On-chip generation and reaction of unstable intermediates—monolithic nanoreactors for diazonium chemistry: Azo dyes
Robert C. R.
Wootton
,
Robin
Fortt
and
Andrew J.
de Mello
*
Imperial College of Science, Technology and Medicine, Exhibition Road, South Kensington, London, UK SW7 2AY. E-mail: a.demello@ic.ac.uk; Fax: +44 (0)20 7594 5833; Tel: +44 (0)20 7594 5820
First published on 22nd January 2002
Abstract
Monolithic nanoreactors for the safe and expedient continuous synthesis of products requiring unstable intermediates were fabricated and tested by the synthesis of azo dyes under hydrodynamic pumping regimes.
Introduction
Reactive intermediates form the mainstay of chemistry at the laboratory scale, and form a large part of the fine and bulk chemical industries. The difficulties involved in the scale-up of processes involving reactive intermediates tend to devolve from the instability of the intermediates themselves. In many cases elaborate precautions must be taken to guard against reactive hazards and loss of reaction specificity.The use of micro- and nanoreactor technology to combat reactive chemical hazards is an area of increasing interest in current research.1 The term ‘microreactor’ has been used to denote reaction systems with a volume most readily measured in units of millilitres or in sub-millilitre units. As this covers a broad spectrum of units, the designation ‘nanoreactor’ has been coined to denote reactors with an instantaneous reaction volume most conveniently measured in nanolitres. Nanoreactors have been used to study reactive intermediates in such reactions as the Ugi multicomponent condensation,2 where evidence for the debated nitrilium intermediate was found but little work has been done on the development of safe continuous flow multi stage syntheses using monolithic structures. Previously, multistep peptide syntheses have been undertaken using simple microfluidic devices under electroosmotic flow (EOF) regimes, but the difficulties invoked using EOF for multiparallel scale-out are significant.3 Not least amongst these difficulties are the limited range of solvents and chip materials compatible with EOF techniques. The potential for arcing in high voltage supplies cannot be lightly dismissed either, as although the reactors themselves pose little ignition hazard, this is rarely the case for an industrial environment in which a scale-out rig would be operated. Consequently, we proposed to study the reactive quenching of diazonium intermediates using a hydrodynamic pumping regime.
Due to the small scale of the reactors and the minute instantaneous volumes involved in continuous flow syntheses the hazard presented by each reactor is minimal and provided a simple, monolithic design is used reactors can be cheaply mass produced and run in multiparallel to provide a simulated large scale flow reactor.
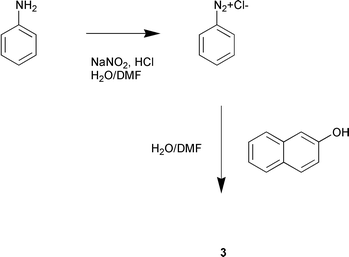
In order to study the application of nanoreactor technology to multi stage synthesis involving reactive intermediates we chose to study the formation of diazonium salts, and their in situ transformation into azo dyes. The highly coloured nature of the products renders detection facile and the reaction is rapid enough to avoid excessive delay in dye formation. This reaction has previously been used for the analysis of nitrites by spectrophotometric measurement of an azo derivative of sulfanilamide.4 An EOF approach to this synthesis has been reported using a preformed diazonium intermediate but the formation and reactive quench of diazonium salts has not been performed in a serial, controlled manner on-chip.8
Azo dyes came to prominence in medicinal chemistry after the discovery that sulfanilamide (1) (see Fig. 1), the active metabolite of the azo dye Prontosil Rubrum, had in vivo antibacterial properties. Despite being superseded by the penicillin antibiotics in most antimicrobial applications sulfa drugs such as sulfasalazine (2) still find application in the treatment of Crohn's disease and ulcerative colitis.5 The Sudan series of azo dyes, typified by Sudan I (3), are commonly used as microbial stains. Azo linkages are generally formed by the coupling reaction of diazonium salts, such as shown in Scheme 1 below.
 | ||
| Scheme 1 | ||
The difficulty facing the industrial chemist in this is the potentially explosive, thermally unstable nature of the diazonium salts. Incidents of explosive decomposition of diazonium salts caused by salt deposition6 or vapour phase reaction7 have been reported, and use of diazonium intermediates in industry is normally subject to stringent safety procedures.
