Chemical reactions in microdroplets by electrostatic manipulation of droplets in liquid media†
Tomohiro Taniguchi*, Toru Torii and Toshiro Higuchi
Department of Precision Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo, 113-8656, Japan
First published on 22nd January 2002
Abstract
A microchemical reaction method involving microdroplets is proposed. Microdroplets are formed in a chemically stable medium on electric panel devices. These devices are substrates which have electrode arrays or electrode dots, and its surfaces are coated by an insulating film (such as Teflon or polypropylene) to prevent discharge and electrolysis of solutions. Microdroplets can be separately manipulated by a traveling electric field, which arises on applying a sequential voltage to the electrodes. Droplets moved smoothly at 1 Hz and voltage 400 V0-p. Reagents were then put in droplets that were collided and coalesced, resulting in chemical reactions that included alkalization of phenolphthalein and the luciferin–luciferase reaction.
Introduction
In conventional μTAS designs, microchemical reactions are performed under conditions of continuous flow, requiring microfluid devices such as pumps and valves as well as samples and reagents. Therefore there are some problems, such as the structure of devices being complex, the dead volume large, the continuity conditions preventing complex fluid control, and so on.As a solution for problems concerned with dead volume and the continuity conditions, there have been reports on methods based on the discrete flow in microchannels.1,2 In these devices sample and reagent were treated as droplets in a microchannel punctuated by air. Sammarco et al. used a hydrophilic channel and injected a small volume sample, and manipulated the small volume samples (droplets) using thermocapillary pumping.1 Hosokawa et al. manipulated droplets using a pneumatic drive, and used a HMCV (hydrophobic microcapillary vent) as a microvalve.2 They achieved positioning and mixing of droplets in a micro channel. These methods have advantage in sample volume, reaction time, however, since the droplets were transported in air, evaporation of liquid sample is a problem. Also, since the droplets were manipulated in a microchannel network, manipulating many sample droplets independently, and simultaneously, is difficult.
There have been reports on a method using an airborne droplet reaction chamber.3 Nilsson et al. used a method that prepared picolitre scale sample-containing droplets by a piezo-electric ejector, and levitated droplets with a ultrasonic levitator. A method using droplets in oil was also reported.3 Mizuno et al. treated the sample as a droplet in oil, and manipulated droplets by laser trapping.4 They achieved manipulation of DNA sample droplets and operation of PCR in microdroplets. However, independent and simultaneous manipulation of many sample droplets in one device is difficult in these methods.
On the other hand, solid particles can be manipulated electrostatically. Moesner and Higuchi5 used devices such as the electric panel, the electric tube, and electric dots, and achieved two-dimensional manipulation and precise positioning of particles, both in open air and in liquid.
There have also been reports on microdroplet based chemical reactors using electrostatic manipulation.6,7 In this the devices for transport were substrates on which arrays of electrodes were fabricated. Microdroplets on the devices were transported by electric fields. These methods for use with microdroplets, based on discrete flow, have advantages over conventional methods in reagent volume, reaction time, and efficiency of reaction; also, microfluid devices are not needed.
However, since these methods involve microdroplets in open air, problems remain such as evaporation of microdroplets and contamination. Also, to maximize the electric force on droplets and minimize interaction between droplets and device surfaces, those surfaces must be designed to be water-repellent.6,7
In this paper, a method is described for manipulating microdroplets in a chemically stable liquid medium. Since the microdroplets are surrounded by the medium, problems of evaporation are avoided. The microdroplets in the medium are almost spherical, so that transport is more efficient. Microdroplets were manipulated on an electric panel device, having electrode arrays or electrode dots. This method derives from the electrostatic solid particle microhandling technique of Moesner and Higuchi.5 Microdroplets on electrode array devices are transported together perpendicular to electrode arrays, whereas each microdroplet on electrode dot devices can be manipulated independently, and manipulated two-dimensionally by controlling the sequential voltages applied to the electrodes.
Microdroplets (about 0.1–1 μL in volume, surrounded by oil) were manipulated, were collided with each other, and were mixed on electric panel devices.
Electrostatic manipulation in liquid media
The electrostatic manipulation of droplets is shown in Fig. 1. Microdroplets are surrounded by a chemically stable medium, such as oil, on the electric panel devices. These devices are substrates which have multi-phase electrode arrays or electrode dots. Specifically, the device shown in Fig. 1(a) has six-phase electrode arrays. Droplets were transported by applying sequential voltages to the electrodes, as shown in Fig. 1(b) (in the figure, the sequential voltages have a six-phase rectangular profile: [+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0].
