A Hantzsch synthesis of 2-aminothiazoles performed in a heated microreactor system
Eduardo
Garcia-Egido
*,
Stephanie Y. F.
Wong
and
Brian H.
Warrington
GlaxoSmithKline Pharmaceuticals, New Frontiers Science Park (North), Harlow, Essex, UK CM19 5AW. E-mail: Eduardo_2_Garcia-Egido@gsk.com; Fax: +44 (0)1279 622500; Tel: +44 (0)1279 627993
First published on 18th January 2002
Abstract
This paper presents the first example known to the authors of a heated organic reaction performed on a glass microreactor under electro-osmotic flow control. The experiments consisted of the preparation of a series of 2-aminothiazoles by means of a Hantzsch reaction of ring-substituted 2-bromoacetophenones and 1-substituted-2-thioureas carried out in microchannels, with the aim of investigating the generic utility of the reactor in carrying out analogue reactions. The reactions were performed on T-design microchips etched into a thin borosilicate glass plate and sealed over with a thick borosilicate top plate containing reservoirs. The mobility of the reagents and products was achieved using electro-osmotic flow (EOF), with the driving voltages being generated by a computer-controlled power supply. During the experiments the T-shaped chip was heated at 70 °C using a Peltier heater, aligned with the channels and the heat generated by this device was applied to the lower plate. The degree of conversion was quantified by LC-MS using UV detection by comparison with standard calibration curves for starting materials and final products. In all cases, conversions were found to be similar or greater than those found for equivalent macro scale batch syntheses, thus illustrating the potential of this heated microreactor system to generate a series of compounds which contain biologically active molecules.
Introduction
The drug industry is expected to develop safe drugs that address unmet medical needs, modify disease or otherwise show substantial socio-economic benefit. Ultimately this requires the chemist to seek the unequivocally best lead as a starting point for optimisation. Automated chemistry has allowed diversity campaigns based on the production of a very large number of small arrays of single compounds to be realised. However, the fact remains that the numbers produced provide only a minuscule level of sampling of diversity space. Thus, although leads are found, it is highly unlikely that any of them is the best lead. The chances of finding the best lead increases as the number of truly diverse compounds synthesised increases, but if reactions are carried out on a macro scale the point is rapidly reached where the logistic difficulties in the supply and storage of proprietary reagents become impossible.Microchannel flow reactors potentially provide a basis for carrying out ultra-high throughput chemical synthesis on a greatly reduced scale that is compatible with highly miniaturised modern screening techniques. Their use could bring the reductions in process cycle times, reagent costs and storage overheads, necessary to increase laboratory throughput to many thousands of compounds a day. Recently published descriptions of microchannel based syntheses of azacompounds,1 Wittig products,2 Suzuki3 and Ugi reactions,4 and peptide synthesis5 demonstrating these time and scale economies are therefore propitious.
All these published microchannel based syntheses were carried out at room temperature. We considered conducting reactions at higher temperature in order to increase the scope of microchannel based technologies. The reaction considered was a Hantzsch′s thiazole synthesis,6 particularly focused to the synthesis of 2-aminothiazoles (Scheme 1).
 | ||
| Scheme 1 General reaction scheme for Hantzsch’s 2-aminothiazole synthesis. | ||
Experimental
Reactions were performed in generic microreactors of a type previously described by Daykin and Haswell7 in which a borosilicate bottom plate is etched with a T-design channel (Fig. 1, 300 μm wide and 115 μm deep) and then sealed over with a borosilicate top plate using a thermal bonding technique. A computer-controlled power supply was used to mobilise reagents and products using electro-osmotic flow (EOF). | ||
| Fig. 1 Construction details of the T-shaped glass microreactor. | ||
Voltages applied throughout the experiment were of the same value on reservoirs A and B and ground connected to reservoir C. During the 30 min period the T-shaped chip was heated at 70 °C using an in-house designed T-shaped Peltier heater, aligned with the channels and the heat generated by this device was applied to the lower plate (Fig. 2).
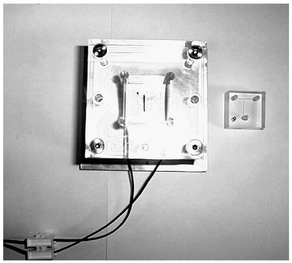 | ||
| Fig. 2 T-shaped Peltier heater and T-shaped chip. | ||
The synthesis of 2-aminothiazoles in the microreactor system was carried out from 14 mM solutions of ring-substituted 2-bromoacetophenones (Reservoir A, 100 mol%) and 21 mM solution of 1-substituted-2-thioureas (Reservoir B, 150 mol%). After a 30 min period, reaction products were identified and conversions quantified on reservoir C by LC-MS using a UV detector. Previously, calibration curves were prepared from starting materials and synthesised pure samples of final products.
Reactions in macro scale were carried out using the same experimental conditions as in the microreactors. A generic procedure is as follows. A 14 mM solution in 1-methyl-2-pyrrolidinone (NMP, 2 mL) of the ring-sustituted 2-bromoacetophenone is added to a 21 mM solution in NMP (2 mL) of the 1-sustituted-2-thiourea heated at 70 °C. The resulting solution was stirred and heated at 70 °C for 30 min. After that a sample of the crude product was analysed by LC-MS as previously described for microreactors experiments.
