the lipid.2 However, incorporation of ionophoric compounds into phospholipid bilayers mediates ion transport across the bilayer membrane. It is well known that naturally occurring antibiotic ionophores such as monensin,3 valinomycin,4 gramicidin,5 and alamethicin6 have the abilities for selective ion transport across phospholipid bilayer membranes. In the past decade, a variety of synthetic ionophores have been designed for mimicking selective ion transport in biological membrane systems.7We have recently reported that calixarene-based ionophores (p-tert-butylcalix[n]arene ester derivatives) are easily incorporated into phospholipid bilayer membranes, and they can mediate ion transport across the bilayer membranes.8 It has been shown that the ester derivatives of calix[4]arene and calix[5]arene have ion transport activities for Na+ and K+ in phospholipid bilayer systems.8a,b,d,9 In the case of the calix[4]arene ester, the rates of Na+ transport across the bilayer are comparable to the rates of monensin-mediated Na+ transport.8a Although, the calix[5]arene esters show K+ transport activity in phospholipid bilayers, they do not have a high enough K+/Na+ selectivity to be used as selective K+ carriers in biological membranes.8b In spite of numerous new ionophores synthesized,10 valinomycin is still used as the most effective K+ ionophore in biological membrane systems.1,11
The calixarene-based K+ ionophores (calix[4]arene-crown-5 derivatives),12 first reported by Ungaro and co-workers are unique ionophores in which a calix[4]arene is combined with a crown ether bridge. Reinhoudt and co-workers12d have investigated their ion transport properties in supported liquid membranes, and found that calix[4]arene-crown-5 derivatives can act as selective K+ carriers in the membrane. They also found that a 1,3-alternate calix[4]arene-crown-5 conformer has a high K+/Na+ selectivity and its selectivity is better than that of valinomycin in a supported liquid membrane.12e However, there has been no study of ion transport by calix[4]arene-crown-5 derivatives in lipid bilayer systems. The objective of the present study is to determine whether calix[4]arene-crown-5 derivatives have a similar capacity to valinomycin for selective K+ transport across phospholipid bilayer membranes.
In this work, we have investigated alkali-metal ion transport across a planar phospholipid bilayer by p-tert-butylcalix[4]arene-crown-5 derivatives (1,2; Scheme 1), p-tert-butylcalix[5]arene ester 3, dibenzo-18-crown-6 and valinomycin. The ion transport activities and ion permeabilities of the compounds in the bilayer were determined using a voltage clamp method.2 Here we report that, among the synthetic calixarene ionophores studied, p-tert-butylcalix[4]arene-crown-5 (1) is a most effective K+ carrier in a phospholipid bilayer system.
 | ||
| Scheme 1 Synthetic ionophores tested for K+ transport activity in a planar phospholipid bilayer system. | ||
Results and discussion
Planar bilayer experiments using a patch clamp amplifier constitute a highly sensitive method for determining the ion transport activity of electrogenic ionophores, because the ion fluxes across the bilayer can be detected at pA level of membrane current.2 Planar bilayers were formed across an aperture in a Teflon film between two aqueous salt solutions in the cis and trans chamber. Fig. 1 shows the generation of membrane currents when an aliquot of a DMSO solution of 1 was added to the cis chamber. Upon addition of 1, membrane conductance significantly increased and gave a stable level about 1 min after the addition. A control experiment was performed where only neat DMSO was added: a control level (0.5 pA at 100 mV) of the membrane current did not change upon addition of 200 μl of DMSO. The insert in Fig. 1 shows the dependence of the membrane current on KCl concentration. The plot of current versus the concentration of KCl gave a saturation curve, suggesting that the ion transport by 1 takes place by a carrier mechanism.11 Similar saturation behavior of K+ flux has been reported in a system in which 1 is incorporated into a supported-liquid membrane.12d | ||
| Fig. 1 Increase in membrane conductance by the addition of 20 μl of 1 (100 μM DMSO solution). The ionophore was added to the cis chamber with stirring, where two chambers were filled with KCl solution (100 mM) adjusted to pH = 7.2 by a HEPES–Tris buffer. The insert shows the dependence of the membrane current on KCl concentration. | ||
To confirm the transport of K+ ions by 1, we measured resting membrane potentials when different concentrations KCl were placed on opposite sides of the planar bilayer membrane. If the planar bilayer membrane containing 1 is permeable only to K+ ions, the resting membrane potentials should be expressed by the Nernst equation (1).2

| (1) |
When the concentration ratios of [K+]cis/[K+]trans were increased to >1, the resting membrane potentials were positive on the side of the lower concentration, and the plot of the membrane potentials versus ln[K+]cis/[K+]trans showed a linear relationship (Fig. 2). It can be seen that the slope of the membrane potential is almost the same as the slope (59 mV decade−1) of the theoretical Nerstian plot. Thus the selectivity, i.e. the permeability ratio for K+/Cl− is very large, suggesting that Cl− anions cannot be transported by 1 across the bilayer.