0].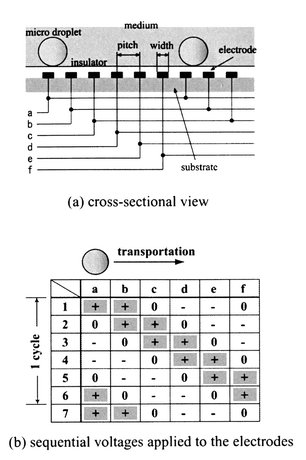 | ||
| Fig. 1 Principle of electrostatic manipulation. | ||
Since microdroplets on the electrode array devices are transported together in the direction perpendicular to the arrays, some guiding methods are necessary to control droplet motion. By contrast, each microdroplet on electrode dot devices can be manipulated independently by controlling the two-dimensional voltage profile applied to the electrodes. By this means many chemical reactions can be induced at once on the same device, and applications to combinatorial chemistry are facilitated (Fig. 2).
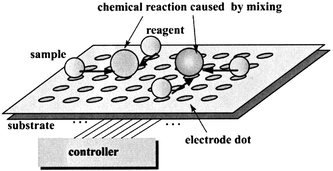 | ||
| Fig. 2 Application of the concept for combinatorial chemistry. | ||
For efficient transport, microdroplets must be spherical so that any contact angle is large. For transport in open air, special techniques are necessary including the use of superhydrophobic materials as such as alkylketene dimer (AKD) and plasma-polymer as surfaces, and surface morphology techniques.7,8 Droplets in chemically stable liquids are almost spherical, as with the dispersed phase of water–oil emulsion.
As mentioned, droplets in medium do not evaporate, causing problems. These problems are very severe for microdroplets of volume 0.1–1.0 μL.
Experimental
Electric panel device
We used a six-phase or three-phase electrode array device and a nine-phase (3 × 3) electrode dot device.A three-phase and a six-phase electrode array device are shown in Fig. 3. These devices consist of a polyester substrate 300 μm thick, electrode arrays of silver paste printed using a silkscreen printmaking process, and an insulating covering film. The electrode arrays have multi pitch length of 0.5 mm, 0.75 mm, 1.0 mm, 2.0 mm and width 0.2 mm. The insulating covering film was Teflon or polypropylene (PP) tape of thickness 90 μm (the devices shown in the figure were not covered with tape). The external terminals shown at the left were not covered.
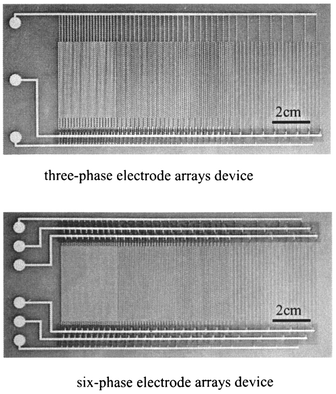 | ||
| Fig. 3 The electrode array devices. | ||
The electrode dot device is shown in Fig. 4. It comprises a four-layer glass-reinforced epoxy-printed circuitboard. The first layer has a nine-phase electrode dot matrix with an average pitch of 1.0 mm and a width of 0.5 mm. The entire electrode dot matrix consists of many 3 × 3 units, and its area is 150 mm × 150 mm (Fig. 4(a)). Each electrode has a hole through its center, and these holes are connected to electric circuits of the layers below. From the electric circuits of the lowest three-layer, the electrode dots were connected to external terminals (upper left of Fig. 4(a)). Manipulation of droplets took place by applying sequential voltages to the three-phase electrode columns or three-phase electrode lines.
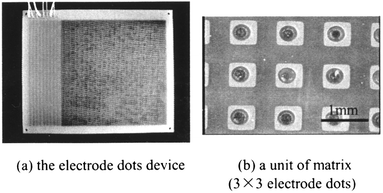 | ||
| Fig. 4 The nine-phase electrode dots device. | ||
System
A programmable multichannel high voltage supply was connected to the electrodes of the devices. All sequential voltages used in this paper have a six-phase rectangular profile. The supply source consists of PhotoMOS relay switches with adjustable positive and negative high voltage sources with an absolute DC voltage-output of 1.1 kV. Switching was performed by signals through the Centronics interface of the PC. Software was used to program the pattern and frequency of the sequential voltages (for example [+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0], 1 Hz: 1 cycle output). A similar system was used for electrostatic particle microhandling by Moesner and Higuchi.5
0], 1 Hz: 1 cycle output). A similar system was used for electrostatic particle microhandling by Moesner and Higuchi.5Sequential voltages
The sequential voltages we used have a six-phase rectangular profile (Fig. 1(b)). Some voltage sequences, such as [+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0] and [+
0] and [+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −], were first evaluated, and as a result all further experiments used the sequence [+
−], were first evaluated, and as a result all further experiments used the sequence [+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ], which proved to be the most efficient sequence for droplet transport.
