Synthesis of soluble halogenated aryloxy substituted indium phthalocyanines
Received (in Cambridge, UK) 24th August 2001, Accepted 8th November 2001
First published on 3rd December 2001
Abstract
Phthalocyanines solubilised by either 8 or 16 aryloxy or haloaryloxy groups are described. A series of phthalocyanine derivatives were prepared containing indium. 1,2-Dinitriles and the corresponding diiminoisoindolines were used as precursors. A naphthalocyanine metallated with indium and solubilised with four tert-butyl groups is reported.
Introduction
Optical limiters or reverse saturable absorbers are materials which display diminishing transmittance with increasing incident light intensity. This attenuation of the optical throughput can be used to protect various sensors against damage from pulsed laser sources.1–5 Phthalocyanines are promising candidates for certain applications.6 Peripheral substituents can fine-tune the ground state absorption to help create a good visible window by red shifting the long wavelength Q band absorption. A range of different metals can also be incorporated to help achieve desirable excited state characteristics. Population of triplet excited states from singlet states by intersystem crossing is desirable because more strongly absorbing triplet states can have a longer lifetime and enhance the limiter performance. Heavy metals, halogens and paramagnetic groups are known to promote intersystem crossing and so the
synthesis of suitably functionalised phthalocyanines containing these features is of interest.6 Phthalocyanines containing the metals Al, Si, V and In have been studied and shown to possess desirable properties.6Results and discussion
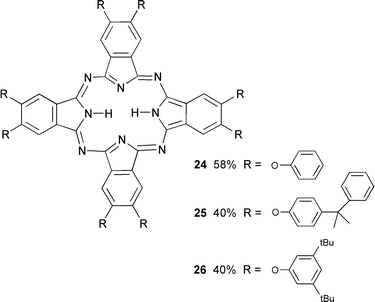
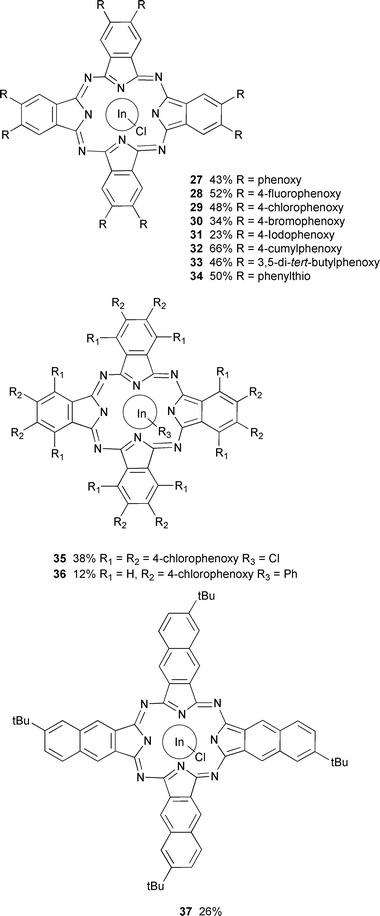
This paper reports the synthesis of unmetallated aryloxy substituted phthalocyanines 24–26, metallated phthalocyanines 27–33 and 35–36 containing indium, a phenylthio substituted phthalocyanine 34 containing indium and a naphthalocyanine (a tetrabenzo[b,k,t,c1]phthalocyanine) 37 containing indium. Aryloxy groups substituted with fluoro, chloro, bromo or iodo groups were incorporated to allow the influence of remote halogens upon optical limiter performance to be investigated. The oxygen group itself causes a bathochromic shift of the long wavelength absorption maximum7–10 which helps to widen the
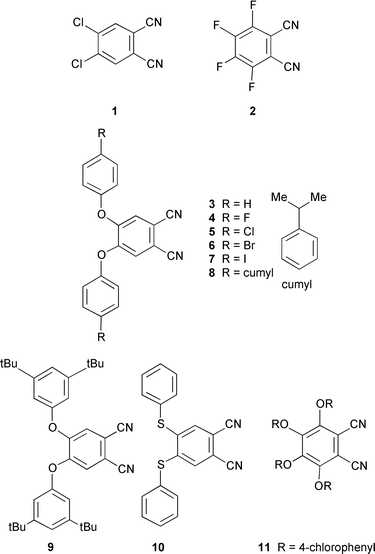
visible window. 4,5-Diaryloxy substituted dicyanobenzenes 3–9 were prepared by treatment of 4,5-dichloro-1,2-dicyanobenzene 1 with an appropriate phenol and K2CO3 in DMSO. Compound 1 was prepared by a literature route from 4,5-dichlorobenzene-1,2-dicarboxylic acid.11 Dinitrile 10 was prepared by treating 1 with thiophenol and base. Tetrakis(4-chlorophenoxy)-1,2-dicyanobenzene 11 was prepared by treating commercially available tetrafluorophthalonitrile 2 with 4-chlorophenol and K2CO3 in DMSO. Related tetrakis(aryloxy)-1,2-dicyanobenzenes have been reported previously.12
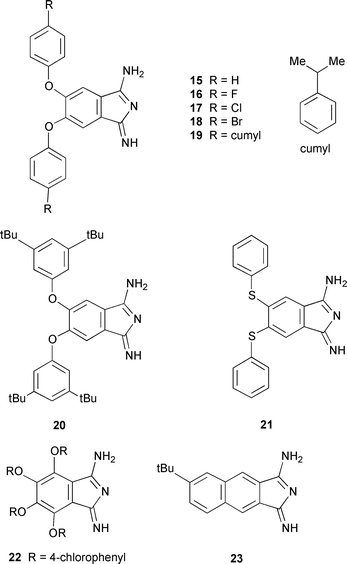
6-tert-Butyl-2,3-dicyanonaphthalene 14 was prepared by the literature route shown in Scheme 1.13 This involved free radical bromination of o-xylene 12 to give tetrabromoxylene 13. Treatment with NaI in hot DMF eliminates bromine to give an intermediate diene which is intercepted by cycloaddition with fumaronitrile. Elimination of two more moles of HBr gives naphthalonitrile 14. Treatment of the above dinitriles with ammonia gas in NaOMe–MeOH14–17 gave the corresponding diiminoisoindolines 15–23. The progress of the reaction can be monitored by IR observing the disappearance
of the nitrile peak from 2230–2250 cm−1. A large broad peak in the range 3200–3500 cm−1 corresponding to the NH stretch is observed. The diiminoisoindolines were used for the synthesis of InCl substituted phthalocyanines.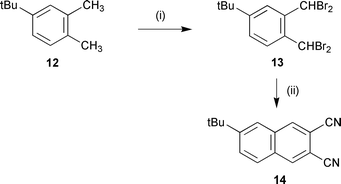 |
| | Scheme 1 Reagents and conditions (i) NBS–AIBN, 56%; (ii) NaI–fumaronitrile–DMF, 43%. | |
