Differential measurement with a microfluidic device for the highly selective continuous measurement of histamine released from rat basophilic leukemia cells (RBL-2H3)
Ryoji
Kurita
a,
Katsuyoshi
Hayashi
b,
Tsutomu
Horiuchi
b,
Osamu
Niwa
*b,
Kazutaka
Maeyama
c and
Katsuyuki
Tanizawa
d
aNTT Advanced Technology, 3-1 Morinosato Wakamiya, Atsugi, Kanagawa, 243-0124, Japan
bNTT Lifestyle and Environmental Technology Laboratories, 3-1 Morinosato, Wakamiya, Atsugi, Kanagawa, 243-0198, Japan
cDepartment of Pharmacology, Ehime University, School of Medicine, Ehime, 791-0295, Japan
dInstitute of Scientific and Industrial Research, Osaka University, Ibaraki, Osaka, 567, Japan
First published on 18th January 2002
Abstract
We fabricated a micro-fluidic device for the highly selective detection of the histamine released from rat basophilic leukemia (RBL) 2H3 cells. The device has two thin layer flow channels, each with one working electrode. One electrode was modified with Os-polyvinylpyridine based mediator containing horseradish peroxidase (Os-gel-HRP) and histamine oxidase (HAOx), the other was modified with Os-gel-HRP without any HAOx. We employed the device for differential measurement by using the HAOx modified electrode for detection and the unmodified electrode as a reference. The detection limit was greatly improved from 190 to 25 nM since the baseline noise level was suppressed. We used differential measurement to observe the histamine released from RBL-2H3 cells when stimulated with dinitrophenylated bovine serum albumin (DNP-BSA) as an antigen. We injected 5 μM of histamine solution into our device and it remained stable for more than 8 h.
Introduction
Histamine is a well-known monoamine that plays important roles in relation to immune response and gastric secretion. Histamine also functions as a neurotransmitter in the central nervous system.1–4 It is important to detect the histamine concentration in seafood because histamine often causes a severe allergic reaction.5,6 Many different methods have already been proposed for measuring histamine. Most approaches use liquid chromatography (LC) combined with OPA derivatization7–9 or the AOAC method, which uses fluorescence detection.10,11 Most of these previously reported methods were designed for relatively large volume samples. If we wish to measure histamine contained in a cell, we must reduce the detection volume.Zare et al. reported a chip-based capillary electrophoresis system with laser induced fluorescence detection. Their approach involves manipulating a few cells into the flow channel with optical tweezers using a YAG laser. The cells are then lysed and labeled. Finally, the contents of the cells are separated electrophoretically and detected with an He-Cd laser.12
Although this method is highly sensitive for very small sample volumes, the separation time required for each measurement should be taken into account. Then we find that it is difficult to employ the method for measuring histamine released from cells due to the poor temporal resolution.
Electrochemical measurement is a useful approach for the real-time measurement of histamine released from cells. There are two electrochemical methods for measuring histamine continuously. Pihel et al. used a carbon fiber microelectrode to detect histamine and 5-hydroxytryptamine (or serotonin: 5-HT) secreted from mast cells.13,14 With a carbon fiber electrode at pH 7.4, histamine and 5-HT were oxidized at 1400 and 600 mV, respectively. Since the anodic potential limit overlaps the histamine oxidation peak, they quantified the concentration peak by background subtraction. They also reported that histamine and 5-HT are co-released from mast cells and the amount of histamine is much greater than that of 5-HT. The detection limits for histamine and 5-HT were 1.4 μM and 12 nM, respectively. In their paper, they suggested that the much lower 5-HT detection limit was due to the adsorption of 5-HT at the electrode. However, the higher oxidation potential of histamine should raise the detection limit.