], which proved to be the most efficient sequence for droplet transport.Next, the effect of voltage on transport was investigated. The behavior of 1 μL water microdroplets was observed on the six-phase electrode arrays device, altering the voltage value from 200 V0-p to 600 V0-p. The sequence [+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ] was used, and insulation comprised a 90 μm thickness PP film. Droplets were found to be transported smoothly when the voltage value was greater than 350 V0-p. At voltages greater than 350 V0-p, droplets of most other aqueous solutions also moved smoothly. Since too large a voltage caused cross-electrode discharge, the voltage value used in our experiments was 400 V0-p. However, with thinner insulating layers than the PP films used here, smaller voltages can be used.
] was used, and insulation comprised a 90 μm thickness PP film. Droplets were found to be transported smoothly when the voltage value was greater than 350 V0-p. At voltages greater than 350 V0-p, droplets of most other aqueous solutions also moved smoothly. Since too large a voltage caused cross-electrode discharge, the voltage value used in our experiments was 400 V0-p. However, with thinner insulating layers than the PP films used here, smaller voltages can be used.
Microdroplets in liquid media
To prevent the mixture of water droplets and the medium, and the diffusion of components of droplet, chemically stable/inert liquids, such as oil and fluorinert (Sumitomo 3 M), must be used as media. In experiments mentioned in this paper, a commercial vegetable oil was used as a medium. Properties of this oil are shown in Table 1. Since its density is less than that of most aqueous solutions, the microdroplets sank in it and rested on the electric panel devices. The microdroplets (0.1–1.0 μL) were placed into the medium using a commercial normal pipette (NPX-2, NichipetEX).| Edible oil | Water | |
|---|---|---|
| Density | 0.92 g cm−3 | 1.0 g cm−2 |
| Viscosity | 60 mPa s | 1.0 mPa s |
Microdroplets in a chemically stable medium such as oil are almost spherical. A water droplet in oil, of diameter 1 mm, is shown in Fig. 5. This droplet was on the PP film, which is not a superhydrophobic material, such as AKD or plasma-polymer. Friction and droplet–surface interaction can be minimized, and transport and manipulation made more efficient. In fact, microdroplets in the oil medium were transported at lower voltages than in air, given otherwise identical experimental conditions.
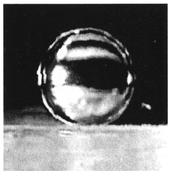 | ||
| Fig. 5 A water microdroplet in oil (a microdroplet was 1 mm in diameter). | ||
Droplet manipulation on the electrode arrays device
Microdroplets on the three-phase and six-phase electrode array devices could be transported with the frequency of the sequential voltages applied to the electrodes in the range 0.5–3.0 Hz. Transport of droplets was more efficient as the ratio of electrode width to pitch increased. In fact, droplets showed the smoothest motion on the electrode array area of pitch 0.5 mm.On the electrode array devices, microdroplets were transported only perpendicular to the electrode arrays. A guiding technique is therefore necessary to control the motion of the droplets. We used thin polymer films along which the microdroplets were guided. These films were about 30 μm thick, based on trials with 1 μL microdroplets. PET films, which have a thickness of 30 μm, were used in the experiments described below.
Droplet manipulation on the electrode dots device
With the electrode dot device, each microdroplet was manipulated independently. Manipulation of a single droplet is shown in Fig. 6. Two microdroplets consisting of 1 μL of water-based ink were put into the medium. Sequential voltages were applied only to the electrode column A, and not to column B. Fig. 6 shows that only the microdroplet on column A was manipulated downward from the upper side of the figure, and that on column B remained stationary.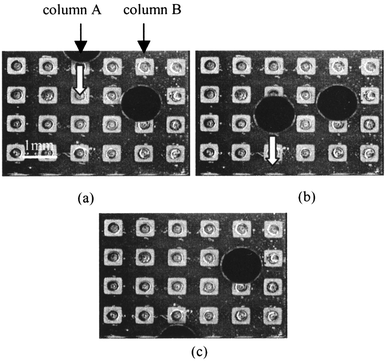 | ||
| Fig. 6 Droplet manipulation on the electrode dots device. | ||
Next, mixing of microdroplets on the electrode dot device was achieved. Mixing of three microdroplets is shown in Fig. 7. One water droplet, of volume 0.1 μL, was located on a center electrode dot, and two other water droplets of the same volume were located on a dot to the right and a lower dot in Fig. 7(a). Upon applying the voltage sequence to the center electrode line of dots, the droplet to the right approached the center dot, and coalesced with the droplet on it (Fig. 7(b) and (c)). Finally, on applying the voltage sequence to the center electrode column of dots, the droplet on the lower dot approached the center dot and coalesced.