Non-metallated phthalocyanines 24–26 substituted with 8 aryloxy groups were prepared by cyclotetramerisation of dinitriles 3, 8 and 9 respectively with the base DBU in hot pentanol.11,18–20 On cooling the precipitated product was isolated by filtration. Metallated phthalocyanines 28–31 containing indium were prepared by cyclotetramerisation of the corresponding dinitriles by heating with InCl3 in quinoline.21 The other indium containing phthalocyanines were most satisfactorily prepared by heating the appropriate diiminoisoindoline with InCl3 in quinoline. Treatment of phthalocyanine 29
with PhMgBr displaces the chlorine group with a phenyl group to give compound 36. Similar methods have been used to prepare a range of indium containing phthalocyanines substituted with different axial groups.21 Naphthalocyanine 37 was prepared as a mixture of isomers by cyclotetramerisation of dihydrodiimino-1H-benz[f]isoindole 23 by heating with InCl3 in quinoline.
The UV spectrum of each phthalocyanine is important because a low absorption cross section in the visible region provides the optical window for an RSA device. The octasubstituted aryloxyphthalocyanines 24–26 have a long wavelength absorption at around 700 nm. Substitution with InCl produces little overall change in this absorption maximum. The octakis(arylthio)phthalocyanine 34 containing InCl has a long wavelength maximum at 735 nm which is shifted bathochromically by about 50 nm compared to the value for the corresponding octakis(aryloxy)phthalocyanine 27. Sulfur substituents produce a greater bathochromic shift than oxygen substituents. Phthalocyanine 35 substituted with 16 aryloxy groups shows a maximum at 738 nm which is shifted bathochromically compared to the octakis(aryloxy)phthalocyanine 27. Naphthalocyanines are of particular interest for optical limiters because of the much wider visible window that they exhibit. Naphthalocyanine 37 has a main maximum at 793 nm. This peak is bathochromically shifted compared to the InCl containing phthalocyanines.
In summary a range of different phthalocyanines have been prepared which will allow a study of the influence of structural factors upon optical limiting performance to be evaluated.
Experimental
Ultraviolet spectra were recorded on a Perkin-Elmer Lambda 15 UV-VIS spectrometer using dichloromethane as the solvent. Infrared spectra were recorded on an ATI Mattson FTIR spectrometer. 1H and 13C NMR spectra were obtained at 250 MHz and 62.9 MHz respectively on a Bruker AC 250 spectrometer and at 400 MHz and 100.5 MHz respectively on a Varian 400 spectrometer. Chemical shifts, δ, are given in ppm relative to SiMe4. Coupling constants, J, are given in Hz. Mass spectra were carried out at the University of Wales, Swansea using either the low resolution electron impact or fast atom bombardment methods. Butterworth Laboratories, using a PE 2400 CHN analyser, conducted elemental analyses. Melting points were carried out using a Kofler hot-stage microscope and are uncorrected.4,5-Disubstituted 1,2-dicyanobenzenes
General procedure. 4,5-Dichloro-1,2-dicyanobenzene 1 (1.97 g, 10 mmol) and 60 mmol of a phenol or thiophenol were heated at 90 °C in DMSO (20 ml) with stirring under N2. K2CO3 powder ( 2.76 g, 20 mmol) was added in portions (8 × 20 mmol) every 5 min. The mixture was heated for a further 30 min. After cooling the mixture was added to ice–H2O (200 ml), filtered and the product was recrystallised from MeOH.
4,5-Diphenoxy-1,2-dicyanobenzene 3. Yield 70%, mp 148–149 °C (lit. 149 °C11) (Found: C, 76.8; H, 3.9; N, 9.1. C20H12N2O2 requires C, 76.9; H, 3.85; N, 9.0%); λmax(EtOH)/nm 296; νmax (KBr)/cm−1 688, 747, 818, 876, 906, 1074, 1162, 1210, 1246, 1304, 1397, 1457, 1501, 1561, 1591, 1609 and 2236; δH (250 MHz; CDCl3) 7.08 (4H, d, J 7.6), 7.15 (2H, s), 7.30 (4H, t) and 7.45 (2H, t); δC (62.9 MHz; CDCl3) 110.4, 115.0, 120.0, 122.0, 126.0, 130.6, 152.0 and 154.1; m/z (EI) 312 (M+, 100%). 4,5-Bis(p-fluorophenoxy)-1,2-dicyanobenzene 4. Yield 72%, mp 186–188 °C (Found: C, 68.7; H, 3.0; N, 7.8. C20H10N2O2F2 requires C, 69.0; H, 2.9; N, 8.0%); λmax(EtOH)/nm 291; νmax (KBr)/cm−1 784, 854, 863, 1011, 1087, 1190, 1207, 1298, 1384, 1495, 1569, 1617 and 2229; δH (400 MHz; CDCl3) 7.01 (4H, m), 7.10 (4H, m) and 7.12 (2H, s); δC (100.5 MHz; CDCl3) 111.6, 115.9, 118.3, 118.5, 122.5, 122.6, 150.8, 150.9, 152.9, 160.0 and 162.5; m/z (EI) 348 (M+, 100%).
