Rapid enzymatic transglycosylation and oligosaccharide synthesis in a microchip reactor†
Ken-ichi
Kanno
*a,
Hideaki
Maeda
b,
Showta
Izumo
a,
Masakazu
Ikuno
a,
Kazuhito
Takeshita
a,
Asuka
Tashiro
a and
Masayuki
Fujii
ac
aDepartment of Bio-Environmental Chemistry, Kyushu School of Engineering, Kinki University, Kayanomori 11-6, Iizuka-shi, Fukuoka, 820-8555, Japan
bNational Institute of Industrial Science and Technology (AIST), Suku-machi 807-1, Tosu, Saga, 841-0052, Japan
cHenkel Kindai Laboratories (HKL), Center of Advanced Technology, Kayanomori 11-6, Iizuka-shi, Fukuoka, 820-8555, Japan
First published on 17th January 2002
Abstract
Glycosidase-promoted hydrolysis was performed in a microreaction channel. The result was compared with the reaction in a micro-test tube. Transgalactosylation on p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside was also examined in a microreaction channel, because transglycosylation is a useful method for oligosaccharide synthesis. We examined both the forward reaction, i.e., hydrolysis, and the reverse reaction, i.e., transglycosylation, in the microreaction channel. The results showed that both hydrolysis and transglycosylation were enhanced in the microreaction channel compared with the batch reaction.
Introduction
Glycoconjugates are one of the most important class of bio-molecules, because glycoconjugates are often essential for biological reactions, for instance, cell adhesion,1 virus infection,2 and cell migration.3 These bio-active glycoconjugates and their mimics have been synthesized chemically, enzymatically, or chemo-enzymatically to study their biological roles and the mechanisms. Some of this research has developed antidotes and strong inhibitors for virus or bacterial infections.4–6Carbohydrate-related enzymes, especially glycosyltransferase and glycosidase, have been cloned and some of them have been utilized for various oligosaccharide syntheses during the past couple of decades,7–9 and those investigations have made carbohydrate-related enzymes convenient tools for oligosaccharide syntheses. However, enzymatic methods present a number of problems, i.e., instability of enzyme and slow reaction rate depending on the type of enzyme, although the enzymatic method has many advantages, i.e., short reaction steps, a non-tedious reaction, and no pollution of heavy metals. It is important to investigate a new device for enzymatic oligosaccharide syntheses to resolve the problems remaining in enzymatic synthesis. Recently, enzymatic reactions in a microchip for analytical or synthetic chemistry have been reported.10–13 From those reports, it seems to be that the microchip might also be a feasible tool for the enzymatic synthesis of bio-active molecules. In this study, we focus on the microchip as a new device for enzymatic oligosaccharide synthesis.
Experimental
Materials
p-Nitrophenyl-β-D-galactopyranoside, p-nitrophenyl-2-acetamide-2-deoxy-β-D-gluco-pyranoside and β-galactosidase from E. Coli (E.C.3.2.1.23) were purchased from Wako. The microreaction channel was mechanically fabricated on PMMA using Robodrill equipped with a flat end mill (∅ 100 μm). The top plate assembly was achieved by baking at 100 °C under vacuum for 1 h. The geometry of the channel was 200 μm width, 200 μm depth, and 40 cm length. The microreactor equipment is shown in Fig. 1. The reaction temperature was fixed using a hot plate. The temperature around the microchip was regulated with the distance between the microchip and the hot plate. The reaction was quenched with boiling water. The reaction products from the output of the microchannel reactor were collected after the first 20 μl fraction. The samples were applied on a HPLC column after inactivation of the enzyme. The reaction mixture was applied on a LC-MS using an ODS-column by means of a Hewlett-Packard series 1100 connected with an LC-Q system. The ratios between p-nitrophenol and p-nitrophenyl-β-D-galactopyranose in the reaction mixtures were calculated from the absorption at 302 nm for p-nitrophenylglycosides and 405 nm for p-nitrophenol. | ||
| Fig. 1 The microchannel reactor system. | ||
Hydrolysis of p-nitrophenyl-β-D-galactopyranoside in a microreaction channel
0.32 mM p-nitrophenyl-β-D-galactopyranoside in phosphate buffer (pH 8) and β-galactosidase (20 U) in 10 ml of the same buffer were stored in their respective microsyringes. Both solutions were charged into the microreaction channel by pumping with an identical flow rate which was controlled using syringe pumps. The reaction was carried out at 37 °C using a hot plate. The reaction time (minutes), which is the residence times for the fluid elements passing through the microreactor channel, was estimated from the pumping speed of the syringe pump (μl min−1). The reaction mixture was dropped into a hot water bath at 80 °C to inactivate the enzyme.Hydrolysis of p-nitrophenyl-β-D-galactopyranoside in a commercial micro-test tube
Into the solution of 0.32 mM p-nitrophenyl-β-D-galactopyranoside in phosphate buffer (pH 8) was added the equivalent volume of β-galactosidase (20 U) in 10 ml of the same buffer, and the mixture stored at 37 °C. The reaction was terminated using a hot water bath at 80 °C to inactivate the enzyme.Hydrolysis of p-nitrophenyl-β-D-galactopyranoside in a pre-treated microreaction channel
