Pressure pinched injection of nanolitre volumes in planar micro-analytical devices
Xiaoxia Baia, Hye Jin Leea, Joel S. Rossiera, Frederic Reymondb, Helwig Schaferc, Michael Wossnerc and Hubert H. Girault*a
aLaboratoire d′Electrochimie, Ecole Polytechnique Federale de Lausanne, 1015, Lausanne, Switzerland. E-mail: hubert.girault@epfl.ch; Fax: +41.21.693.36.67; Tel: +41.21.693.31.51
bDiagnoSwiss SA, Rte de l′Ile-au-Bois 2, c/o CIMO S.A. - CP, 1870, Monthey, Switzerland. E-mail: info@diagnoswiss.com; Fax: +41.24.471.49.01; Tel: +41.24.471.49.00
cMetrohm Ltd., CH-9101 Herisau, Switzerland. E-mail: info@metrohm.ch; Fax: +41.71.353.89.01; Tel: +41.71.353.85.85
First published on 22nd January 2002
Abstract
A new method for injecting and driving fluids by means of a multi-port injection valve and syringe pumps in a micro-channel network is described. A structure composed of two micro-channels arranged as a cross is connected with capillary tubes to an external multi-port injection valve. The fluid flows are driven by pressure and the multi-port valve controls the direction of the flow within the different sections of the structure. The first position of the multi-port valve allows the preparation of the loading of the sample, which is pinched in the cross section of the two micro-channels. The second position allows the precise injection of nL volumes. No dead volume exists between injection and separation modes. The system can be used to prepare a sample plug by pressure in order to perform chromatography with a broad range of buffered or non-buffered solutions. Thanks to the insensitivity to the ionic strength of the sample, this injection method is useful for the injection of complex biological samples in microchip analysis. In order to demonstrate the feasibility of the method, different solutions of ionic or fluorescent molecules were injected and detected in a photoablated planar polymer device.
1. Introduction
Miniaturisation of analytical systems has played an important role in the development of fast analysis systems using very small amounts of sample. During the last decade, intensive efforts have been devoted to the design of miniaturised complete laboratory systems, mainly for applications in capillary electrophoresis and electrochromatography.1,2 However, developments in micro-analysis have been hindered to some extent by the lack of efficient fluid pumping systems, especially when the matrix of the sample solution cannot be controlled precisely, as is the case for complex protein mixtures. The fluid movement in micro-channel networks built in glass, silica or polymeric materials is conventionally driven by capillary flow or by electro-osmotic pumping.3 The former process relies on chemical modifications of the surface properties, which in turn establish restrictions on the flow direction and rate. The latter process involves high-voltage inputs through electrodes in contact with a solution to generate electro-osmosis, which is usually referred to as electrokinetic pumping.One of the most critical parameters in high-resolution electrophoresis or electrochromatography is the sample injection. In standard capillary electrophoresis, two injection methods are generally used. The first one is electrokinetic injection, which is based on the application of a high voltage for pumping the sample solution by generation of an electro-osmotic flow. This method is very easy to perform, but requires that the capillary is filled with the sample solution prior to the application of the high voltage. With this injection mode in micro-analysis, several potentials were applied in the different channels in order to force the solution to flow and to design well-defined sample plugs. The first capillary electrophoresis (CE) chip employed a T-injector design,4,5 but recent devices include cross6 and double-T7 injectors because of the difficulty in controlling the sample plug. Pinched injection,8,9 gated injection10 and floating injection11,12 were also developed for the generation of well-defined plugs in various analytical applications. A lot of effort has been devoted to the optimisation of this electrokinetic injection mode. Particularly, the use of only one high voltage to simplify the complicated instrumentation13 (also adapted to the injection of neutral species14), the optimisation of the micro-structure geometry,8,15 the development of a narrow sample channel injector to increase the system efficiency16 and optically gated electrophoresis with reduced injection bias17 have been proposed.
However, when some of the species to be analysed have very different electrophoretic mobilities, electrokinetic injection methods are not always suited. In such a situation, some of the species will migrate during the injection, providing bias in the final concentration ratio between the analytes whereby neutral species would be injected preferentially to anionic species.18 The other principal drawback of the electrokinetic method is the necessity to work with well-defined sample conductivity, viscosity and thermal properties. For instance, the injection of a sample in pure water is very difficult because of a strong stacking effect. Also, the injection of very complex mixtures with, for example, high protein concentrations is difficult, because the proteins may change the zeta potential in the sample micro-channel and hence induce instability in the intensity or even change the direction of the electro-osmotic flow. It may be expected that novel fluid handling procedures based on pressure injection, which are less sensitive to sample composition, can be used in micro-analytical devices.