4,5-Bis(p-chlorophenoxy)-1,2-dicyanobenzene 5. Yield 70%, mp 188–190 °C (Found: C, 63.2; H, 2.5; N, 7.3. C20H10N2O2Cl2 requires C, 63.0; H, 2.6; N, 7.35%); λmax(EtOH)/nm 293; νmax (KBr)/cm−1 772, 824, 1010, 1090, 1187, 1223, 1303, 1324, 1399, 1483, 1502, 1581, 1599 and 2226; δH (250 MHz; CDCl3) 7.0 (4H, d, J, 9.2), 7.2 (2H, s) and 7.4 (4H, d, J 9.2); δC (62.9 MHz; CDCl3) 111.1, 114.7, 116.7, 121.0, 122.4, 130.7, 151.5 and 152.7; m/z (EI) 380/382/384 (M+, 100%).
4,5-Bis(p-bromophenoxy)-1,2-dicyanobenzene 6. Yield 78%, mp 212–214 °C (Found: C, 51.2; H, 1.9; N, 5.9. C20H10N2O2Br2 requires C, 51.1; H, 2.1; N, 6.0%); νmax(EtOH)/nm 292; νmax (KBr)/cm−1 773, 862, 1012, 1067, 1221, 1305, 1323, 1398, 1484, 1503, 1576, 1597 and 2225; δH (250 MHz; CDCl3) 7.0 (4H, d, J 8.9), 7.2 (2H, s) and 7.5 (4H, d, J 8.9); δC (62.9 MHz; CDCl3) 111.2, 114.7, 118.9, 121.4, 122.6, 133.7, 151.4 and 153.3; m/z (EI) 468/470/472 (M+, 100%).
4,5-Bis(p-iodophenoxy)-1,2-dicyanobenzene 7. Yield 76%, mp 214–217 °C (Found: C, 42.2; H, 1.6; N, 5.0. C20H10N2O2I2 requires C, 42.4; H, 1.8; N, 4.95%); λmax(EtOH)/nm 292; νmax (KBr)/cm−1 768, 830, 1009, 1220, 1305, 1324, 1395, 1481, 1502, 1564, 1598 and 2224; δH (250 MHz; CDCl3) 7.73 (4H, d, J 8.85), 7.21 (2H, s) and 6.80 (4H, d, J 8.85); δC (62.9 MHz; CDCl3) 89.6, 111.2, 114.7, 121.7, 122.7, 139.6, 151.3 and 154.1; m/z (EI) 564 (M+, 100%).
4,5-Bis(cumylphenoxy)-1,2-dicyanobenzene 8. Yield 82%, mp 166–168 °C (Found: C, 83.15; H, 5.8; N, 5.3. C38H32N2O2 requires C, 83.2; H, 5.8; N, 5.1%); λmax(EtOH)/nm 292; νmax (KBr)/cm−1 701, 798, 1015, 1215, 1319, 1385, 1499, 1561, 1590, 2236 and 2959; δH (250 MHz; CDCl3) 1.71 (12H, s), 6.96 (4H, m), 7.16 (2H, m) and 7.18–7.31 (14H, m); δC (62.9 MHz; CDCl3) 30.7, 42.7, 109.9, 115.2, 119.4, 121.7, 125.9, 126.8, 128.2, 129.0, 148.7, 150.0, 151.8 and 152.1; m/z (EI) 548 (M+, 100%).
4,5-Bis(3,5-di-tert-butylphenoxy)-1,2-dicyanobenzene 9. Yield 91%, mp 240–241 °C (Found: C, 80.4; H, 8.0; N, 5.4. C36H44N2O2 requires C, 80.6; H, 8.2; N, 5.2%) λmax(EtOH)/nm 308; νmax (KBr)/cm−1 706, 898, 918, 952, 1077, 1220, 1291, 1418, 1435, 1505, 1576, 1605, 2230, 2867, 2903 and 2962; δH (250 MHz; CDCl3) 1.33 (36H, s), 6.90 (4H, s), 7.10 (2H, s) and 7.35 (2H, s); δC (62.9 MHz; CDCl3) 31.4, 35.0, 109.7, 114.7, 115.4, 120.5, 152.5, 153.4, 153.9, 155.0; m/z (EI) 536 (M+, 100%).