In contrast, enzyme modified electrodes have been studied with a view to measuring monoamines and diamines including histamine.6,15–20 Monoamine or diamine oxidase, methylamine dehydrogenase and putrescine oxidase combined with an oxygen electrode or platinum electrode have been used to construct such electrochemical biosensors. However, most sensors react not only to histamine but also to diamines such as putrescine and cadaverine. Therefore, the total amount of histamine and diamines has been measured.19
Ohashi et al. reported a more selective histamine biosensor designed to measure seafood samples.5 They used an oxygen electrode combined with a fungal amine oxidase, namely a Cu-containing enzyme that was prepared from mycelium extracted from Aspergillus niger. Their sensor was sensitive to histamine but insensitive to putrescine and cadaverine. More recently, Niculescu et al. reported a biosensor for measuring fish freshness using amine.20 They modified the electrode with redox hydrogel containing HRP and amine oxidase from grass peas. We reported a microfabricated flow type biosensor that we designed to detect histamine released from RBL-2H3 cells.21 The sensor consists of two glass plates; one with a microflow channel, the other with a carbon film based electrochemical cell. We modified the working electrode with Os-gel based mediator containing HRP22 and recombinant histamine oxidase from Escherichia coli cells.23 Histamine oxidase is a Cu2+–quinoprotein enzyme that has been found in the cells of Arthrobacter globiformis.24,25 This enzyme is very sensitive to histamine and much less sensitive to aliphatic monoamines and diamines. We used our sensor to detect the histamine released when the cultured mast cells were stimulated with an antigen. This system might be used to screen for chemicals that cause allergic reactions. However, for such applications, we must be able to distinguish signals from various types of interference. This is because we cannot separate the samples before detection as with capillary electrophoresis. The types of interference include baseline current fluctuation caused by electrostatic and pumping noise. An additional problem is the capacitive current that results from the ionic strength differences that occur when we add another solution containing an allergen. We reported that a sharp capacitive current could be observed when stimulating cultured nerve cells with potassium chloride solution.26,27
In this paper, we describe the differential measurement of histamine using a microfluidic device consisting of a sample and a reference dual channel thin layer flow channel designed to eliminate various types of interference. We also report the application of this device to the continuous measurement of histamine released from RBL-2H3 cells.
Experimental
Chemicals and materials
Recombinant histamine oxidase (HAOx) was prepared by one of the authors as described in a previous report.23 Histamine was purchased from Wako (Tokyo, Japan). Bovine serum albumin (BSA) was purchased from Sigma (St. Louis, MO). Heat-inactivated fetal bovine serum was purchased from Hyclone. Osmium-poly(vinylpyridine) wired horseradish peroxidase (Os-gel-HRP) was purchased from Bioanalytical Systems (West Lafayette, IN). UV-curable resin was purchased from Loctite (Yokohama, Japan). A silicon-based positive photoresist and THB-516-L positive photoresist (THB) were obtained from NTT Advanced Technology (Tokyo, Japan) and JSR (Tokyo, Japan), respectively. The external medium used for cell measurements contained 119 mM NaCl, 5 mM KCl, 1 mM CaCl2, 0.4 mM MgCl2, 5.6 mM glucose, and 25 mM PIPES and was adjusted to pH 7.2.Microfluidic device
Fig. 1 is a photograph of our microfluidic device for measuring histamine and a schematic representation of the electrochemical cell. The device structure is basically the same as that in our previous report on the selective detection of L-glutamate27 except for the enzyme modified electrode designed to detect histamine. The total width of the dual flow channel was 1 mm and it was 20 μm deep. We calculated the surface area of each working electrode to be 0.2 mm2. We coated the two working electrodes with Os-gel-HRP using a small brush. We dried them at room temperature for 1 h, and then coated HAOx film on one of the Os-gel-HRP modified electrodes also using a brush. The HAOx solution contained 0.76 units μl−1 HAOx and 2% BSA, and 0.2% glutaraldehyde, which we used to crosslink the film. We then used the same method to coat the other working electrode with 2% BSA and 0.2% glutaraldehyde solution without HAOx. We coated the reference electrode with silver paste (in toluene).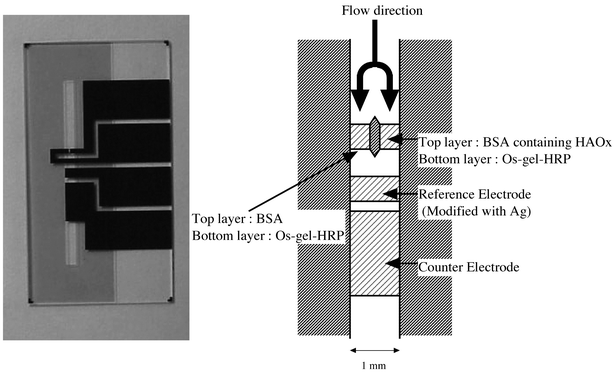 | ||
| Fig. 1 A photograph of our microfluidic device for measuring histamine and a schematic representation of the electrochemical cell. | ||
We also microfabricated a flow sensor for the continuous measurement of histamine. The sensor structure is described in ref. 21. In this case, the device had a rectangular flow channel 400 μm wide and 100 μm deep. The enzyme-modified layer in the channel was about 3 mm long and the working electrode was 0.4 mm2.
