Laser trapping and Raman spectroscopy of single cellular organelles in the nanometer range†
Katsuhio
Ajito
* and
Keiichi
Torimitsu
NTT Basic Research Laboratories, NTT Corporation, 3-1, Morinosato-Wakamiya, Atsugi, Kanagawa, 243-0198, Japan. E-mail: ajito@will.brl.ntt.co.jp
First published on 24th January 2002
Abstract
The laser trapping technique combined with near-infrared Raman (NIR) spectroscopy was used for the analysis of single cellular organelles in the nanometer range. The samples were synaptosomes, nerve-ending particles (about 500–700 nm in diameter) isolated from a neuron in a rat brain, dispersed in the phosphate buffer solution. The NIR laser Raman trapping (NIR-LRT) system trapped a single synaptosome without photochemical damage and provided a Raman spectrum of the sample with less fluorescence background. After the background subtraction from the Raman spectrum, two large peaks appeared, which are attributed to the peaks of the CH2 deformation mode and the amide I mode. This indicates the laser-trapped synaptosomes include some types of lipids and proteins. The result demonstrates that the NIR-LRT system can determine biological molecules in single cellular organelles in the nanometer range. Further improvement of the detection sensitivity will enable us to get detailed information about the functions of single cellular organelles in the brain, which will be valuable for neuroscience.
Introduction
The laser trapping technique (also known as the optical tweezers) has been used to capture and manipulate single organic and biological particles in a micrometer range, such as latex beads, aerosol particles, microdroplets, microcapsules, and biological samples (bacteria, blood cells, etc.) in solution.1–5 The laser trapping technique is based on optical radiation pressure generated by the laser beam and was first reported by Ashkin in 1970.6 Then, in 1986, a more practical method using an optical microscope was introduced by Chu and co-workers.7 In the method, a microparticle is grabbed by the force of the radiation pressure gradient generated from the tightly focused laser beam under the objective lens. Initially, a visible laser beam was used for laser trapping. Nowadays though, a low energy near-infrared laser (NIR) light ranging from 700 to 1100 nm wavelength is widely used because there is less likelihood of sample damage due to photochemical reactions than with visible laser light. When an NIR laser beam is focused on a small particle using an objective lens, the particle is trapped by the force of the radiation pressure gradient without photochemical damage.Laser trapping in analytical chemistry is very important for determining the species and structure of molecules contained in a single microparticle. Chemical reactions within single microdroplets or microcapsules have been studied by using laser trapping combined with electrochemistry,8 absorption spectroscopy,9 fluorescence spectroscopy,8,9 and Raman spectroscopy.10–12 Raman spectroscopy is advantageous as an analytical tool for trapped particles because it provides information about their structure and conformation of various kinds of molecules in the particle. Another advantage in terms of instrumentation for Raman measurements is that the focused laser beam can be used for both laser trapping and Raman spectroscopy. In addition, morphology-dependent resonances have been used to elastically enhance Raman scattering light from microparticles whenever their size and refractive index lead to interference of the light in them.11,12 However, these systems combine Raman spectroscopy with laser trapping by visible laser light10–13 which makes them unsuitable for biological samples because of photochemical damage and high fluorescence background in Raman spectra. We have already built a new type of microscope system called the NIR laser Raman trapping (NIR-LRT) system, which combines laser trapping with Raman microscopy using NIR laser light,14 and confirmed the sensitivity of the system by analyses of single microdroplets15–17 and single polymer nanoparticles.18
This paper describes the first application of the NIR-LRT system to the analysis of single cellular organelles in the nanometer range. The system enables us to trap single cellular organelles without photochemical damage and provides their Raman spectra with less fluorescence background. The two Raman peaks of the CH2 deformation mode and the amide I mode clearly appear in the spectra.