Simple, single function reactor chips have been used previously to generate diazonium salts in solution and to react preformed diazonium salts. For example Harrison et al. described an elegant system for the formation of azo dyes under EOF control but this involved preformed diazonium tetrafluoroborates.8 Leaving aside the differences between our pumped approach and the EOF technique favoured by Harrison, our aim here in the current Communication is to show that hazardous reactive materials can be generated on chip and used in situ. While stabilised diazo compounds can be handled with relative ease, transport of more reactive diazo intermediates between chip reactors must be carried out at low temperatures to avoid thermal decomposition of the intermediate. This leads to unnecessarily elaborate support equipment. A simpler and more elegant approach is to minimise transport distances by incorporating both generation of the unstable intermediate and reactive quenching into one monolithic chip design. Below is a description of a first generation monolithic device for multi stage synthesis using reactive intermediates.
As shown in Fig. 2 the nanoreactor consists of two Y junctions separated by a serpentine delay section. A second delay section precedes the exhaust, at which samples may be collected for analysis. The mixing of reagents on chip is totally diffusive, which has the advantage of leading to the simplest structures. Diffusion rates can be accurately calculated for the low Reynold's number regimes in such micromachined structures.9 For a typical structure with a channel width of 150 μm and a channel depth of 50 μm the limiting flow rate for complete diffusive mixing over the 8 cm delay sections in the structure shown was calculated to be 3.5 μl min−1 through the first two inlets and a balancing flow of 7 μl min−1 through the third inlet.
 | ||
| Fig. 2 Schematic of microreactor channel pattern used in all syntheses. | ||
Initial results were obtained on a microscope stage, by viewing channels on a Leica DMIL inverted microscope where formation of a persistent red colouration in the effluent delay channel was evinced by the formation of the azo dye. Identical procedures were followed with three separate aryl amines, each being coupled to β-naphthol. After visual detection of the products, the reactors were run for extended periods to collect samples for analysis.
Experimental procedure
The following is a general method for the preparation of azo dyes using continuous flow nanoreactors:Using the fluidic network as shown in Fig. 1 above, solutions A and B were introduced into the first mixer segment at a rate of 3.5 μl min−1 and solution C was introduced into the second mixer segment at a rate of 7 μl min−1 using a Harvard Apparatus PHD 2000 syringe pump. Azo dye formation was evidenced by the immediate formation of a persistent red coloration in the efferent streams of the second serpentine section.
1-(phenylazo)-2-naphthol (Sudan I, 3)
CAS registry no. 847-07-9.Solution A: Aniline (0.1 ml), hydrochloric acid (conc., 0.35 ml), water (2 ml) and N,N-dimethylformamide (12 ml).
Solution B: Sodium nitrite (0.75 g) in water (4 ml), N,N-dimethylformamide (20 ml).
Solution C: β-Naphthol (0.15 g), sodium hydroxide solution (10%, 9 ml), water (20 ml) in N,N-dimethylformamide (290 ml), 52% conversion.
λ max 478 (418 ) nm. Lit. 476 (418) nm.10
1-(2-methylphenylazo)-2-naphthol (4, R1 = Me, R2 = H)
CAS registry no. 2646-17-5.Solution A: o-Toluidine (0.1 ml), hydrochloric acid (conc., 0.35 ml), water (2 ml) and N,N-dimethylformamide (12 ml).
Solution B: Sodium nitrite (0.75 g) in water (4 ml), N,N-dimethylformamide (20 ml)
Solution C: β-Naphthol (0.15 g), sodium hydroxide solution (10%, 9 ml), water (20 ml) in N,N-dimethylformamide (290 ml), 23% conversion.