![Membrane potentials as a function of the ratio of KCl concentrations in the cis and the trans chamber separated by a planar bilayer containing 1. Conditions: [K+]trans
= 100 mM, pH = 7.2, lipid/1
= 100 ∶ 1 (w/w). The solid line shows a theoretical Nernstian slope.](/image/article/2002/P2/b106153b/b106153b-f2.gif) | ||
| Fig. 2 Membrane potentials as a function of the ratio of KCl concentrations in the cis and the trans chamber separated by a planar bilayer containing 1. Conditions: [K+]trans = 100 mM, pH = 7.2, lipid/1 = 100 ∶ 1 (w/w). The solid line shows a theoretical Nernstian slope. | ||
From the view of biological applications, it is of importance to compare the ion transport activity of the synthetic K+ ionophores with that of valinomycin. Fig. 3 shows the dependence of the K+ current at 100 mV on the ionophore concentrations (in the cis chamber). The K+ current decreased in the order: valinomycin >1 > 3 > 2 > dibenzo-18-crown-6. The ion transport activity may be evaluated by the value of the membrane current with respect to the ionophore concentration in the cis chamber: 300 and 14.3 pA μM−1 for valinomycin and 1, respectively. The K+ transport activity of 1 was less than that of valinomycin by a factor of ca. 20. Among the calixarene ionophores, compound 1 showed the highest K+ transport activity in the bilayer membrane. It should be noted that the dependences of the K+ current on the concentration of the calixarene ionophores (1–3) showed linear relationships. This suggests that the calixarene ionophores form a 1 ∶ 1 complexes with K+ ions in the bilayer, in a similar manner to the case of valinomycin. Interestingly, dibenzo-18-crown-6 did not show the K+ transport activity in the bilayer. The poor activity of the dibenzo-18-crown-6 can be explained by lower lipophilicity in comparison to the other synthetic ionophores. Indeed, leaching of dibenzo-18-crown-6 into the aqueous phase has been observed in a supported liquid membrane system.12d In the case of 1 and valinomycin, these compounds are very lipophilic, which prevents leaching into the aqueous phase: the partition coefficient (log P) in octanol–water is reported as 15 and 8.6 for 1 and valinomycin, respectively.12d Thus, the lower K+ transport activity of 1versus valinomycin may be attributed to the lower binding constant of the K+ complex (KK+ = 3.8 × 108 for 112c and KK+ = 2.2 × 109 for valinomycin12e in CDCl3 saturated with H2O).
 | ||
| Fig. 3 Dependences of K+ current on ionophore concentration: (■) valinomycin; (□) 1; (△) 3; (○) 2; and (▽) dibenzo-18-crown-6. Ionophores were added as DMSO solutions (100 μM) to the cis chamber. | ||
To evaluate the ion transport selectivities for 1, we measured current–voltage curves under symmetrical ionic conditions [Fig. 4(a)]. The membrane conductivity at 100 mV decreased in the order: K+ > Rb+ > Cs+ > Na+ > Li+. This result shows that 1-mediated ion transport is selective toward K+ ion. To obtain the values of the ion permeability, we examined the current–voltage relationships under unsymmetrical ionic conditions [Fig. 4(b)]. The reversal potentials (which correspond to zero current voltages) in the MCl(cis)/KCl(trans) system is related to the ion concentrations on the two sides of the membrane according to the Goldman–Hodgkin–Katz equation (2),2,11,13
 | (2) |

| (3) |
![Current–voltage relationships for a planar bilayer containing 1: (a) symmetrical ionic conditions; [MCl]cis
= [MCl]trans
= 100 mM, pH = 7.2, lipid/1
= 100 ∶ 1 (w/w). (b) unsymmetrical ionic conditions; [MCl]cis
= [KCl]trans
= 100 mM, pH = 7.2, lipid/1
= 200 ∶ 1 (w/w).](/image/article/2002/P2/b106153b/b106153b-f4.gif) | ||
| Fig. 4 Current–voltage relationships for a planar bilayer containing 1: (a) symmetrical ionic conditions; [MCl]cis = [MCl]trans = 100 mM, pH = 7.2, lipid/1 = 100 ∶ 1 (w/w). (b) unsymmetrical ionic conditions; [MCl]cis = [KCl]trans = 100 mM, pH = 7.2, lipid/1 = 200 ∶ 1 (w/w). | ||
The values of the reversal potentials are summarized in Table 1. Fig. 5 shows the relative ion permeability for 1, together with the results for 3 and valinomycin. Both 1 and 3 show ion transport selectivity for K+, while valinomycin shows ion transport selectivity for Rb+. The K+/Na+ selectivities of these compounds in the bilayer system are 23, 12, and 2 for valinomycin, 1, and 3, respectively. Although the K+/Na+ selectivitiy of 1 is less than that of valinomycin by a factor of ca. 2, compound 1 has the highest K+ selectivity among the calixarene-based synthetic ionophores.