Experimental
The experimental set-up of the NIR-LRT system has been described in previous papers.16–18 Briefly, a Raman microprobe spectrometer (Ramascope, Renishaw) containing two holographic notch filters (HNFs) and a single grating polychromator was specially modified for NIR laser light. The excitation light source is a continuous-wave, single-frequency Ti:sapphire ring laser (Titan-CW, Schwartz Electro-Optics) tuned to 730 nm in the TEM00 mode. The pump source for the Ti:sapphire ring laser is the 532 nm line (pumping power: 5 W) of a solid-state visible continuous-wave laser (Millenia, Spectra-Physics Lasers). The laser beam (power: about 100 mW) passes through the optical microscope (BH-2, Olympus) and is focused onto the sample by the oil-immersed objective lens. The oil-immersed objective lens has a numerical aperture of 1.3. The objective lens is also used to collect light scattered from the sample at 180° with respect to the incident light. A charge coupled device (CCD) camera (02-06-1-225, Wright Instruments) containing a Peltier-cooled (200 K) CCD chip is used for Raman measurement. An additional CCD camera and a HNF were fitted with the optical microscope to obtain optical images. The two-slit confocal arrangement is used for Raman measurement, which reduces the background Raman scattering from the region unfocused by the laser beam.The cellular organelle samples used in the Raman measurement were nerve-ending particles isolated from neuron in rat brain. They are called synaptosomes and remain active several hours after isolation. The cerebral cortex was removed from a 7 or 8 day-old Wister rat. Then homogenization and centrifugal separation processes were carried out using the Percoll gradients method.19,20 Briefly, the cerebral cortex in the sucrose aqueous solution (0.32 M) was mixed using a homogenizer (Hom, Iuchi). Then, a synaptosome layer was isolated in sucrose aqueous solution by using an ultracentrifuge (Optima TLX, Beckman) and then purified in Percoll (Amersham Pharmacia Biotech) gradient solution (23, 10, 3 vol%). Finally, the synaptosomes were dispersed in phosphate-buffered solution (D-PBS, Gibco) for the Raman measurement.
Images of the dried synaptosomes were taken with a scanning electron microscope (SEM, JSM-890, Jeol) at a voltage of 3 kV. The glutaldehyde solution (5 wt.%) was added to the phosphate-buffered solution containing the synaptosomes. Then, the sysnaptosomes were transferred form the phosphate-buffered solution to the ethanol aqueous solutions (from 10 to 100 wt.%) and dried on a glass coverslip.
The optical arrangement of the sample cell is shown in Fig. 1. The phosphate-buffered solution containing synaptosomes was fixed in the glass cell, and then the glass cell was covered with a 0.17 mm thick fluorescence-free glass coverslip. The NIR laser beam was focused on the sample solution in the glass cell through the immersion oil and the glass coverslip for laser trapping and Raman measurements.
 | ||
| Fig. 1 Schematic of the optical arrangement for laser trapping of the synaptosomes. | ||
Results and discussion
The SEM images of the synaptosomes are shown in Fig. 2. Each synaptosome is spherical and about 500 to 700 nm in diameter as shown in Fig. 2A, which is in good agreement with the literature.20Fig. 2B shows the synaptosome image at higher magnification. The surface of the synaptosome in Fig. 2B is rough, which indicates that it contains many small synaptic vesicles. These small vesicles probably have high refractive indexes and make it possible to trap a synaptosome by the radiation pressure generated from the laser light under the objective lens. | ||
Fig. 2
Images of synaptosomes obtained using the scanning electron microscope with (A) a magnification of ×40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, (B) a magnification of ×100 000, (B) a magnification of ×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000.
| ||
The images of light reflected from single and aggregated synaptosomes during laser light irradiation are shown in Fig. 3. The concentration of the sample solution in image A to E in Fig. 3 are 103, 102, 10, 1, and 0.1, respectively. Image A is fuzzy because many synaptosomes are aggregated in the focal spot of the laser beam. The aggregation reduced with decreasing sample solution concentration, as shown in images B and C. Furthermore, a circular pattern appeared around the bright spot, as shown in images D and E, when the concentration is low. The fuzzy images, images A to C, were obtained just after laser irradiation. However, the circular pattern in image D or image E was obtained about 10–30 min after laser irradiation. We have already reported that single nanometer-scale polystyrene latex beads showed such a circular pattern during laser-trapping, which was made by the interference between the light reflected from the top and the bottom of the laser-trapped nanoparticle.18 Therefore, it can be concluded that the circular patterns in image D (1 μg ml−1) and E (0.1 μg ml−1) were generated from single laser-trapped synaptosomes.