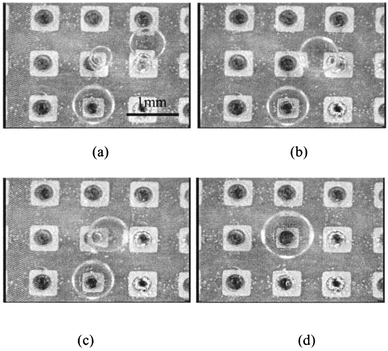 | ||
| Fig. 7 Mixing of three droplets on the electrode dots device. | ||
Since each microdroplet can be manipulated independently using the electrode dot device, the timing and sequence of microdroplet mixing can be controlled. This method has advantages for combinatorial chemistry devices.
Chemical reaction caused by mixing
By putting both sample and reagent into microdroplets, chemical reactions can be controlled by the mixing of droplets. We studied two chemical reactions.First, alkalization of phenolphthalein took place in a mixed droplet. This experiment used the nine-phase electrode dot device and is shown in Fig. 8. A microdroplet of NaOH aqueous solution (1.0 × 10−3 mol L−1) and phenolphthalein solution were put into the medium on the nine-phase electrode dots device (Fig. 8(a)). Both droplets had volume 1 μL. By applying the sequential voltage to a center electrode line of dots, a NaOH solution droplet (to the right in the figure) moved left (Fig. 8(b) and (c)). Next, upon applying the sequential voltages to a center column, the phenolphthalein solution droplet moved upward, and coalesced with the NaOH solution droplet (Fig. 8(d)). The mixing of the two droplets caused a chemical reaction, and the mixed droplet became red in color (Fig. 8(e)).
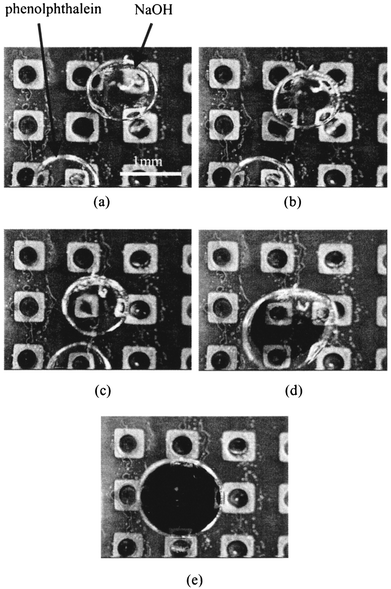 | ||
| Fig. 8 Alkalization of phenolphthalein caused by mixing. | ||
A problem arose in this experiment, concerning concentration of the solution. The surface tension of some aqueous solutions, such as NaOH solution, and Na2CO3 solution, decreases as its concentration increases. Consequently, application of a large voltage caused significant deformation of droplets of these solutions, and a droplet having large concentration (and small surface tension) was broken. Deformation and breakage of NaOH aqueous solution (1.0 × 10−2 mol L−1) droplets is shown in Fig. 9. Droplets in the medium (Fig. 9(a)) were deformed by applying large voltages (about 500 V0-p under these experimental conditions), and broke (Fig. 9(b)). The result was that many very small droplets were created, too small to be visible in these figures.