In pressure-induced injections, valves are used to limit the volume of the injected sample. However, due to the difficulty in designing, fabricating and integrating such devices in μ-systems, pressure-induced injection has rarely been used until now. Indeed, only a few processes, each with their own advantages and disadvantages, have been investigated for non-electrokinetic injection. Pneumatic pumping19 was used to control the sample injection through a shut-off valve. In this case, the volume of sample solution injected was quite large, ranging from 50 to 100 μL with a total dead volume of about 10 μL. Another report20 described the use of a centrifugal force to pump fluids into micro-channels disposed on a disk. The main drawback of this system relied on the fact that the flow direction could not be reversed or changed. In a similar way, off-chip sample introduction21 has been achieved by connecting a flow injection system to a microchip capillary electrophoresis apparatus, but this method still leads to rather large dead volumes (about 160 μL). Finally, a commercially available nL injection valve has been directly coupled with to a capillary electrophoresis system, demonstrating the pressure-induced injection of volume of 6–60 nL.22
In standard chromatography, the injection is performed by means of a multi-port two-way valve system where an injection loop is filled, after the solution has been injected into the column, by switching the valve. This method enables the injection of sample with a minimum perturbation of the flow. Nevertheless, this system generates a fairly large dead volume due to the presence of tubing connecting the injection loop to the chromatography column, thereby rendering such an injection method difficult to integrate in micro-total analysis systems. In order to overcome this problem, a novel way to inject nL samples on a planar micro-chip is presented here, which combines the use of a multi-port valve with a special disposition of the pressure pumping system. Three syringe pumps connected through an 8-port two-way valve to a cross intersection of a micro-channel network are used here to describe this novel pressure-induced injection method. The performance of this methodology is demonstrated by pressure-pinched injection of nL plugs of ionic and fluorescent solutions.
2. Experimental
2.1 Chemicals
A solution of Eosin B (Aldrich, Milwaukee, WI, USA) and sodium chloride (Fluka, Buchs, Switzerland) was used to visualise the injection, and fluorescein (Fluka) in phosphate buffer (Sigma, St. Louis, MO, USA) was used for the fluorescence detection. All chemicals were of analytical grade, and ultra-pure water (Millipore, Bedford, MA, USA) was used for dilution.2.2 Fabrication of the device
A micro-channel network was fabricated in a polymeric substrate (polyethylene terephthalate (PET), Dupont Mylar A) by the use of UV excimer laser photoablation following a method already described elsewhere.23 Schematic diagrams of the analytical micro-chip device designed and tested in this work are shown in Fig. 1(a). The fabricated μ-structures comprise an injector, a separation channel and a detector. In general, at first, the carbon ink is cast into a 50 μm wide and 20 μm deep channel to construct the microelectrode for conductivity detection.24 A 6 cm long channel with 200 μm width and 60 μm depth is then perpendicularly photoablated to the electrode band in order to form two face-to-face electrodes on the side surface of the separation channel (see Fig. 1(b)). Afterwards, to define a injection cross made of two 1-cm long channels, a 2 cm channel with the same cross section dimension as the separation channel is perpendicularly drilled across the separation channel as shown in Fig. 1(a). The micro-channels are then sealed by lamination of a 30 μm thick polyethylene/polyethylene terephthalate (PE/PET) layer and thus compose a set of capillaries.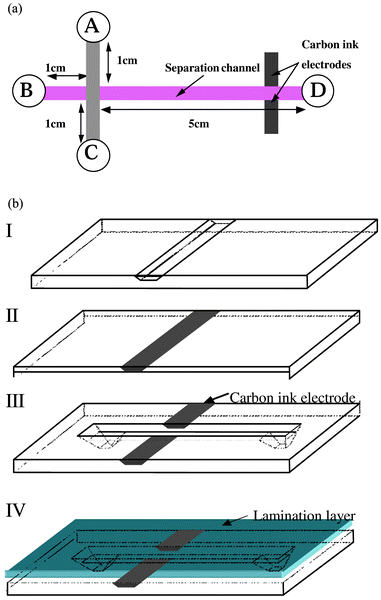 | ||
| Fig. 1 Schematic diagram showing the structure of polymer μ-chip devices used to demonstrate the concept of the present pressure-driven injection method. The structure in part (a) is composed of an asymmetrical cross of sealed micro-channels that are connected to external tubes via the reservoirs A–D. On the right hand side of the structure, the longer micro-channel serves as separation column, and two carbon ink micro-electrodes are inserted in the vertical walls of this micro-channel in order to detect the injected plugs by conductivity. The structure of part (b) shows the fabrication principle for the incorporation of the electrodes on the microchip for the conductivity detection. | ||
It is worth noting here that the cross presents a double depth, since this small portion of the polymer was exposed twice to the laser beam during the fabrication of the micro-structure. Due to the typical trapezoidal form generated with this type of micro-fabrication method,25 the volume of the upper layer of the cross can be evaluated to 2 nL. Therefore, the estimated maximum volume of this double depth cross is ∼4 nL. The injected volume can be approximately deduced from the volume of the intersection. However, it is important to note that nanoliter volumes or sub nanoliter samples can be injected due to the triangle shape within the cross intersection in the time independent injection mode that will be shown later.
Four inlet reservoirs of the micro-channel network were connected to a two-way micro-8-port valve (VICI AG, Houston TX, USA). As described below, the two positions of the valve allow both the sample loading and the injection (see also the detailed configuration in Fig. 2). Capillary tubes and three syringe pumps were also connected to the micro-channel network, through the micro 8-port valve, and the flow rate of syringe pumps for sample driving and buffer driving are fixed at 3.33 μL min−1 and 0.8 or 0.9 μL min−1, respectively.
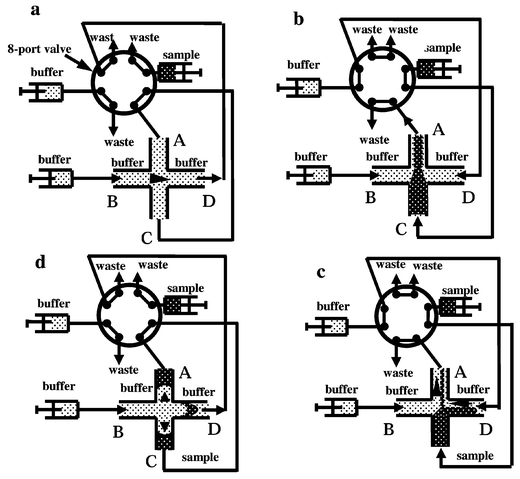 | ||
| Fig. 2 Schematic diagram of a two-way micro-8-port valve connected to a micro-channel network as shown in Fig. 1, which describes the movement of a sample and an elution solution as a function of the position of the valve. This figure illustrates how a sample can be injected into a micro-channel network: a, the standby state before sample loading; b and c, configuration of the pressure pinched injection, where the sample solution is loaded into the cross and where the loaded plug is pressed by two countercurrent flows. A symmetrical triangle (b) or a non-symmetrical (c) shape of the loaded plug can be obtained using the same pinch pressures or different ones at B and D connections. d, Configuration of the separation position where the sample plug is injected under pressure and evolves in a parabolic shape. | ||
2.3 Injection procedure for time independent injection
The injection method necessitates the use of two solutions (running buffer and sample solution) that are pumped independently into the micro-channel network. At the beginning, the system is in standby mode, as shown in Fig. 2a. Upon switching (see Fig. 2b), the sample solution is pumped from the inlet C to the outlet A whereas both sides of the main channel (connections B and D) are connected to two syringe pumps delivering the buffer solution. In this case, the sample solution is pumped linearly throughout the structure. In order to prevent the volume of the sample plug depending on the injection time, it is possible to force the running buffer from both sides of the sample flow by applying a pressure through the syringes that are linked to the connections B and D.The running buffer at the connection B is connected directly to the structure, without passing through the multi-port valve as presented in Fig. 2. This means that the same pressure is imposed to the inlet B during the injection loading as well as during the separation. The other connecting capillary tubes are connected as presented in Fig. 2b, where the flow is imposed to the three micro-channels B, C, D towards the intersection and where one single channel section A is connected to the waste, therefore a pinched symmetrical triangle plug is constructed at the intersection by balancing the flow rates from B and D.