 | ||
| Fig. 3 Images of light reflected from aggregated and single synaptosomes during laser-trapping in solution. The sample concentrations are (A) 103, (B) 102, (C) 10, (D) 1, and (E) 0.1, respectively. | ||
Fig. 4 shows the Raman spectra of aggregated (A, B, and C) and single (D and E) synaptosomes, which correspond to images A–E in Fig. 3. The exposure time of each spectrum was 100 s. The background spectrum of phosphate-buffered solution was subtracted from each spectrum. The two large peaks, which are very often seen in Raman spectra of brain tissues, are clearly observed.21,22 The peaks at 1445 cm−1 are assigned to the CH2 deformation mode and the peaks at 1657 cm−1 are assigned to the amide I mode.23 The peak of the CH2 deformation mode indicates that the trapped synaptosome contains lipids, which consist of cell membrane. The amide I mode is well known as one that appears for many types of proteins. Therefore, the peak at 1657 cm−1 in the spectrum indicates that the trapped synaptosome contains some types of proteins. In addition, the CH2 bending mode of some proteins sometimes shows Raman peaks near the CH2 deformation mode of lipids. If the peak of the CH2 bending mode appears, this peak probably mixed with the peak of the the CH2 deformation mode at 1445 cm−1. Fig. 5 shows the sample concentration dependence of the Raman peak intensities of the CH2 deformation mode at 1445 cm−1 and the amide I mode at 1657 cm−1. The Raman intensity decreased as sample concentration decreased below 1 μg ml−1 then became constant. This is consistent with the discussion about the images in Fig. 3; some synaptosomes are aggregated in the focal spot of the laser beam at the concentration larger than 1 μg ml−1 and a single synaptosome was trapped at the concentration of 1 μg ml−1 or below. The result indicates that the system can trap single cellular organelles (synaptosomes) in the nanometer range without photochemical damage and shows the existence of lipids and proteins in them very clearly. We have demonstrated the performance of the NIR-LRT system for the analysis for single cell organelles in the nanometer range. Further improvement of the system sensitivity will enable us to differentiate membrane proteins, clarify interactions between proteins and membranes, and better understand the role of neurotransmitters in the brain. This will be valuable for neuroscience.
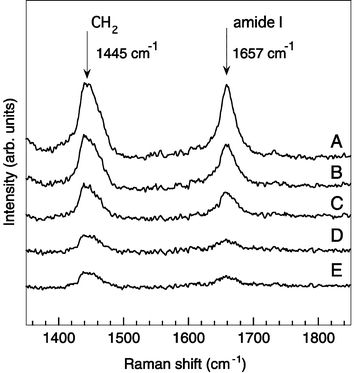 | ||
| Fig. 4 Raman spectra of aggregated and single synaptosome correspond to the images in Fig. 3. | ||
 | ||
| Fig. 5 Dependence of the Raman peak intensities of the amide I mode and the CH2 deformation mode on the synaptosome concentration in solution. | ||
Conclusions
The near-infrared (NIR) Raman spectroscopy of single synaptosomes, cellular organelles in neurons, was demonstrated for the first time using the laser trapping technique. The NIR laser Raman trapping (NIR-LRT) system, which combines laser trapping with NIR Raman microscopy, was able to trap single synaptosomes without photochemical damage and provide their Raman spectrum with less fluorescence background. Two large peaks appeared, which correspond to the peaks of the CH2 deformation mode and the amide I mode. The peaks in the Raman spectrum of single synaptosome of the CH2 deformation mode and the amide I mode in the Raman spectra indicates the synaptosomes contain some type of lipids and proteins, respectively. The result indicates that the system is very useful for determining molecular species in single cellular organelles in a nanometer range. Very recently the laser trapping technique has been applied to micro total analysis systems (μTAS).24 We believe that further improvement of our technique will enable us to get detailed information about the functions of single cellular organelles in the brain and to use Raman microscopy in a microchip system for biological analyses.Acknowledgements
The authors thank Ms Yuriko Furukawa (The Core Research for Evolutional Science and Technology Program, Japan Science and Technology Corporation) for the assistance in the synaptosome sample preparation and Mr Yoshio Ohki (NTT Advanced Technology Corporation) for support in the use of the scanning electron microscope, Dr Nohoko Kasai for the helpful discussions, Dr Hideaki Takayanagi and Dr Masao Morita (NTT Basic Research laboratories) for their encouragement, and Mr David Steenken (Kurdyla and Associates) for English consultation.References
- K. Svoboda and S. M. Block, Annu. Rev. Biophys. Biomol. Struct., 1994, 23, 247 CrossRef CAS.
- M. W. Berns, Sci. Am., 1998, 278(4), 62 Search PubMed.
- Laser Tweezers in Cell Biology; Methods in Cell Biology, ed. M. P. Sheetz, Academic Press, California, 1998, vol. 55 Search PubMed.