Measurement
When we evaluated the performance of our device by using standard histamine solution, we placed the end of the sampling capillary in buffer solution and connected the other capillary to a syringe. We installed the syringe in a CMA 102 dual syringe pump (Stockholm, Sweden) to introduce the buffer solution into the device in a suction mode. We introduced the sample solution into the device by drawing it from a plastic container. We connected the four pads of the microfabricated sensor to two LC-4C potentiostats (BAS) and stored the data in a computer using an analog/digital converter DA-5 (BAS). The potentials of both working electrodes were held at −50 mV vs. Ag. We performed standard solution measurements at room temperature.When measuring the histamine released from RBL-2H3 cells, we fixed the device to a manipulator (Narishige, Tokyo) to control the distance between the cultured cells and the end of the sampling capillary under microscope observation. The culture dish with RBL-2H3 cells and the various solutions were maintained at 37 °C by using refluxed ethyleneglycol. The RBL-2H3 cells were cultured for a week in a culture dish in Eagle′s minimum essential medium with 15% fetal bovine serum in a humid atmosphere consisting of 5% CO2 in air at 37 °C.
Before measuring the histamine released from the RBL-2H3 cells, we sensitized the cultured RBL-2H3 cells with 0.5 μg ml−1 of monoclonal IgE against dinitrophenylated bovine serum albumin (DNP-BSA) for 24 h. After sensitization, the cells were rinsed with 2 ml of PIPES buffer and incubated for 10 min. We measured variations in the histamine concentration near the cell by drawing the solution near the cells at a flow rate of 1 μl min−1 while adding DNP-BSA (20 ng ml−1).
Results and discussion
Standard measurement
Fig. 2 shows the response of 0.5 μM of histamine in the microfluidic device at a flow rate of 1 μl min−1. The cathodic current at one electrode modified with BSA-HAOx/Os-gel-HRP started to increase after we injected the histamine and reached a steady state (Fig. 2(a)). In contrast, we observed no current change at the other working electrode, which we modified with BSA/Os-gel-HRP containing no HAOx (Fig. 2(b)). This shows that the hydrogen peroxide generated at the BSA-HAOx modified electrode does not diffuse to the other electrode despite the short distance (200 μm) between the two working electrodes. This is due to the separator that we fabricated between the two working electrodes. | ||
| Fig. 2 The response of 0.5 μM histamine in the microfluidic device at a flow rate of 1 μl min−1. Trace (a) shows the current at the working electrode modified with a bilayer of BSA-HAOx/Os-gel-HRP. Trace (b) shows the current at the other electrode modified with a bilayer of BSA/Os-gel-HRP. Trace (c) shows the current obtained by deducting the current shown in (b) from that shown in (a). | ||
As described in the Introduction, various kinds of noise are present when we detect bio-chemicals with high sensitivity. These include electrostatic noise or baseline noise caused by pumping induced pressure fluctuations or the capacitive current observed when another solution is added to stimulate cultured cells. The magnitude of the noises observed at the two electrodes and the shapes of their curves should be similar if the electrode size, surface condition and flow rates at the two electrodes are the same. Since the surfaces of both working electrodes were well defined by photolithography and also pre-treated with oxygen plasma before being modified with Os-gel-HRP, we could use our sensor to observe solely the current caused by the enzymatic reaction of histamine by subtracting the signal at the electrode without HAOx from that at the HAOx modified electrode.
Fig.2(c) shows a trace we obtained when we deducted the current shown in (b) from that shown in (a). It clearly reveals that the electrostatic noise and pumping noise were greatly reduced making it possible to observe just the histamine concentration change. We calculated a detection limit of 0.19 μM (S/N = 3) from trace (a). However, we obtained a lower detection limit of 25 nM (S/N = 3) from trace (c) because of the noise reduction realized by using the responses at the dual electrodes. In the past we reported that the detection limit of our single working electrode based microfabricated histamine sensor was 67 nM.21 This detection limit is lower than that of our new device if we calculate the limit from curve (a), but much lower if we use the differential response (curve c). The higher detection limit obtained from curve (a) is due to its low conversion efficiency since the working electrode is small. In addition, the enzyme layer (3 mm long) is immobilized in our previously reported sensor, and this increases the signal. However, the detection limit of our new device is about 3 times lower than that of our previous sensor in spite of the higher enzyme loading in its channel. Therefore, this shows that differential measurement is effective in achieving not only high selectivity but also a low detection limit with a smaller sample volume.