When the multi-port valve is switched to the original position, as presented in Fig. 2d, the running buffer flows from the inlet B to the outlet D and brings the plug previously positioned at the intersection. The side arms (A and C) are connected together and hence the net flow in these side arms is zero. However the buffer may induce a transient push-back effect in both side channels during the switching of the valve, allowing the injection of a small and very well-defined plug. The solution is then pushed through the separation channel and the plug transforms to a parabolic shape, as expected for conventional pressure-driven tubular flows. The portion of the valve connecting the two side channels, A and C, can eventually be obstructed to prevent fluid flow caused by pressure imbalance. In conclusion, this time-independent injection process as shown in Fig. 2 proceeds via the steps sequenced in Fig. 2a, b and d. Repetitions of the injection procedure can be performed. A certain waiting time, 1–2 min, need to be considered between two sequential injections to exclude the sample plug from the waste port of the injection valve in order to prevent the feedback of the sample in the next assay.
2.4. Injection procedure for time dependent injection
Based on the same principle, another time dependent injection mode was also operated. The only difference is that the pressure in the buffer reservoir B (see Fig. 2c) on the left hand side of the structure is larger than that imposed in the waste reservoir D at the end of the sample loading. By decreasing the flow rate of syringe pump connected to reservoir D, part of the sample plug was pushed into the main separation channel, depending on the duration time of this process and the pressure difference induced by the different flow rates pressed on B and D. At the end of the injection duration, the valve is also switched to positions as shown in Fig. 2d. In contrast to the time-independent injection mentioned above, this injection runs through the sequences depicted in Fig. 2a–2d.2.5 Imaging and conductivity detection of an injected plug
A set of images of the cross section at the different stages of the injection were obtained by means of a microscope (Axiovert 25, Zeiss) and were recorded on videotape.Generally speaking, the face-to-face carbon electrodes which are connected with a conductimeter (Metrohm, AG, Switzerland) measured the resistance of the solution flowing through them. A calibration demonstrated this conductivity detection on microchip. Different concentrations of sodium chloride solutions ranging from 1 μM to 50 mM were filled in the microchannel by vacuum pumping. The conductivity of the static solution was then measured. The linear correlation coefficient in the concentration range between 1 and 10 mM is 0.9995, while the detection limit is 1 μM. This result proves the applicability of this microband electrode as a conductivity detector.
3. Results
3.1. Time independent pinched injection
In order to demonstrate the feasibility of the present concept, the intersection of the μ-channel network is placed under a microscope: visible and fluorescence images are taken at different times during the injection and the separation. The photographs presented in Fig. 3 clearly demonstrate the feasibility of driving the solution as presented in the injection pattern of Fig. 2. Indeed, it can be seen in Fig. 3A that the side channels are equally pressurised, which induces the creation of a symmetrical injection plug. The sample solution (2 mM sodium chloride + 10 mM Eosin B) is pinched between two side solutions (pure water) that are colourless. The sequence of photographs shows the behaviour of the solution at the μ-channel intersection upon switching the multi-port valve, from the position of Fig. 2b to that of Fig. 2d. It is interesting to note here the parabolic shape of the injected plug flowing into the separation channel. The sample solution itself can be pushed back by the novel pressure, ensuring the complete separation between the plug and the rest of the sample solution (see Fig. 3D). | ||
| Fig. 3 Photographs of the micro-channel intersection during the pressure-driven injection of a solution of 2mM sodium chloride + 10 mM Eosin B in water under the configuration presented in Fig. 2. The photographs were taken at the following times after positioning of the multi-port valve in the configuration of Fig. 2b: A, 0 s; B, 0.6 s; C, 1 s; and D, 3 s. The feature of Fig. 3A remained stable during several minutes and injection can be operated at any time after first stabilisation of the flows. | ||
This experiment demonstrates that a pinched injection similar to that developed by Ramsey8 for electrically-driven flow injection can be obtained by mechanical means. Indeed, in both cases, the sample solution is pumped through the intersection, whereas two counter-current flows are pinching it from the side. As a consequence, the sample solution has no time to diffuse inside the main channel and the volume of the injection plug volume is much less dependent on the duration of the injection. The fundamental difference of the present injection system is that no electrical field is necessary inside the structure in order to pump the solution. Indeed, the solution flows are fully controlled by pressure, so that no electroosmosis flow has to be generated in the μ-system. Thanks to the pressure-driven flow, the present device can be used for the manipulation of solutions that do not contain salts or contain very complex mixtures of solute molecules, including high concentrations of charged species. Conversely, due to the parabolic shape of the flows, the theoretical plates obtained during the separation are lower with the present pressure-induced injection than with the electrokinetic injection. However, it can be optimised as described by Dutta et al.,26 who demonstrated different shapes of channel to reduce the parabolic evolution of the plug by pressure flow.