A pre-treated chip was prepared by the following three steps. First, 300 μl of β-galactosidase in phosphate buffer, without substrate, was charged into the microreactor channel. The flow rates of the enzyme solution were the same as the hydrolysis reactions in the microreaction channel. Second, the microreaction channel was rinsed with phosphate buffer, and the enzymatic activity of this microreaction channel pre-treated with the enzyme was examined as the next step. Third, into this pre-treated microchip was charged p-nitrophenyl-β-D-galactopyranoside in phosphate buffer (pH 8), without the enzyme, at the same flow rates as those of the hydrolysis reactions.Transgalactosylation on p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside in a microreaction channel
The mixture of p-nitrophenyl-β-D-galactopyranoside (0.32 mM) and p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside (3.2 mM) in 50% phosphate buffer (pH 8)–acetonitrile solvent system and β-galactosidase (20 U) in 10 ml of the same solvent system was charged into the microreaction channel from respective microsyringes at identical flow rates. The reaction was carried out at 37 °C. The products were analyzed by using LC-MS: m/z calcd. C20H28KN2O13 [M+K]+: 543.5, found: 543.3.Transgalactosylation on p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside in a commercial micro-test tube
Into the mixture of p-nitrophenyl-β-D-galactopyranoside (0.32 mM) and p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside (3.2 mM) in 50% phosphate buffer (pH 8)–acetonitrile solvent system, was added 20 U β-galactosidase in 10 ml of the same buffer, and the mixture was stored at 37 °C. The reaction was quenched by a hot water bath at 80 °C.Results and discussion
We have chosen a microchannel reactor as the reaction channel, although a capillary is much cheaper than a microchip, to keep laminar flow at the input of the channel. The enzyme solution and the substrate solution are mixed at the input, and it may be difficult to mix both solutions together without disorder of the flow if we use a capillary. As written in the Experimental section, the first 20 μl of the fractions were not collected, because the early fraction from the output might be produced in a different fluidic environment affected by the air at the top of flow.In this study, we examined the enzymatic hydrolysis of p-nitrophenyl-β-D-galactopyranoside with β-galactosidase (E. Coli) in the microreaction channel (see Scheme 1). The composition of the reaction mixture at equilibrium is p-nitrophenol, p-nitrophenyl-β-D-galactopyranoside, and D-galactose. The time course of the reaction in the micro-test tube was compared with that in the microreaction channel. In Fig. 2, vertical line presents the ratio between p-nitrophenyl (PNP) and p-nitrophenyl-β-D-galactopyranoside (PNPGal), and the horizontal axis presents the reaction time. As shown in Fig. 2, the reaction in the microreaction channel was about 5 times faster than the batch reaction. As shown in Fig. 2, the time course of the hydrolysis in the mirochannel reactor seemed to reach equilibrium after 10 min. However, It can not be ensured that the curve reached equilibrium after 10 min, because we do not have any data after 30 min due to the following reasons. A longer reaction time (slower flow rate) in the microchannel reactor causes vaporization of the reaction solvent giving deposition of the products and buffer salts at the output of the microreaction channel. A longer channel causes loss of pressure. By those considerations, we fixed the length of the channel and the reaction times for every experiment as follows: length of channel; 40 cm, reaction time; from 0 to 30 min to avoid vaporization and deposition.
 | ||
| Scheme 1 Hydrolysis of p-nitrophenyl-β-D-galactopyranoside with β-galactosidase. | ||
 | ||
| Fig. 2 Initial reaction of the hydrolysis: (■) hydrolysis in the microchip, (●) hydrolysis in commercial micro-test tube, (△) hydrolysis with pre-treated chip. | ||
In this experiment, we did not shake the micro-test tube during the whole reaction time, although, in the first few seconds the micro-test tubes were shaken by hand in order to mix the enzyme solution and substrate solution together. We also examined the reaction in the micro-test tubes stirring with a magnetic stirrer. However, we could not get any clear data from the experiments under stirring that is probably caused by the inactivation of the enzyme at the air/water interface increased by vigorous stirring.
The first possible reason for the difference between the reaction rate in the microreaction channel and that of the batch reaction might be the different Reynolds number. the second possible reason might be absorption of the enzyme on the channel wall. It is well known that the diffusion constant in the microchannel is higher than that in the bulk channel, because of increasing Reynolds number. This fact suggests that the diffusion of the enzyme molecules in the whole reaction mixture in the microchannel is faster than that in the bulk fluid.
In order to confirm the second possible reason, we examined the hydrolytic activity of the ‘pre-treated chip’. The ‘pre-treated chip’ was prepared by treating the microreaction channel with 300 μl of the enzyme solution, rinsing with the same amounts of buffer. As shown in Fig. 2, the pre-treated microchip has weak enzymatic activity, however, it seems not to be of enough activity to explain the acceleration of the hydrolysis reaction in the microreaction channel.