λ max 487 (423) nm. Lit. 488 (427) nm.11
1-(3-methylphenyl)-2-naphthol (4, R1 = H, R2 = Me)
CAS registry no. 6656-98-0.Solution A: m-Toluidine (0.1 ml), hydrochloric acid (conc., 0.35 ml), water (2 ml) and N,N-dimethylformamide (12 ml).
Solution B: Sodium nitrite (0.75 g) in water (4 ml), N,N-dimethylformamide (20 ml).
Solution C: β-Naphthol (0.15 g), sodium hydroxide solution (10%, 9 ml), water (20 ml) in N,N-dimethylformamide (290 ml), 9% conversion.
λ max 499 (419) nm. Compares well with literature spectrum.12
Results and discussion
IR spectral data show close agreement with data given in the literature.13,14 Samples were identical to those prepared by standard laboratory methods.The reactions above were not optimised for the diazotisation reaction and this is probably responsible for the variation in conversion. Preliminary data from a more in-depth study show a close concordance between the extent of diazotisation of the aminoarenes and the conversion rates given above. This suggests that optimisation of the diazotisation step would lead to enhanced yields. External monitoring of the extent of diazotisation can be accomplished by spectroscopic methods, rendering optimisation more facile, though such studies fall outside the scope of this Communication. As a proof of principle the use of hydrodynamic regimes for the continuous flow generation and reactive quenching of diazonium salts is easily accomplished. The simple structures required and the safe nature of the procedure as described show clear advantages over bulk scale syntheses and with optimisation for diazonium formation may offer an alternative to existing industrial routes.
Conclusion
In conclusion the utility of nanoreactors in the continuous flow multistage syntheses involving unstable, reactive intermediates has been demonstrated. The benefits of the technique include room temperature handling of thermally unstable intermediates and increased safety over existing procedures. Further work involving unstable or reactive intermediates is underway in our laboratories.Acknowledgements
The authors thank EPSRC UK, the Department of Trade and Industry (DTI), and the Lab-on-a-Chip consortium for financial support. RF is grateful to SmithKlineBeecham Pharmaceuticals for an EPSRC CASE studentship.References
- R. D. Chambers and R. C. H. Spink, Chem. Commun., 1999, 883 RSC; S. J. Haswell, ‘Miniaturisation—What's in it for Chemistry?’ Micro Total Analytical Systems 2001, Kluwer Academic Publishers, Dordrecht/Boston/London, p. 637 Search PubMed.
- M. C. Mitchell, V. Spikmans and A. J. de Mello, Analyst, 2001, 126, 24 RSC.
- P. Watts, C. Wiles, S. J. Haswell, E. Pombo-Villar and P. Styring, Chem. Commun., 2001, 990 RSC.
- G. M. Greenway, S. J. Haswell and P. H. Petsul, Anal. Chim. Acta, 1999, 387, 1 CrossRef CAS.
- M. A. Peppercorn, Ann. Intern. Med., 1984, 3, 377 Search PubMed.
- W. H. Doyle, Loss Prev., 1969, 3, 14 Search PubMed.
- Anon., Sichere Chemiearb., 1993, 45(1), 8 Search PubMed.
- H. Salimi-Moosavi, T. Tang and D. J. Harrison, J. Am. Chem. Soc., 1997, 119, 8716 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) Significant reference.
Significant reference. - J. Crank, The Mathematics of Diffusion, Clarendon Press, Oxford, 2nd edn., 1975 Search PubMed.
- Aldrich Catalogue Handbook of Fine Chemicals, Aldrich Chemical Company, Gillingham, Dorset, UK, 1999, p. 1583 Search PubMed.
- J. N. Ospenson, Acta Chem. Scand., 1950, 4, 1331.
- A. Burawoy, A. G. Salem and A. R. Thompson, J. Chem. Soc., 1952, 4793 RSC.
- The Aldrich Library of FT-IR Spectra, 1st edn., Aldrich Chemical Company, Milwaukee, WI, USA, 1985 Search PubMed.
- K. J. Morgan, J. Chem. Soc., 1961, 215 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |