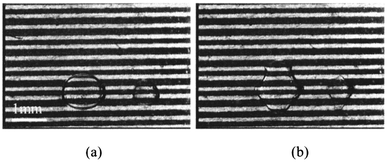 | ||
| Fig. 9 Deformation and breaking of NaOH solution droplets. | ||
Next, a luciferin–luciferase enzyme reaction was achieved in a mixed droplet. This reaction is a luminescence reaction shown as follows:
| reduced luciferin + ATP + O2 → (luciferin, Mg2+)→oxidized-luciferin + PP + AMP + H2O + hν |
This experiment used the six-phase electrode array device and is shown in Fig. 10. Fig. 10(b), (c) and (d) were taken by a supersensitive CCD camera (IK-TU50, TOSHIBA). A microdroplet of luciferin and ATP (adenosine triphosphate) aqueous solution and of luciferase aqueous solution were placed into the medium, on the six-phase electrode arrays device with guidance films (Fig. 10(a); the luciferase droplet is not visible). Both droplets have a volume of 1 μL. Upon applying the sequential voltage to the electrodes, the droplet of luciferase solution was transported along the guidance film (from the left in the figure), and approached the droplet of luciferin and ATP (Fig. 10(b)). Mixing of the two droplets caused a chemical reaction, and the luciferin luminesced in the mixed droplet. (Fig. 10(c))
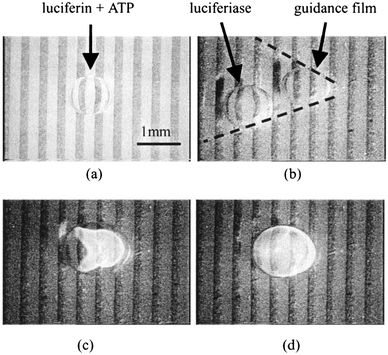 | ||
| Fig. 10 Luciferin luminescence caused by mixing of droplets. | ||
Conclusion
A method has been described for manipulating microdroplets in a medium so as to control chemical reactions between droplets of reaction constituents. This method has the advantages of reduced sample and reagent volume, reaction time, and cost. Furthermore, since the droplets are in a chemically stable liquid medium, another liquid, evaporation is avoided. The microdroplets were manipulated electrostatically on the electric panel devices. Fluid pumps and valves were not necessary, and the configuration of the device can be kept simple.Microdroplets of water, NaOH aqueous solution, luciferin and ATP aqueous solution were manipulated on the electrode array devices and the electrode dot device. Microdroplets on the electrode array devices were transported smoothly. On the electrode dot device, droplets could be manipulated independently in two dimensions. By putting sample and reagent into droplets, mixing of droplets by coalescence induces chemical reactions. In particular, alkalization of phenolphthalein and luminescence of luciferin via the luciferin–luciferase enzyme reaction were both demonstrated experimentally.
Future work
In this paper, μL-scale droplets were treated. The next step is to manipulate nL- or pL-scale droplets, and this development will increase the advantages of our method. Also, consideration is needed of materials for the medium, insulator, and guidance film, to treat droplets which contain hydrophobic or amphiphilic compounds.References
- T. S. Sammarco, Y. D. Fields, B. N. Johnson, D. K. Jones, D. T. Burke, C. H. Mastrangelo and M. A. Burns, Microfabrication of an Integrated DNA Analysis System, in Proc. Micro Total Analysis System '96, pp. 150–152 Search PubMed.
- K. Hosokawa, T. Fujii and I. Endo, Droplet-based Nano/picoliter Mixer using Hydrophobic Microcapillary Vent, in Proc. Micro Electro Mechanical Systems '99, pp. 307–310 Search PubMed.
- S. Nilsson, S. Santession, E. Degerman, T. Johansson, T. Laurell, J. Nilsson and J. Johansson, Airborne Chemistry For Biological Micro Analysis, in Micro Total Analysis System 2000, pp. 19–24 Search PubMed.
- S. Katsura, A. Yamaguchi, N. Harada, K. Hirano and A. Mizuno, Micro-reactor based on water-in-oil emulsion, in IEEE Industry Application Society Annual Meeting, Phoenix, AZ, 1999 Search PubMed.
- F. M. Moesner and T. Higuchi, Devices for Particle Handling by an AC Electric Field, in Proc. IEEE Micro Electro Mechanical Systems Conf., 1995, Amsterdam, The Netherlands, pp. 66–71 Search PubMed.
- M. Washizu, IEEE Trans. Ind. Appl., 1998, 34(4), 732–737 CrossRef CAS.
- A. Torkkeli, A. Häärä, J. Saarilahti, H. Härmä, T. Soukka and P. Tolonen, Droplet Manipulation on a Superhydrophobic Surface for Microchemical Analysis, in Proc. Transducers ′01 Eurosensors XV, Int. Conf. on Solid-State Sensors and Actuators,Munich, Germany, 2001, pp. 1150–1153 Search PubMed.
- A. Torkkeli, J. Saarilahti and A. Häärä, Electrostatic Transportation of Water Droplets on Superhydrophobic Surfaces, in Proc. IEEE Int. Conf. on Micro Electro Mechanical Systems, MEMS2001, Interlaken, Swizerland, 2001 Search PubMed.
Footnote |
| † Presented at the International Symposium on Microchemistry and Microsystems (ISMM 2001), Kawasaki, Japan, September 16–18, 2001. |
| This journal is © The Royal Society of Chemistry 2002 |