4,5-Bis(phenylthio)-1,2-dicyanobenzene 10. Yield 46%, mp 157–159 °C (lit. 159 °C7) (Found: C, 69.6; H, 3.4; N, 8.0; S, 18.5. C20H12N2S2 requires C, 69.8; H, 3.5; N, 8.1; S, 18.6%); λmax(EtOH)/nm 293; νmax (KBr)/cm−1 1210 and 2227; δH (250 MHz; CDCl3) 7.0 (2H, s) and 7.54 (10H, m); δC (62.9 MHz; CDCl3) 111.8, 115.4, 128.6, 130.0, 130.7, 135.2 and 144.2 (one resonance is missing); m/z (EI) 344 (M+, 100%). 3,4,5,6-Tetrakis(p-chlorophenoxy)-1,2-dicyanobenzene 11. 4-Chlorophenol (19.3 g, 150 mmol) and 3,4,5,6-tetrafluoro-1,2-dicyanobenzene (3.93 g, 19.63 mmol) in DMSO (50 ml) at 90 °C under N2 were treated with 12 portions of K2CO3 (2.76 g, 20 mmol) at 5 min intervals. After heating for a further 1.5 h the mixture was allowed to cool, added to ice–H2O (400 ml) and filtered to give the product as a colourless solid which was recrystallised from MeOH. Yield 20%, mp 208–210 °C (Found: C, 60.6; H, 2.4; N, 4.4. C32H16N2O4Cl4 requires C, 60.8; H, 2.5; N, 4.4%); νmax(EtOH)/nm 290; νmax (KBr)/cm−1 650, 824, 982, 1012, 1089, 1166, 1205, 1302, 1375, 1440, 1486, 1561, 1588 and 2231; δH (250 MHz; CDCl3)
6.43 (4H, d, J 8.85), 6.72 (4H, d, J 8.85), 7.11 (4H, d, J 9.2) and 7.22 (4H, d, J 9.2); δC (62.9 MHz; CDCl3) 108.4, 111.1, 116.8, 117.5, 129.5, 129.6, 129.8, 129.9, 146.5, 149.1, 153.9 and 154.8; m/z (EI) 632/634/636/638 (M+, 100%).
1,2-Bis(dibromomethyl)-4-tert-butylbenzene 13. 13 A mixture of 4-tert-butyl-o-xylene 12 (5.0 g, 31 mmol), N-bromosuccinimide (NBS) (21.9 g, 123 mmol) and a small quantity of AIBN in CCl4 was heated under reflux for 10 h. The reaction mixture was filtered while hot and the solvent was removed in vacuo and the crude product used without further purification owing to the reactivity of the bis(dibromomethyl) groups. Yield 56%, δH (250 MHz; CDCl3) 1.34 (9H, s), 7.11 (2H, s), 7.18 (1H, s), 7.39 (1H, d, J 8.2) and 7.60 (1H, d, J 8.2); δC (62.9 MHz; CDCl3) 31.1, 36.7, 125.8, 126.0, 127.1, 127.3, 127.8, 129.6, 130.9 and 153.8; m/z (FAB) 474/476/478/480/482 (M+,
100%). 6-tert-Butyl-2,3-dicyanonaphthalene 14. A mixture of crude 1,2-bis(dibromomethyl)-4-tert-butylbenzene 13 (14.7 g, 31 mmol), fumaronitrile (2.4 g, 31 mmol) and sodium iodide (30 g, 0.2 mol) in DMF was heated at 75–80 °C for 7 h. The reaction mixture was cooled to room temperature and an aqueous solution of Na2SO4 was added. The precipitate was filtered and washed with CH2Cl2 to give the title compound (3.1 g, 43%) mp 193–194 °C (lit. 194–195 °C13); νmax (KBr)/cm−1 471, 840, 901, 926, 1262, 1384, 1624, 2229, 2871, 2904 and 2954; δH (250 MHz; CDCl3) 1.43 (9H, s), 7.90 (3H, m), 8.29 (1H, s) and 8.31 (1H, s); δC (62.9 MHz; CDCl3)
30.9, 35.5, 109.3, 109.9, 116.2, 123.9, 128.4, 130.0, 131.5, 133.4, 135.4, 135.9 and 154.6 (one resonance is missing); m/z (FAB) 234 (M+, 100%). 5,6-Disubstituted 1,3-diiminoisoindolines
General procedure. 14–17 The dicyanobenzene (7 mmol) derivative was gently refluxed in NaOMe–MeOH solution (200 ml, 0.7 g Na in 500 ml MeOH) for 5 h with NH3 bubbled through the solution. The end point was determined by observing the disappearance of the CN stretch on aliquots examined by IR. The reaction mixture was allowed to cool and concentrated under vacuum to give the crude product. They were used immediately in the preparation of the corresponding InCl phthalocyanines without further purification. 5,6-Diphenoxy-1,3-diiminoisoindoline 15. Yield 97%, λmax(EtOH)/nm 284; νmax (KBr)/cm−1 565, 741, 871, 1030, 1069, 1211, 1270, 1385, 1458, 1490, 1597, 1637, 2853, 2925 and 3430; δH (400 MHz; CDCl3) 6.80 (3H, m, 3 × NH), 7.00 (4H, m), 7.11 (4H, m), 7.20 (2H, s) and 7.34 (2H, m); δC (100.5 MHz; CDCl3) 114.6, 116.5, 120.0, 121.3, 125.8, 131.1, 152.2 and 157.1; m/z 329 (M+, 100%).
5,6-Bis(p-fluorophenoxy)-1,3-diiminoisoindoline 16. Yield 97%, λmax(EtOH)/nm 293; νmax (KBr)/cm−1 1201 and 3428; δH (400 MHz; CDCl3) 6.91 (3H, m), 7.11 (4H, m), 7.21 (2H, s) and 7.26 (4H, m); δC (100.5 MHz; CDCl3) 112.7, 118.2, 119.9, 120.1, 126.3, 126.4, 152.3, 152.4, 154.0, 160.2 and 162.9; m/z (EI) 365 (M+, 100%).