- H.-B. Kim, S. Yoshida, A. Miura and N. Kitamura, Anal. Chem., 1998, 70, 111 CrossRef CAS.
- P. Zemánek, A. Jonás, L. Srámek and M. Liska, Opt. Lett., 1999, 24, 1.
- A. Ashkin, Phys. Rev. Lett., 1970, 24, 156 CrossRef CAS.
- A. Ashkin, J. M. Dziedzic, J. E. Bjorkholm and S. Chu, Opt. Lett., 1986, 11, 288 CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) The paper shows the conventional laser trapping technique using a single laser beam and an objective lens.
The paper shows the conventional laser trapping technique using a single laser beam and an objective lens. - K. Nakatani, T. Uchida, H. Misawa, N. Kitamura and H. Masuhara, J. Phys. Chem., 1993, 97, 5197 CrossRef CAS.
- N. Kitamura, H.-B. Kim, S. Habuchi and M. Chiba., Trends Anal. Chem., 1999, 18, 675 CrossRef CAS.
- C. Esen, T. Kaiser and G. Schweiger, Appl. Spectrosc., 1996, 50, 823 CAS.
- M. Trunk, J. Popp, M. Lankers and W. Kiefer, Chem. Phys. Lett., 1997, 264, 233 CrossRef CAS.
- J. Musick, J. Popp, M. Trunk and W. Kiefer, Appl. Spectrosc., 1998, 52, 692 CrossRef CAS.
- K. D. Crawford and K. D. Hughes, J. Phys. Chem. B, 1998, 102, 2325 CrossRef CAS.
- K. Ajito, Appl. Spectrosc., 1998, 52, 339 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) The paper is the first
report of the near-infrared Raman spectroscopy with laser trapping technique.
The paper is the first
report of the near-infrared Raman spectroscopy with laser trapping technique. - K. Ajito, in Recent Research Developments in Applied Spectroscopy, ed. S. G. Pandalai, Research Signpost, Trivandrum, 2000, vol. 3, pp. 135–143 Search PubMed.
- K. Ajito, M. Morita and K. Torimitsu, Anal. Chem., 2000, 72, 4721 CrossRef CAS.
- K. Ajito and K. Torimitsu, Trends
Anal. Chem., 2001, 20, 255 Search PubMed .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) The paper is the first report of the liquid–liquid extraction process in single picoliter droplets.
The paper is the first report of the liquid–liquid extraction process in single picoliter droplets. - K. Ajito and K. Torimitsu, Appl. Spectrosc., in press Search PubMed.
-
T. S. Shira, in Regulatory Protein Modification: Techniques and Protocols, ed. H. C. Hemmings, Jr., Humana Press, Totowa, NJ, 1997, pp. 67–119 Search PubMed.
![[*]](https://www.rsc.org/images/entities/char_e103.gif) Significant reference.
Significant reference. - P. R. Dunkley, P. E. Jarvie, J. W. Heath, G. J. Kidd and J. A. P. Rostas, Brain Res., 1986, 372, 115 CrossRef CAS.
- A. Mizuno, T. Hayashi, K. Tashibu, S. Maraishi, K. Kawauchi and and Y. Ozaki, Neurosci. Lett., 1992, 141, 47 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper describes the advantages of near-infrared Raman spectroscopy
for brain tissue analysis.
This paper describes the advantages of near-infrared Raman spectroscopy
for brain tissue analysis. -
Y. Guan, E. N. Lewis and I. W. Levin, in Analytical Applications of Raman Spectroscopy, ed. M. J. Pelletier, Blackwell Science, Oxford, UK, 1999, ch. 7, pp. 277–327 Search PubMed.
![[*]](https://www.rsc.org/images/entities/char_e103.gif) The chapter reviews the biological application of Raman spectroscopy and includes 125 references.
The chapter reviews the biological application of Raman spectroscopy and includes 125 references. - M. G. Shim and B. C. Wilson, Photochem. Photobiol., 1996, 63, 662 CAS.
- Micro Total Analysis Systems, ed. A. van den Berg, W. Olthuis and P. Bergveld, Kluwer Academic, Dordrecht, The Netherlands, 2000 Search PubMed.
Footnote |
| † Presented at the International Symposium on Microchemistry and Microsystems (ISMM 2001), Kawasaki, Japan, September 16–18, 2001. |
| This journal is © The Royal Society of Chemistry 2002 |