| Ionophore | −ϕrev/mV | |||
|---|---|---|---|---|
| Li+/K+ | Na+/K+ | Rb+/K+ | Cs+/K+ | |
| a Ionic conditions: [MCl]cis = [KCl]trans = 100 mM, pH = 7.2. | ||||
| Valinomycin | 108 | 80 | −27 | 22 |
| 1 | 67 | 63 | 11 | 33 |
| 3 | 77 | 18 | 3 | 18 |
 | ||
| Fig. 5 Ion permeability across a planar lipid bilayer containing calixarene ionophores (1,3) and valinomycin. The values of the ion permeability are normalized by the largest value of the ion permeability observed. | ||
Mechanisms for ionophore-mediated ion transport across bilayer membranes are divided into two categories: carrier and channel mechanisms.11 The calix[4]arene-crown-5 (1) has a cylindrical structure based on a calix[4]arene moiety and its molecular length along the cylindrical structure is ca. 12 Å (estimated from CPK models). Thus there is a possibility that two molecules of 1 aligning across a bilayer form a channel-like structure. If such a channel exists, a single-channel current fluctuation2,13 should be observed under conditions of very low ionophore concentration. Unfortunately, we could not observe such a single-channel current fluctuation for 1. The carrier mechanism for the 1-mediated transport of K+ across phopholipid bilayer membranes is supported by the following findings: (1) the K+ transport activity of 1 is less than that of the natural antibiotic carrier, valinomycin, by a factor of ca. 20; (2) the K+ current (flux) of 1 shows saturation behavior at high concentrations of KCl solution; (3) the K+ current of 1 increases linearly with the concentration of 1 added to the cis chamber, suggesting that 1 forms a 1 ∶ 1 complex with K+ ions in a bilayer.
Conclusion
Synthetic ion carriers active in phospholipid bilayers must have high lipophilicities as well as high ion selectivities. We have demonstrated that the calix[4]arene-crown-5 (1) can act as a synthetic K+ carrier in a phosholipid bilayer membrane. Because of the high lipophilicity of 1, this compound can be easily incorporated into the phospholipid bilayer membrane, and gives a stable K+ flux across the bilayer. Among the synthetic ionophores studied, compound 1 shows K+ transport ability with the highest K+/Na+ selectivity, while its selectivity is less than that of valinomycin by a factor of ca. 2. In the case of dibenzo-18-crown-6, no K+ transport activitiy was detected. Although the ion transport ability of 1 is not as good as that of valinomycin, compound 1 can be regarded as a first example of a synthetic K+ carrier active in lipid bilayer membranes. Reinhoudt et al. have found that several dialkoxycalix[4]arene crowns in the 1,3-alternative conformation have better K+/Na+ selectivity than 1 and valinomycin using supported liquid membranes.12e The systematic investigation of the relationships between the conformations of calix[4]arene crowns and their ion transport properties in bilayer systems will be the subject of future papers.Experimental
Chemicals
Valinomycin and dibenzo-18-crown-6 were purchased from Aldrich Chemicals. The calix[4]arene-crown-5 derivatives (1,2) and calix[5]arene ester 3 were prepared according to the literature methods.12,14 All compounds were identified by their 1H NMR spectra and mass spectroscopy. Soybean phospholipids were purchased from Nakarai Tasque (Kyoto, Japan) and purified by the literature method.15 Analytical reagent grade LiCl, NaCl, KCl, RbCl, and CsCl were purchased from Wako and used without further purification.Planar bilayer formation