5,6-Bis(p-chlorophenoxy)-1,3-diiminoisoindoline 17. Yield 97%, λmax(EtOH)/nm 288; νmax (KBr)/cm−1 824, 1010, 1090, 1143, 1213, 1284, 1407, 1485, 1543, 1637, 2854, 2932, 2965 and 3417; δH (400MHz; CDCl3) 6.87 (3H, m), 7.12 (4H, d), 7.24 (2H, s) and 7.49 (4H, d); δC (100.5 MHz; CDCl3) 112.3, 116.2, 118.4, 123.1, 124.6, 131.4, 154.3 and 154.6; m/z (EI) 397/399/401 (M+, 100%).
5,6-Bis(p-bromophenoxy)-1,3-diiminoisoindoline 18. Yield 97%, λmax(EtOH)/nm 289; νmax (KBr)/cm−1 822, 1009, 1070, 1164, 1211, 1297, 1411, 1482, 1580 and 3416; δH (400 MHz; CDCl3) 6.80 (3H, m), 7.02 (4H, d), 7.32 (2H, s) and 7.59 (4H, d); δC (100.5 MHz; CDCl3) 112.5, 116.3, 120.8, 122.4, 124.9, 136.3, 152.7 and 155.0; m/z (EI) 485/487/489 (M+, 100%).
5,6-Bis(cumylphenoxy)-1,3-diiminoisoindoline 19. Yield 97%, λmax(EtOH)/nm 286; νmax (KBr)/cm−1 701, 867, 961, 1080, 1202, 1298, 1363, 1421, 1479, 1594, 1655, 2867, 2904, 2964 and 3421; δH (250 MHz; CDCl3) 1.66 (12H, s), 6.87 (4H, d, J 8.85), 7.17 (4H, d, J 8.85) and 7.25 (15H, m); δC (100.5 MHz; CDCl3) 30.9, 42.6, 77.3, 112.3, 118.3, 125.8, 126.8, 128.1, 128.4, 146.7, 150.4, 151.2, 154.0 and 165.2; m/z (EI) 565 (M+, 100%).
5,6-Bis(3,5-di-tert-butylphenoxy)-1,3-diiminoisoindoline 20. Yield 97%, λmax(EtOH)/nm 291; νmax (KBr)/cm−1 457, 709, 860, 1015, 1030, 1047, 1118, 1204, 1308, 1364, 1425, 1454, 1556, 1596, 2830, 2868, 2968 and 3436; δH (400 MHz; CDCl3) 1.26 (36H, s), 6.88 (7H, m), 7.19 (2H, s) and 7.21 (2H, s); δC (100.5 MHz; CDCl3) 32.4, 36.1, 112.1, 114.7, 115.4, 119.6, 134.5, 152.7, 154.1 and 156.4; m/z (EI) 553 (M+, 100%).
5,6-Bis(phenylthio)-1,3-diiminoisoindoline 21. Yield 97%, λmax(EtOH)/nm 285; νmax (KBr)/cm−1 738, 855, 905, 1023, 1066, 1143, 1174, 1225, 1270, 1318, 1379, 1439, 1533, 1644, 2831, 2854, 2970 and 3427; δH (400 MHz; CDCl3) 6.76 (3H, m), 7.07 (2H, s) and 7.30–7.60 (10H, m); δC (100.5 MHz; CDCl3) 115.2, 117.6, 128.0, 131.0, 131.9, 135.5, 144.8 and 152.5; m/z 361 (M+, 100%)
4,5,6,7-Tetrakis(p-chlorophenoxy)-1,3-diiminoisoindoline 22. Yield 97%, νmax (KBr)/cm−1 1200 and 3417; λmax(EtOH)/nm 290 and 322; m/z (EI) 649/651/653 (M+, 100%).
6-tert-Butyl-2,3-dihydro-1,3-diimino-1H-benz[f]isoindole 23. Yield 97%, νmax (KBr)/cm−1 472, 578, 726, 815, 904, 1030, 1159, 1257, 1384, 1458, 1542, 1626, 2852, 2925, 2952 and 3422; δH (400 MHz; CDCl3) 1.04 (9H, s), 6.91 (3H, s, br), 7.16 (4H, s, br), 7.42 (1H, s, br); δC (100.5 MHz; CDCl3) 31.0, 34.6, 112.0, 120.2, 120.4, 124.1, 125.8, 128.7, 130.8, 131.4, 131.7, 133.8, 149.7 and 171.3; m/z (FAB) 251 (M+, 100%)
2,3,9,10,16,17,23,24-Octasubstituted phthalocyanines
General procedure. 18–20 The appropriate dicyanobenzene (4 mmol) and DBU (0.6g, 4 mmol) in pentanol (50 ml) were refluxed for 48 h. The dark green solution was filtered and the precipitated product Soxhlet extracted with MeOH, then dried under vacuum. 2,3,9,10,16,17,23,24-Octaphenoxyphthalocyanine 24. 11 Yield 58%, mp >275 °C (Found: C, 76.6, H, 3.9; N, 9.0. C80H50N8O8 requires C, 76.8; H, 4.0; N, 8.95%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 344, 673 and 700 (5.2); νmax (KBr)/cm−1 702, 833, 1007, 1203, 1272, 1385, 1399, 1438, 1491, 1618, 1637 and 3416; δH (400 MHz; CDCl3) −0.80 (2H, s), 7.16 (16H, d), 7.24 (16H, t), 7.37 (8H, t) and 8.82 (8H, s); δC (100.5 MHz; CDCl3) 112.7, 115.9, 121.3, 126.5, 128.2, 130.9, 153.1 and 156.7; m/z 1250 (M+, 100%). 2,3,9,10,16,17,23,24-Octakis(cumylphenoxy)phthalocyanine 25. Yield 40%, mp 112–114 °C (Found: C, 82.8; H, 5.8; N, 5.1. C152H130N8O8 requires C, 83.1; H, 6.0; N, 5.1%); νmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 328, 606, 637, 667 and 701 (5.3); νmax (KBr)/cm−1 700, 763, 1014, 1215, 1278, 1351, 1429, 1501, 1599, 2869, 2928, 2966 and 3426; δH (400 MHz; CDCl3) −0.78 (2H, s), 1.68 (48H, s), 7.11–7.27 (72H, m) and 8.75 (8H, s); δC (100.5 MHz; CDCl3) 31.9, 43.7, 118.7, 119.7, 120.1, 126.8, 126.9, 127.8, 129.0, 129.1, 129.2, 129.7, 151.7 and 156.4; m/z (FAB) 2194 (M+, 100%).