The disadvantage of using the microfluidic device for cell measurement rather than the enzyme modified microelectrode is its temporal resolution. The mixing of the solution in the microchannel slows the response. However, this situation can be improved by miniaturizing the flow channel since the Reynolds number decreases in the smaller channel and suppresses the mixing of the solution. The response time is fast and the temporal resolution is good with a bare microelectrode. Short time events such as the quantum release of catecholamine have been observed using a carbon fiber electrode.28 However, the electrode response was much slower when we modified the microelectrode with enzyme and mediator. We compared the response for histamine and electroactive species (ferrocene derivatives: aq-ferr) to determine whether it was the inner volume of our device or the modified electrode that worsened the temporal resolution.
Fig. 3 shows the normalized response curves of 1 μM aq-ferr with a bare electrode (trace a) and 1 μM histamine with an HAOx modified electrode (trace b). We held the working electrode potentials at −50 mV for histamine and 700 mV for aq-ferr, respectively. It required 10 s for the anodic current to start to increase after we injected aq-ferr into the device. This delay time is not a serious problem if the intermixing in the channel is suppressed. By contrast, if we compare the currents from the point when the current starts to the point where it reaches a steady state, the histamine response is much slower than that of aq-ferr. The current generated by histamine reaches a steady state in 52 s, whereas it takes only 26 s for aq-ferr. These response times are due to the rate at which the concentration gradient forms on the electrode. Therefore, these results suggest that the temporal resolution is mainly due to the formation of the concentration gradient in the enzyme film and the enzymatic reaction of HAOx.
 | ||
| Fig. 3 The normalized response curves of 1 μM aq-ferr with a bare electrode (trace a) and 1 μM histamine with an HAOx modified electrode (trace b). | ||
Device performance
It is very important to reduce the sampling volume when measuring chemicals released from cells. This is because large volume sampling reduces the analyte concentration due to dilution. Small volume sampling is also effective in improving spatial resolution. In contrast, the electrode sensitivity decreases as the flow rate decreases since the diffusion layer is thicker at a lower flow rate, which reduces the analyte diffusion to the electrode surface.Fig. 4 shows the flow rate dependence of the limiting currents for 5 μM of histamine with our device. The potential of the working electrode was −50 mV versus Ag, and we changed the flow rate in the 0.1 to 4 μl min−1 range. The sensitivity of the device was unchanged at flow rates of 1 to 4 μl min−1. However the sensitivity decreased rapidly when the flow rate was less than 1 μl min−1. The histamine diffusion to the modified electrode decreased with decreasing flow rate. However, the reaction efficiency of the HAOx increased with decreasing flow rate, thus compensating for the decrease in the analyte diffusion to the electrode. As described above, a low flow rate is better for our application. We measured the histamine calibration curve at a flow rate of 1 μl min−1. Fig. 5 shows the results. The cathodic current was proportional to the histamine concentration over a wide range of 0.5 to 500 μM. The detection limit (25 nM) is comparable to that (23 nM) when we used a 6 mm diameter glassy carbon electrode modified with Os-gel-HRP and HAOx in the thin layer radial flow cell. However, this result shows that we achieved the same performance with a much smaller device.
 | ||
| Fig. 4 The variation in the current as a function of the flow rate with 5 μM of histamine | ||
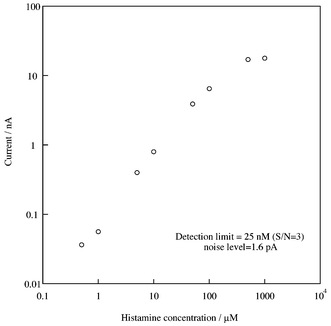 | ||
| Fig. 5 The calibration curve for histamine at a flow rate of 1 μl min−1. | ||
We measured 5 μM of histamine for 8 h to determine the time dependence of the histamine signal. The device showed excellent stability in spite of the smaller amount of enzyme loaded on the electrode and the injection of a relatively high concentration histamine solution. The relative standard deviation was 2.9%. The signal reduction was also small when we measured the device after keeping it in a refrigerator overnight.