Planar bilayer membranes (soybean phospholipids) were prepared by the folding method.2,16 Bilayers were formed at an aperture (diameter, 0.2 mm) in a Teflon film (thickness, 12.5 μm) which separated two Teflon chambers (internal volume of each chamber is 1.7 ml with surface area of 1 cm2). The side to which compounds were added was defined as the “cis” chamber and the opposite side was the “trans” chamber. The aqueous salt solution (alkali metal chlorides) containing HEPES [N′-(2-hydroxyethyl)piperazine-N-(ethane-2-sulfonic acid]–Tris [tris(hydroxymethyl)aminomethane] buffer (25 mM, pH 7.2) (1.5 ml) was injected into both chambers with two syringes so that the water surface was just below the aperture. Then a 15 μl aliquot of phospholipid or phospholipid–calixarene mixtures dissolved in hexane (10 mg ml−1) was placed on the surface of the solution in both chambers. After (ca. 10 min), the hexane was evaporated off leaving a phospholipid monolayer at the air–water interface in both chambers. The bilayer membrane was then formed by raising the water level sequentially in both chambers above the aperture.Incorporation of the ionophore into planar bilayer membranes
Two methods were used to incorporate the ionophores into the planar bilayer membranes. In the measurements of concentration dependences of ionophores on membrane currents, microliter aliquots of ionophores dissolved in DMSO were added to the cis chamber after the formation of the bilayers. In the measurements of current–voltage curves, planar bilayer membranes were directly prepared from the mixture of lipids and ionophores.Electrical recording
A patch clamp amplifier (CEZ-2300; Nihon Kohden, Ltd., Tokyo, Japan) was used in voltage clamp mode to amplify the currents and to control the voltages across the bilayer membranes. The command voltage was fed to the trans chamber via an Ag/AgCl electrode through an agar bridge and the cis chamber was earthed via an Ag/AgCl electrode through an agar bridge. The voltage was referenced to the cis side with respect to the trans side. The output signal from the amplifier was filtered at 1kHz and recorded using a Digidata 1200A A/D converter (Axon Instruments, Inc, USA). All measurements were performed at 25 °C.Acknowledgements
The author thanks Mr E. Yamada (NMR center, faculty of engineering, Hokkaido University) for the measurements of 1H NMR spectra. The author also thanks Mr T. Ohta (R.I.E.S.) for technical support in the bilayer set up.References
- B. Alberts, D. Bray, J. Lewis, M. Raff, K. Roberts and J. D. Watson, Molecular Biology of the Cell, 3rd edn., Gerland Publishing, New York, 1994 Search PubMed.
- W. Hanke and W.-R. Schlue, Planar Lipid Bilayers, Academic Press, San Diego, 1993 Search PubMed.
- R. Sandeaux, J. Sandeaux, C. Gavach and B. Brun, Biochem. Biophys. Acta, 1982, 684, 1274 Search PubMed; F. G. Riddell and S. Arumugam, Biochem. Biophys. Acta, 1988, 945, 65 Search PubMed; M. Inabayashi, S. Miyauchi, N. Kamo and T. Jin, Biochemistry, 1995, 34, 3455 CrossRef CAS.
- P. Mueller and D. O. Rudin, Biochem. Biophys. Res. Commun., 1967, 26, 398 CrossRef CAS; R. Benz and P. Läuger, J. Membr. Biol., 1976, 27, 178 Search PubMed.
- S. B. Hladky, V. B. Myers and D. A. Haydon, Biochem. Biophys. Acta, 1972, 274, 294 Search PubMed; D. A. Haydon, Biochem. Biophys. Acta, 1972, 274, 313 Search PubMed.
- R. Nagaraj and P. Balaram, Acc. Chem. Res., 1981, 14, 356 CrossRef CAS; S. C. Quay and R. Latorre, Biophys. J., 1982, 37, 154 Search PubMed.