2,3,9,10,16,17,23,24-Octakis(3,5-di-tert-butylphenoxy)phthalocyanine 26. Yield 46%, mp >275 °C (Found: C, 80.6; H, 8.7; N, 5.5. C144H178N8O8 requires C, 80.5; H, 8.4; N, 5.2%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 348, 607, 640, 669 and 703 (5.3); νmax (KBr)/cm−1 706, 961, 1011, 1087, 1199, 1298, 1385, 1423, 1439, 1459, 1588, 2867, 2904, 2963 and 3430; δH (400 MHz; CDCl3) −0.89 (2H, s), 1.61 (144H, s), 7.41 (16H, s), 7.53 (8H, s) and 9.33 (8H, s); δC (100.5 MHz; CDCl3) 31.7, 35.3, 113.0, 114.2, 117.7, 133.1, 151.4, 152.8 and 157.1 (one resonance is missing); m/z (FAB) 2146 (M+, 100%).
Metallated phthalocyanines
General procedure. 21,13,15 The appropriate dicyanobenzene (6.5 mmol, for compounds 28–31) or diiminoisoindoline (6.5 mmol, for compounds 27 and 32–37) and InCl3 (2.2 mmol) in dry quinoline (15 ml) were refluxed for 2 h. After cooling MeOH was added to precipitate the product. The product was washed thoroughly with MeOH (500 ml) and dried under vacuum. 2,3,9,10,16,17,23,24-Octaphenoxyphthalocyaninatoindium chloride 27. Yield 43%, mp >275 °C (Found: C, 68.4; H, 3.2; N, 7.9. C80H48N8O8InCl requires C, 68.6; H, 3.5; N, 8.0%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 346, 612 and 683 (5.4); νmax (KBr)/cm−1 688, 743, 891, 1030, 1088, 1162, 1204, 1272, 1332, 1399, 1450, 1489 and 1589; δH (400 MHz; CDCl3) 7.00–7.41 (40H, m) and 8.80 (8H, s); δC (100.5 MHz; CDCl3) 126.8, 129.1, 130.7, 133.0, 133.2, 135.1, 136.7 and 153.4; m/z (FAB) 1398/1400 (M+, 100%), 1364 (M+
− Cl).
2,3,9,10,16,17,23,24-Octakis(p-fluorophenoxy)phthalocyaninatoindium chloride 28. Yield 52%, mp >275 °C (Found: C, 62.0; H, 2.8; N, 7.2. C80H40N8O8F8ClIn requires C, 62.25; H, 2.6; N, 7.3%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 366, 627 and 697 (5.15); νmax (KBr)/cm−1 831, 894, 1029, 1091, 1136, 1195, 1274, 1339, 1401, 1452, 1502 and 1608; δH (400 MHz; CDCl3) 7.20 (16H, m), 7.21 (16H, m) and 8.74 (8H, s); δC (100.5 MHz; CDCl3) 114.4, 117.4, 117.7, 117.9, 134.2, 152.4, 153.4, 159.3 and 161.7; m/z (FAB) 1542/1544 (M+, 100%), 1508 (M − Cl).
2,3,9,10,16,17,23,24-Octakis(p-chlorophenoxy)phthalocyaninatoindium chloride 29. Yield 0.6 g, 48%, mp >275 °C (Found: C, 57.5; H, 2.6; N, 6.5. C80H40N8O8Cl9In requires C, 57.3; H, 2.3; N, 6.7%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 364, 630, 669 and 690 (5.42); νmax (KBr)/cm−1 1088, 1208, 1268, 1398, 1451, 1485 and 1585; δH (400 MHz; CDCl3) 7.13 (16H, d, J 8.9), 7.39 (16H, d, J 8.9) and 8.97 (8H, s); δC (100.5 MHz; CDCl3) 120.7, 121.1, 130.4, 130.9, 131.1, 134.6, 151.6 and 156.4; m/z (FAB) 1670/1672/1674/1676 (M+, 100%).
2,3,9,10,16,17,23,24-Octakis(p-bromophenoxy)phthalocyaninatoindium chloride 30. Yield 34%, mp 243–245 °C (Found: C, 47.1; H, 1.8; N, 5.4. C80H40N8O8Br8ClIn requires C, 47.3; H, 2.0; N, 5.5%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 364, 629, 667 and 698 (5.05); νmax (KBr)/cm−1 1090, 1209, 1281, 1399, 1451, 1482, 1560, 1579 and 1618; δH (400 MHz; CDCl3) 7.06 (16H, d, J 8.9), 7.53 (16H, d, J 8.9) and 8.00 (8H, s); δC (100.5 MHz; CDCl3) 116.1, 117.8, 120.4, 121.1, 121.5, 134.1, 151.5 and 157.1; m/z (FAB) 2024/2026/2028/2030 (M+, 100%).