In a further experiment, a solution of 10 mM Eosin B and 2 mM of sodium chloride has been injected into the separation channel following the above procedure. Pure water was placed in the buffer syringes and was used as eluent. The detection is performed by conductivity measurement using two carbon ink electrodes that face each other and were inserted at the end of the separation channel as shown in Fig. 1(b). The chromatogram obtained is presented in Fig. 4, the shape of the plug represents a typically pressure-driven detection pattern. Two very sharp peaks appear before the sample peak due to the switching of the injection valve. As was mentioned above, the first peak corresponds to the start of the sample loading, the valve position switching from that of Fig. 2a to that of Fig. 2b, and the second to the injection, the position of Fig. 2b
switching to that of Fig. 2d. Therefore, the sample loading time and the retention time could be directly deduced from the chromatogram according to these two sharp peaks. For the case shown in Fig. 4, the loading time of the injection is 0.51 min, which is enough to have a pinched plug as seen in Fig. 3A, while the retention time is 1.18 min. Depending on the sample flow rate, the total sample consumption during one measurement can be calculated about 5 μL. Compared to the injected volume, this sample consumption is quite large. The main point here is that there is no dead volume between injector and separation channel on a microchip. Whenever a sample plug is formed, it can be injected into the separation channel.
 | ||
| Fig. 4 Conductimetry chromatogram obtained for the separation of a 10 mM Eosin B + 2 mM sodium chloride solution after injection of this mixture in the conditions described in Fig. 3. The flow rate of syringe pump driving the sample solution is 3.33 μL min−1, while the flow rate for buffer driving is 0.9 μL min−1. | ||
3.2 Time dependent pinched injection
An example of the time dependent injection is shown in Fig.5, where fluorescence images have been taken to monitor the injection of a solution of 100 μM fluorescein in 10 mM phosphate buffer at pH 7.4. In Fig. 5A, the sample is pinched from both sides as in Fig. 3A. Then, the flow rate to outlet D is reduced and some sample coming from C and some buffer coming from B are loaded in the separation channel (Fig. 5B). Afterwards, the valve is switched to the position as in Fig. 2d and the sample continues in the separation channel as the solution from B acts as the eluent (Fig. 5C). In this manner, large volumes of samples can be injected, and this injection mode is time dependent. | ||
| Fig. 5 Sequence of fluorescence images showing the injection of 100 μM of fluorescein in 10 mM phosphate buffer at pH 7.4 into the micro-structure of Fig. 1. The first fluorescence image of Fig. 5A is obtained with the configuration of Fig. 2b, where the different flow rates in the channels generate an asymmetrical shape of the plug profile at the end of the sample loading shown in the second image (Fig. 5B). The configuration is switched to the position of Fig. 2d, and Fig. 5C shows the injected plug which exhibits a parabolic shape. This plug is thus injected into the main micro-channel for separation. | ||
It must finally be mentioned that the present injection method can also be sustained by electrical means during the separation and/or the injection in order to separate molecules by electrophoresis. For certain applications, only the injection may be sustained by pressure, whereas the separation may be purely electrophoretic. This approach also needs to be investigated, since it would assuredly increase the number of theoretical plates that can be obtained during the separation.
4. Conclusion
The present method provides a novel means to inject nL plugs in a planar microchip device by means of a multi-port two-way valve. It is a pressure driven sample introduction with reduced dead volume, using syringe pumps. This injection mode does not depend on the properties of the sample and buffer solutions. Through the multi-port valve, fluids can be easily controlled within the cross injector. This injection method can also be applied in other systems, and further work will be devoted to applications of this method in chromatography, capillary electrophoresis and electrochromatography. In addition, the system needs to be further optimized so that, for instance, choice of injection volume, which can be calculated by simulation, is ongoing.Acknowledgement
The authors wish to thank the Swiss Commission for Technology and Innovation (CTI) for financial support.References
- J. P. Kutter, Trends Anal. Chem., 2000, 19, 352–363 CrossRef CAS
.