Transgalactosylation was also accelerated in the microreaction channel, although, transglycosylation is the reverse reaction of the hydrolysis reaction (see Scheme 2). Transgalactosylation was carried out in organic solvent–buffer system as a reaction solvent to reduce the water concentration, and consequently, the equilibrium of the reverse reaction was shifted. In Fig. 3, the vertical axis presents the ratios between galactosylated p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside (Gal-GlcNAcPNP) and p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside (PNPGlcNAc) in the products, and the horizontal axis presents the reaction time. The reaction gives three kinds of stereoisomers, i.e., p-nitrophenyl-2-acetamide-2-deoxy-3-O-(β-D-galactopyranosyl)-β-D-glucopyranoside, p-nitrophenyl-2-acetamide-2-deoxy-4-O-(β-D-galactopyranosyl)-β-D-glucopyranoside,
and p-nitrophenyl-2-acetamide-2-deoxy-6-O-(β-D-galactopyranosyl)-β-D-glucopyranoside. In this study, we have not determined the ratios between those isomers in the products, but the total amounts of disaccharide mixture (galactosylated p-nitrophenyl-2-acetamide-2-deoxy-6-O-(β-D-galactopyranosyl)-β-D-glucopyranoside; Gal-GlcNAcPNP) in the products are plotted in Fig. 3. As shown in Fig. 3, the reaction rate in the microreaction channel seems to be enhanced compared with that in the micro-test tube suggesting that the microchip is also an effective device for transglycosylation. Generally, transglycosylation does not yield an oligosaccharide product in high conversion except for a few examples, i.e., equilibrium controlled reaction,14 lipid coated enzyme mediated reactions,15
but these reactions take many hours. Microchips may be feasible reaction devices for enzymatic oligosaccharide synthesis.
 | ||
| Scheme 2 Synthesis of disaccharide by transglycosylation. | ||
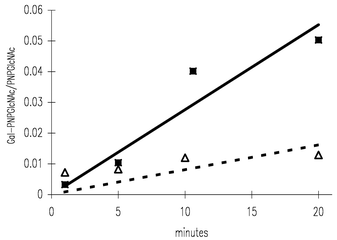 | ||
| Fig. 3 Transglycosylation of β-D-galactopyranoside in the microchip: (■) transglycosylation in the microchip, (△) transglycosylation with the batch reaction. | ||
Acknowledgements
We thank Dr Eiichi Abe for the provision of important information. We thank Mr Ryu Murase for the HPLC measurements.References
- T. A. Springer, Nature, 1990, 346, 425–434 CrossRef CAS.
- Y. Suzuki, Virology, 1992, 189, 121–131 CrossRef CAS.
- L. M. Stoolman, Cell, 1989, 56, 907–910 CrossRef CAS.
- P. I. Kitov, J. M. Sadowska, G. Mulvey, G. D. Armstrong, H. Ling, N. S. Pannu, R. J. Read and D. R. Bundle, Nature, 2000, 403, 669–672 CrossRef CAS.
- B. Faroux-Corlay, L. Clary, C. Gadras, D. Hammache, J. Greiner, C. Sntaella, A. M. Aubertin, P. Vierling and J. Fantini, Carbohydr. Res., 2000, 327, 223–260 Search PubMed.
- P. Arya, K. M. K. Kutterer, H. Qin, J. Roby, M. L. Barnes, S. Lin, C. A. Lingwood and M. G. Peter, Bioorg. Med. Chem., 1999, 7, 2823–2833 CrossRef CAS.
- E. J. Toone, E. S. Simon, M. D. Bednarski and G. M. Whitesides, Tetrahedron, 1989, 45, 5363–5422 CrossRef CAS.
- J. H. Yoon and J. S. Rhee, Carbohydr. Res., 2000, 327, 377–383 CrossRef CAS.
- C. Unverzagt and J. Seifert, Tetrahedron Lett., 2000, 41, 4549–4553 CrossRef CAS.
- Y. Tanaka, M. N. Slyadnev, A. Hibara, M. Tokeshi and T. Kitamori, J. Chromatogr. A, 2000, 894, 45–51 CrossRef CAS.
- M. Miyazaki, H. Nakamura and H. Maeda, Chem. Lett., 2001, 442–443 CrossRef CAS.
- M. N. Slyadnev, Y. Tanaka, M. Tokeshi and T. Kitamori, Anal. Chem., 2001, 73, 4037–4044 CrossRef CAS.
- J. Wang, M. P. Chatrathi and B. Tian, Anal. Chem., 2001, 73, 1733–1739 CrossRef CAS.
- T. Murata and T. Usui, Biosci. Biotechnol. Biochem., 1997, 61, 1059–1066 CrossRef CAS.
- Y. Okahata and T. Mori, J. Chem. Soc., Perkin Trans., 1996, 1, 2861–2866 Search PubMed.
Footnote |
| † Presented at the International Symposium on Microchemistry and Microsystems (ISMM 2001), Kawasaki, Japan, September 16–18, 2001. |
| This journal is © The Royal Society of Chemistry 2002 |