Measurement of histamine release
Since the device provided a low detection limit with a small volume sample and excellent stability, we used it to measure histamine released from RBL-2H3 cells. Fig. 6 shows the response curves we obtained when we stimulated the cells with BSA-DNP (20 ng ml−1) after the cells had been incubated with IgE (0.5 μg ml−1). We observed no clear current increase at the electrode modified with HAOx and Os-gel-HRP (Fig. 6(a)). This is due to the large periodic pumping noise. We also observed a similar noise at the other electrode, which was modified with Os-gel-HRP without HAOx (Fig. 6(b)). However, the differential curve (Fig. 6(c)) shows a much clearer current increase with a magnitude of about 10 pA. The periodic noise was almost canceled out by deducting curve (b) from curve (a). This result shows that differential measurement using our microfluidic device is effective for measuring a low electrochemical signal for real sample measurement. | ||
| Fig. 6 The variation in the histamine concentration from RBL-2H3 cells caused by antigen stimulation. We introduced 20 ng ml−1 of DNP-BSA into the cells, which were incubated with 0.5 μg ml−1 of IgE for 24 h. Traces (a) and (b) show the current at modified dual electrodes with and without HAOx, respectively. Trace (c) shows the current obtained by deducting the current shown in (b) from that shown in (a). | ||
It has been reported that the amount of histamine contained in an RBL-2H3 cell is 2000 pmoles/106 cells (1.2 × 109 molecules cell−1) and 30 to 50% of this histamine was released by antigen stimulation. Using our device, the number of histamine molecules oxidized per second was calculated as follows: 10 × 10−15 (A)/(1.6 × 10−19 (C) × 2 ) = 3.13 × 107 (s−1)
The conversion efficiency at a flow rate of 1 μl min−1 is as follows.
The current at a flow rate of 1 μl min−1 for 5 μM of histamine is 203.9 pA in Fig. 4. However, we estimated the current of 5 μM of histamine at a flow rate of 1 μl min−1 as follows when the conversion efficiency was 100%: 5 × 10−6 (mol l−1) × 2 × 96500 (C mol−1) × 10−6 (l min−1)/2 = 8 × 10−9 (A)
The conversion efficiency is 100 × 203.9 × 10−12 /8 × 10−9 = 2.5 (%)
When we take account of the conversion efficiency at the flow rate (2.5%), the amount of histamine sampled into the device per second is 1.25 × 109 molecules. From this data, we can measure the release from two to three cells per second with our device.
In conclusion, we developed a highly reliable microfluidic device with a dual carbon film electrode modified with HAOx film and without HAOx film. Using a differential measurement mode and by deducting the current at the unmodified electrode from that at the modified electrode, we were able to cancel out the baseline fluctuation and pumping noise. As a result, we were able to measure the histamine released from RBL-2H3 cells continuously with high selectivity.
Acknowledgements
The authors thank Drs Saburo Imamura and Hisao Tabei for encouraging this project. The authors also thank Dr Shoko Iwaki for fruitful discussions and Ms Wakako Tanaka for drawing the photomasks of the device.References
- M. A. Beaven, Monogr. Allergy, 1978, 13, 1 Search PubMed.
- T. Watanabe, Y. Taguchi, S. Shiosaka, J. Tanaka, H. Kubota, Y. Terano, M. Tohyama and H. Wada, Brain Res., 1984, 295, 13 CrossRef CAS.
- K. Maeyama, M. Sasaki and T. Watanabe, Anal. Biochem., 1991, 194, 316 CrossRef CAS.
- Md. K. Alam, M. Sakai, T. Watanabe and K. Maeyama, Anal. Biochem., 1995, 229, 26 CrossRef CAS.
- M. Ohashi, F. Nomura, M. Suzuki, M. Otsuka, O. Adachi and N. Arakawa, J. Food Sci., 1994, 59, 519 CAS.
- I. Karube, I. Satoh, Y. Araki, S. Suzuki and H. Yamada, Enzyme Microb. Technol., 1992, 2, 117 CrossRef.