- G. J. M. Bruin, Electrophoresis, 2000, 21, 3931–3951 CrossRef CAS
.
- N. A. Polson and M. A. Hayes, Anal. Chem., 2001, 73, 312A–319A CAS
.
- A. Manz, D. J. Harrison, E. M. J. Verpoorte, J. C. Fettinger, A. Paulus, H. Ludi and H. M. Widmer, J. Chromatogr., 1992, 593, 253–258 CrossRef CAS
.
- D. J. Harrison, A. Manz, Z. H. Fan, H. Ludi and H. M. Widmer, Anal. Chem., 1992, 64, 1926–1932 CrossRef
.
- D. J. Harrison, K. Fluri, K. Seiler, Z. H. Fan, C. S. Effenhauser and A. Manz, Science, 1993, 261, 895–897 CrossRef CAS
.
- C. S. Effenhauser, A. Paulus, A. Manz and H. M. Widmer, Anal. Chem., 1994, 66, 2949–2953 CrossRef CAS
.
- S. C. Jacobson, R. Hergenroder, L. B. Koutny, R. J. Warmack and J. M. Ramsey, Anal. Chem., 1994, 66, 1107–1113 CrossRef CAS
.
- Z. H. Fan and D. J. Harrison, Anal. Chem., 1994, 66, 177–184 CrossRef CAS
.
- S. C. Jacobson, R. Hergenroder, A. W. Moore and J. M. Ramsey, Anal. Chem., 1994, 66, 4127–4132 CrossRef CAS
.
- J. Khandurina, T. E. McKnight, S. C. Jacobson, L. C. Waters, R. S. Foote and J. M. Ramsey, Anal. Chem., 2000, 72, 2995–3000 CrossRef CAS
.
- O. Salas-Solano, D. Schmalzing, L. Koutny, S. Buonocore, A. Adourian, P. Matsudaira and D. Ehrlich, Anal Chem., 2000, 72, 3129–3137 CrossRef CAS
.
- S. C. Jacobson, S. V. Ermakov and J. M. Ramsey, Anal. Chem., 1999, 71, 3273–3276 CrossRef CAS
.
- J. Palmer, D. S. Burgi, N. J. Munro and J. P. Landers, Anal. Chem., 2001, 73, 725–731 CrossRef CAS
.
- L. L. Shultz-Lockyear, C. L. Colyer, Z. H. Fan, K. I. Roy and D. J. Harrison, Electrophoresis, 1999, 20, 529–538 CrossRef CAS
.
- C. X. Zhang and A. Manz, Anal. Chem., 2001, 73, 2656–2662 CrossRef CAS
.
- J. A. Lapos and A. G. Ewing, Anal. Chem., 2000, 72, 4598–4602 CrossRef CAS
.
- J. P. Alarie, S. C. Jacobson and J. M. Ramsey, Electrophoresis, 2001, 22, 312–317 CrossRef CAS
.
- Z. H. Fan, S. Mangru, R. Granzow, P. Heaney, W. Ho, Q. P. Dong and R. Kumar, Anal. Chem., 1999, 71, 4851–4859 CrossRef CAS
.
- D. C. Duffy, H. L. Gillis, J. Lin, N. F. Sheppard and G. J. Kellogg, Anal. Chem., 1999, 71, 4669–4678 CrossRef CAS
.
- S. Attiya, A. B. Jemere, T. Tang, G. Fitzpatrick, K. Seiler, N. Chiem and D. J. Harrison, Electrophoresis, 2001, 22, 318–327 CrossRef CAS
.
- L. M. Ponton and C. E. Evans, Anal. Chem., 2001, 73, 1974–1978 CrossRef CAS
.
- M. A. Roberts, J. S. Rossier, P. Bercier and H. Girault, Anal. Chem., 1997, 69, 2035–2042 CrossRef CAS
.
- J. S. Rossier, M. A. Roberts, R. Ferrigno and H. H. Girault, Anal. Chem., 1999, 71, 4294–4299 CrossRef CAS
.
- J. S. Rossier, R. Ferrigno and H. H. Girault, Electroanal. Chem., 2000, 492, 15–22 CrossRef CAS
.
- D. Dutta and D. T. Leighton, Anal. Chem., 2001, 73, 504–513 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2002 |