2,3,9,10,16,17,23,24-Octakis(p-iodophenoxy)phthalocyaninatoindium chloride 31. Yield 23%, mp 220–222 °C (Found: C, 39.9; H, 1.5; N, 4.8. C80H40N8O8I8ClIn requires C, 39.9; H, 1.7; N, 4.7%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 363, 629, 671 and 699 (5.1); νmax (KBr)/cm−1 894, 1007, 1098, 1208, 1281, 1400, 1449, 1479, 1560 and 1618; δH (400 MHz; CDCl3) 6.96 (16H, d, J 8.9), 7.83 (16H, d, J 8.9), 8.98 (8H, d); δC (100.5 MHz; CDCl3) 96.1, 116.2, 121.4, 122.3, 140.0, 140.3, 153.8 and 156.5; m/z (FAB) 2406/2408 (M+, 100%).
2,3,9,10,16,17,23,24-Octakis(cumylphenoxy)phthalocyaninatoindium chloride 32. Yield 2.8 g, 66%, mp 139–141 °C (Found: C, 77.6; H, 5.3; N, 4.5. C152H128N8O8InCl requires C, 77.85; H, 5.5; N, 4.8%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 366, 629 and 699 (5.17); νmax (KBr)/cm−1 764, 893, 1029, 1084, 1172, 1211, 1270, 1385, 1399, 1476, 1637, 2868, 2924 and 2965; δH (400 MHz; CDCl3) 1.71 (48H, s), 7.08–7.28 (72H, m) and 9.02 (8H, s); δC (100.5 MHz; CDCl3) 32.1, 43.7, 118.9, 119.8, 126.8, 127.9, 129.2, 129.4, 134.9, 147.2, 151.7, 152.4, 153.7 and 156.4; m/z 2342/2344 (M+, 100%).
2,3,9,10,16,17,23,24-Octakis(3,5-di-tert-butylphenoxy)phthalocyaninatoindium chloride 33. Yield 1.83 g, 46%, mp >200–202 °C (Found: C, 74.9; H, 7.75; N, 4.7. C144H176N8O8InCl requires C, 75.3; H, 7.7; N, 4.9%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 356, 633 and 703 (5.3); νmax (KBr)/cm−1 712, 961, 1036, 1090, 1200, 1270, 1296, 1399, 1458, 1586, 1609, 2866, 2904 and 2964; δH (400 MHz; CDCl3) 1.33 (144H, s), 7.13 (16H, s), 7.25 (8H, s), 9.08 (8H, s); δC (100.5 MHz; CDCl3) 32.5, 36.2, 113.8, 115.4, 118.7, 134.5, 152.7, 153.8, 153.9 and 157.8; m/z (FAB) 2294/2296 (M+, 100%).
2,3,9,10,16,17,23,24-Octakis(phenylthio)phthalocyaninatoindium chloride 34. Yield 50%, mp 169–171 °C (Found: C, 62.7; H, 3.1; N, 7.5; S, 17.0. C80H48N8S8InCl requires C, 62.9; H, 3.2; N, 7.3; S, 16.8%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 357, 659 and 735 (5.16); νmax (KBr)/cm−1 688, 741, 838, 943, 1023, 1063, 1111, 1175, 1278, 1324, 1368, 1402, 1475, 1580, 1677 and 1719; δH (400 MHz; CDCl3) 7.12–7.35 (40H, m) and 8.72 (8H, s); δC (100.5 MHz; CDCl3) 126.8, 129.1, 130.8, 133.0, 135.1, 136.8, 142.2 and 153.8; m/z (FAB) 1526/1528 (M+, 15%), 1491 (M+
− Cl, 100%).
1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-Hexadecakis(p-chlorophenoxy)phthalocyaninatoindium chloride 35. Yield 38%, mp 188–190 °C (Found: C, 57.35; H, 2.2; N, 4.3. C128H64N8O16Cl17In requires C, 57.2; H, 2.4; N, 4.2%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 344, 659, 682 and 738 (5.29); νmax (KBr)/cm−1 1206; δH (400 MHz; CDCl3) 6.41 (16H, d, J 8.9), 6.70 (16H, d, J 8.9), 6.90 (16H, d, J 8.9) and 7.04 (16H, d, J 8.9); δC (100.5 MHz; CDCl3) 117.6, 120.2, 128.0, 129.1, 129.8, 130.2, 130.3, 145.3, 145.4, 152.8, 156.0 and 157.4; m/z (FAB) 2680/2682/2684/2686 (M+,
100%).
Phenyl[2,3,9,10,16,17,23,24-octakis(p-chlorophenoxy)phthalocyaninato]indium 36. A freshly prepared solution of PhMgBr (3 M, 2 ml) was added dropwise to a stirred solution of phthalocyanine 29 (200 mg, 0.074 mmol) in dry THF (20 ml). After monitoring the reaction by TLC (silica gel/toluene), the reaction was stopped when all of the phthalocyanine complex had been consumed. The resulting deep green solution was then poured onto ice, extracted several times with Et2O, washed with water and dried over MgSO4. The solvent was evaporated in vacuo and the residue was subjected to column chromatography. Elution with dichloromethane gave the title compound as a green solid (24 mg, 12%) mp >275 °C (Found: C, 57.5; H, 2.6; N, 6.5. C86H45N8O8Cl9In requires C, 57.3; H, 2.4;
N, 6.7%); λmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 384, 627 and 697 (5.24); νmax (KBr)/cm−1 692, 735, 1020, 1088, 1161, 1209, 1268, 1283, 1398, 1453, 1488 and 1585; δH (400 MHz; CDCl3) 4.00 (2H, s, br), 5.93 (2H, s), 6.11 (1H, s), 7.15 (16H, d, J 8.9), 7.41 (16H, d, J 8.9) and 9.01 (8H, s); δC (100.5 MHz; CDCl3) 121.5, 121.9, 123.0, 123.1, 126.6, 130.9, 131.3, 131.7, 131.9, 134.9, 152.3 and 156.7 (4 resonances are missing); m/z (FAB) 2722/2724/2726 (M+, 100%).