Results and discussion
The optimisation process in the microreactor system was based on the initial reaction of 2-bromo-4′-methylacetophenone and 1-acetyl-2-thiourea (Scheme 2). The selection of the solvent in the microreactor, NMP instead of dimethylformamide (DMF), was based on its higher stability under EOF conditions.8 | ||
| Scheme 2 Optimisation process of Hantzsch’s 2-aminothiazole synthesis was based on this reaction. | ||
A study was made between 100 V and 700 V applying the procedure previously described. Average conversions for each voltage were compared with the conversion obtained from a batch macro scale synthesis carried out under the same conditions . Better results were obtained with voltages between 300 V and 700 V (Table 1). However several by-products were also observed with voltages above 500 V.
| a Better conversions than the macro scale batch synthesis were obtained with voltages between 300 V and 700 V. |
|---|

|
A series of six 2-aminothiazoles in total were synthesised using the procedure previously described for the model compound (Scheme 3), (Table 2). The reaction gave quantitative conversions at 500 V for ring deactivating 2-bromoacetophenones (Table 2, entry 4 and 5). The results obtained in the microreactor were also particularly good at 400 V. These values were compared with the macro batch reactions carried out using the same experimental conditions. Comparable or higher conversions were obtained in the microreactor system.
 | ||
| Scheme 3 General reaction scheme for a series of 2-aminothiazoles synthesised. | ||
| Entry no. | R1 | R2 | R3 | Microreactor conversion (300 V) | Microreactor conversion (400 V) | Microreactor conversion (500 V) | Batch conversion |
|---|---|---|---|---|---|---|---|
| 1 | Acetyl | –H | –H | 42% | 63% | 14% | 44% |
| 2 | Acetyl | –H | –OMe | 53% | 58% | 14% | 53% |
| 3 | Acetyl | –H | –Me | 74% | 77% | 72% | 59% |
| 4 | Acetyl | –Br | –H | 91% | 95% | 99% | 83% |
| 5 | Acetyl | –NO2 | –H | 99% | 99% | 99% | 96% |
| 6 | Phenylethyl | –H | –H | 99% | 99% | 99% | 99% |
The product of 2-bromoacetophenone and 1-phenylethyl-2-thiourea gave the best results of the series with total conversion at all voltages (Table 2, entry 6). This compound, fanetizole, (Fig. 3), is a known pharmacological agent with activity for the treatment of rheumatoid arthritis9 and thus illustrates the potential of this heated microreactor system to generate a series which contains biologically active molecules. The syntheses performed at 400 V usually gave clean products. For example, starting with 2-bromoacetophenone and 1-phenylethyl-2-thiourea (Table 2, entry 6), after 30 min of experiment on microreactor, only the corresponding product (Fanetizole, Fig. 3) was detected in reservoir C (Fig. 4).
 | ||
| Fig. 3 Fanetizole. | ||
 | ||
| Fig. 4 Chromatogram obtained of a sample taken from resevoir C after 30 min of reaction. Fanetizole is the only component detected. | ||
Conclusions
This work demonstrates that heated reactions in glass microreactors are possible under EOF driven flow. The results obtained in these experiments indicate that microreactor based technologies are suitable for the synthesis of a series of 2-aminothiazoles and indicate that this could also be suitable to support the generation of other combinatorial libraries. Synthesis of fanetizole in a microchannel also illustrates the potential of the heated microreactor system to generate a series which contains biologically active molecules. Future objectives are to develop a broad range of chemistries on microreactors using pressure driven flow (as well as EOF) and incorporate the use of polymer supported reagents.Acknowledgements
The authors thank Prof. S. Haswell for some of the software and hardware used. E. G. E. thanks the EU for a postdoctoral Marie Curie fellowship (HMPI-CT-1999-00066).References
- H. Salimi-Moosavi, T. Tang and D. J. Harrison, J. Am. Chem. Soc., 1997, 119, 8716 CrossRef CAS.
- V. Skelton, G. M. Greenway, S. J. Haswell, P. Styring, D. O. Morgan, B. H. Warrington and S. Y. F. Wong, Analyst, 2001, 126, 11 RSC.
- (a) P. Fletcher and S. Haswell, Chem. Br., 1999, 38 Search PubMed; (b) G. M. Greenway, S. Haswell, D. O. Morgan, V. Skelton and P. Styring, Sens. Actuators B, 2000, 63, 153 CrossRef.
- M. C. Mitchell, V. Spikmans and A. J. De Mello, Analyst, 2001, 126, 24 RSC.
- P. Watts, C. Wiles, S. J. Haswell, E. Pombo-Villar and P. Styring, Chem. Commun., 2001, 990 RSC.
- (a) A. R. Hantzsch and J. H. Weber, Ber., 1887, 20, 3118 Search PubMed; (b) J. V. Metzger, Comprehensive Heterocyclic Chemistry, Pergamon Press Ltd., Oxford, 1st edn., 1984, vol. 6, p. 235 Search PubMed.
- R. N. C. Daykin and S. J. Haswell, Anal. Chim. Acta, 1995, 313, 155 CrossRef CAS.
- D. D. Perrin and W. L. F. Armarego, Purification of Laboratory Chemicals, Pergamon Press Ltd., Oxford, 3rd edn., 1988 Search PubMed.
- N. Bailey, A. W. Dean, D. B. Judd, D. Middlemiss, R. Storer and S. P. Watson, Bioorg. Med. Chem. Lett., 1996, 6, 1409 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