- For example, see A. Nakano, Q. Xie, J. V. Mallen, L. Echgoyen and G. W. Gokel, J. Am. Chem. Soc., 1990, 112, 1287 Search PubMed; Y. Kobuke, K. Ueda and M. Sokabe, J. Am. Chem. Soc., 1992, 114, 7618 CrossRef CAS; T. M. Fyles, T. D. James and K. C. Kaye, J. Am. Chem. Soc., 1993, 115, 12315 CrossRef CAS; Q. Xie, Y. Li, G. W. Gokel, J. Hernandez and L. Echgoyen, J. Am. Chem. Soc., 1994, 116, 690 CrossRef CAS; O. Murillo, S. Watanabe, A. Nakano and G. W. Gokel, J. Am. Chem. Soc., 1995, 117, 7665 CrossRef CAS; Y. Tanaka, Y. Kobuke and M. Sokabe, Angew. Chem., Int. Ed. Engl., 1995, 34, 693 CrossRef CAS; T. M. Fyles, D. Loock, W. F. van Straaten-Nijenhuis and X. Zhou, J. Org. Chem., 1996, 61, 8866 CrossRef CAS; M. M. Tedesco, B. Ghebremariam, N. Sakai and S. Matile, Angew. Chem., Int. Ed. Engl., 1999, 38, 540 CrossRef CAS; H.-D. Arndt, A. Knoll and U. Koert, Angew. Chem., Int. Ed. Engl., 2001, 40, 2076 CrossRef CAS.
- (a) T. Jin, M. Kinjo, T. Koyama, Y. Kobayashi and H. Hirata, Langmuir, 1996, 12, 2684 CrossRef CAS; (b) T. Jin, M. Kinjo, Y. Kobayashi and H. Hirata, J. Chem. Soc., Faraday. Trans., 1998, 94, 3135 RSC; (c) T. Jin, Chem. Commun., 1999, 2129 RSC; (d) T. Jin, Chem. Commun., 2000, 1379 RSC.
- N. Kimizuka, T. Wakiyama, A. Yanai, S. Shinkai and T. Kunitake, Bull. Chem. Soc. Jpn., 1996, 69, 3681 CAS.
- R. M. Izatt, K. Pawlak and J. S. Bradshaw, Chem. Rev., 1991, 91, 1721 CrossRef CAS.
- Membrane Transport, ed. S. L. Bonting and J. J. H. M. de Pont, Elsevier, New York, 1981 Search PubMed.
- (a) C. Alfieri, E. Dradi, A. Pochini, R. Ungaro and G. D. Andreetti, J. Chem. Soc., Chem. Commun., 1983, 1075 RSC; (b) P. J. Dijkstra, J. A. J. Brunink, K.-E. Bugge, D. N. Reinhoudt, S. Harkama, R. Ungaro, F. Ugozzoli and E. Ghidini, J. Am. Chem. Soc., 1989, 111, 7567 CrossRef CAS; (c) E. Ghidini, F. Ugozzoli, R. Ungaro, S. Harkama, A. A. El-Fadl and D. N. Reinhoudt, J. Am. Chem. Soc., 1990, 112, 6979 CrossRef CAS; (d) W. F. Nijenhuis, E. G. Buitenhuis, F. de Jong, E. J. R. Sudhölter and D. N. Reinhoudt, J. Am. Chem. Soc., 1991, 113, 7963 CrossRef CAS; (e) A. Casnati, A. Pochini, R. Ungaro, C. Bocchi, F. Ugozzoli, R. J. M. Egberink, H. Struijk, R. Lugtenberg, F. de Jong and D. N. Reinhoudt, Chem. Eur. J., 1996, 2, 436 CrossRef CAS.
- Single-Channel Recording, ed. B. Sakman and E. Neher, Plenum Press, New York, 1983 Search PubMed.
- F. Arnaud-Neu, E. M. Collins, M. Deasy, G. Ferguson, S. J. Harris, B. Kaitner, A. J. Lough, M. A. McKervey, E. Marques, B. L. Ruhl, M. J. Schwing-Weill and E. M. Seward, J. Am. Chem. Soc., 1989, 111, 8681 CrossRef CAS.
- H. Hirata, K. Ohno, N. Sone, Y. Kagawa and T. Hamamoto, J. Biol. Chem., 1986, 261, 9839 CAS.
- M. Montal and P. Mueller, Proc. Natl. Acad. Sci. USA, 1972, 69, 3561 CAS.
| This journal is © The Royal Society of Chemistry 2002 |