Tetra-tert-butyltetrabenzo[b,k,t,c1]phthalocyaninatoindium chloride (tetra-tert-butylnaphthalocyaninatoindium chloride) 37. Yield 26%, mp >275 °C (Found: C, 70.3; H, 5.2; N, 9.9. C64H56N8InCl requires C, 70.6; H, 5.3; N, 10.3%); νmax(EtOH)/nm (log ε/dm3 mol−1 cm−1) 324, 706, 749 and 793 (5.39); νmax (KBr)/cm−1 472, 544, 569, 1022, 1084, 1102, 1259, 1355, 1459, 1560, 1626, 1637, 1655, 2905, 2930 and 2955; δH (400 MHz; CDCl3) 1.80 (36H, s) and 8.05–8.98 (20H, m); m/z (FAB) 1086/1088 (M+, 100%), 1051 (M+
− 35(Cl)).
Acknowledgements
We are grateful to the EPSRC national mass spectrometry service center for mass spectra.References
- “Materials for optical limiting”, ed. R. Crane, K. Lewis, E. van Stryland and M. Khoshnevisan, Proc. Mater. Res. Soc., 1996, 374 Search PubMed.
- K. Mansour, D. Alvarez, K. J. Perry, I. Choong, S. R. Marder and J. W. Perry, Proc. SPIE – Int. Soc. Opt. Eng., 1993, 1853, 132 Search PubMed.
- K. Mansour, P. Fuqua, S. R. Marder, B. Dunn and J. W. Perry, Proc. SPIE – Int. Soc. Opt. Eng., 1994, 2143, 239 Search PubMed.
-
(a) J. W. Perry, K. Mansour, S. R. Marder, C.-T. Chen, P. Miles, M. E. Kenney and G. Kwag, Proc. MRS, 1996, 374, 257 Search PubMed;
(b) P. Le Barny, V. Dentan, P. Robin, F. Soyer and M. Vergnolle, Proc. SPIE – Int. Soc. Opt. Eng., 1996, 2852, 201 Search PubMed.
- P. V. Bedworth, J. W. Perry and S. R. Marder, Chem. Commun., 1997, 1353 RSC.
- J.
W. Perry, K. Mansour, I.-Y. S. Lee, X.-L. Wu, P. V. Bedworth, C.-T. Chen, D. Ng, S. R. Marder, P. Miles, T. Wada, M. Tian and H. Sasabe, Science, 1996, 273, 1533 CrossRef CAS.
- M. J. Cook, A. J. Dunn, S. D. Howe, A. J. Thomson and K. J. Harrison, J. Chem. Soc., Perkin Trans. 1, 1988, 2453 RSC.
- N. B. McKeown, I. Chambrier and M. J. Cook, J. Chem. Soc., Perkin Trans. 1, 1990, 1169 RSC.
- A. N. Cammidge, M. J. Cook, K. J. Harrison and N. B. McKeown, J. Chem. Soc., Perkin Trans. 1, 1991, 3053 RSC.
- G. J. Clarkson, N. B. McKeown and K. E. Treacher, J. Chem. Soc., Perkin Trans. 1, 1995, 1817 RSC.
- D. Wohrle, M. Eskes, K. Shigehara and A. Yamada, Synthesis, 1993, 194 CrossRef.
- W. Eberhardt and M. Hanack, Synthesis, 1997, 95 CrossRef CAS.
- E. I. Kovshev, V. A. Puchnova and E. A. Luk'yanets, J. Org. Chem. USSR, 1971, 364 Search PubMed.
- M. K. Lowery, A. J. Starshak, J. N. Esposito, P. C. Krueger and M. E. Kenney, Inorg. Chem., 1965, 4, 128 CrossRef CAS.
- B. L. Wheeler, G. Nagasubramanian, A. J. Bard, L. A. Schechtman, D. R. Dininny and M. E. Kenney, J. Am. Chem. Soc., 1984, 106, 7404 CrossRef CAS.
- W. E. Ford, M. A. J. Rodgers, L. A. Schechtman, J. R. Sounik, B. D. Rihter and M. E. Kenney, Inorg. Chem., 1992, 31, 3371 CrossRef CAS.
- M. Hanack, P. Haisch, H. Lehmann and L. R. Subramanian, Synthesis, 1993, 387 CrossRef CAS.
- H. Tomoda, S. Saito, S. Ogawa and S. Shiraishi, Chem. Lett., 1980, 1277 CAS.
- H. Tomoda, S. Saito and S. Shiraishi, Chem. Lett., 1983, 313 CAS.
- D. Wohrle, G. Schnurpfeil and G. Knothe, Dyes Pigm., 1992, 18, 91 CrossRef.
- M. Hanack and H. Heckmann, Eur. J. Inorg. Chem., 1998, 367 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2002 |
Click here to see how this site uses Cookies. View our privacy policy here.