- S. Allenmark, S. Bergstorm and L. Enerback, Anal. Biochem., 1985, 144, 98 CrossRef CAS.
- G. Achilli, C. P. Cellerino and G. M. d’Eril, J. Chromatogr., 1994, 661, 201 CrossRef CAS.
- L. G. Harsing, H. Nagashima, D. Duncalf, E. S. Vizi and P. L. Goldiner, Clin. Chem., 1986, 32, 1823 CAS.
- W. F. Staruszkiewicz, J. AOAC, 1977, 60, 1131 CAS.
- W. F. Staruszkiewicz, E. M. Waldron and J. F. Bank, J. AOAC, 1977, 60, 1125 CAS.
- A. R. Wheeler, K. Morishima, D. W. Arnold, A. B. Rossi and R. N. Zare, Proceeding of μ-TAS 2000, 2000, 25 Search PubMed.
- K. Pihel, S. Hsieh, J. W. Jorgenson and R. M. Wightman, Anal. Chem., 1995, 67, 4514 CrossRef CAS.
- K. Pihel, S. Hsieh, J. W. Jorgenson and R. M. Wightman, Biochemistry, 1998, 37, 1046 CrossRef CAS.
- G. C. Chemnitius, M. Suzuki, K. Isobe, J. Kimura, I. Karube and R. D. Schmid, Anal. Chim. Acta, 1992, 263, 93 CrossRef CAS.
- S. Tombelli and M. Mascini, Anal. Chim. Acta, 1998, 358, 277 CrossRef CAS.
- M. G. Loughan, J. M. Hall, A. P. F. Turner and V. L. Davidson, Biosens. Bioelectron., 1995, 10, 569 CrossRef.
- R. Draisci, G. Volpe, L. Lucentini, A. Cecilia, R. Federico and G. Palleschi, Food Chem., 1998, 62, 225 CrossRef CAS.
- K. B. Male, P. Bouvrette, J. H. T. Luong and B. F. Gibbs, J. Food Sci., 1996, 61, 1012 CAS.
- M. Niculescu, C. Nistor, I. Frebort, P. Pec, B. Mattiasson and E. Csoregi, Anal. Chem., 2000, 72, 1591 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper describes a biogenic amine sensor consisting of Os-poly(1-vinylimidazole) containing HRP and amine oxidase. The sensor was used for monitoring fish freshness.
This paper describes a biogenic amine sensor consisting of Os-poly(1-vinylimidazole) containing HRP and amine oxidase. The sensor was used for monitoring fish freshness. - O. Niwa, R. Kurita, K. Hayashi, T. Horiuchi, K. Torimitsu, K. Maeyama and K. Tanizawa, Sens. Actuators
B, 2000, 67, 43 Search PubMed .
![[*]](https://www.rsc.org/images/entities/char_e103.gif)
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper was the first to report on the continuous monitoring of the histamine released from RBL-2H3 cells using histamine oxidase.
This paper was the first to report on the continuous monitoring of the histamine released from RBL-2H3 cells using histamine oxidase. - M. Vreeke, R. Maiden and A. Heller, Anal. Chem., 1992, 64, 3084 CrossRef CAS.
- Y-H. Choi, R. Matsuzaki, T. Fukui, E. Shimizu, T. Yorifuji, H. Sato, Y. Ozaki and K. Tanizawa, J. Biol. Chem., 1995, 270, 4712 CrossRef CAS.
- H. Shimizu, H. Ichise and T. Yorifuji, Agric. Biol. Chem., 1990, 54, 851.
- E. Shimizu, T. Odawara, K. Tanizawa and T. Yorifiji, Biosci.,. Biotechnol. Biochem., 1994, 58, 2118 CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper details the recombinant histamine oxidase used in the present work.
This paper details the recombinant histamine oxidase used in the present work. - O. Niwa, R. Kurita, T. Horiuchi and K. Torimitsu, Electroanalysis, 1999, 11, 356 CrossRef CAS.
- R. Kurita, H. Tabei, K. Hayashi. T. Horiuchi, K. Torimitsu and O. Niwa, Anal. Chim. Acta, 2001, 441, 165 CrossRef CAS.
- R. M. Wightman, L. J. May and A. C. Michael, Anal. Chem., 1988, 60, 769A CAS.
| This journal is © The Royal Society of Chemistry 2002 |
