Controlled growth of layered silver stearate on patterned organic monolayers
Seung Joon
Lee
,
Sang Woo
Han
and
Kwan
Kim
*
Laboratory of Intelligent Interface, School of Chemistry and Molecular Engineering and Center for Molecular Catalysis, Seoul National University, Seoul, Korea 151-742. E-mail: kwankim@plaza.snu.ac.kr
First published on 16th November 2001
Abstract
It is demonstrated that a layered organic–inorganic hybrid material, silver stearate, can be grown in a designed pattern on a solid substrate by a simple process of dipping of a microcontact-printed solid substrate in a mixture of AgNO3 and stearic acid solutions.
In the past decade, the number of organic–inorganic hybrid materials that exhibit alternating two-dimensional (2D) molecular assemblies of organic and inorganic constituents have been expanded considerably, encompassing a host of technologically relevant materials. Specific material properties, e.g., stiffness, strength, weight, nonlinear optical behavior, electrical conductivity, photochemical charge transfer and ferromagnetism, can be manipulated by systematic variations in the structure and properties of the organic and inorganic constituents at the molecular level.1
Silver alkane carboxylates (AgCO2R) are one class of organic–inorganic hetero-structured materials that show a well-developed progression of intense X-ray reflections; these intense X-ray reflections are associated with the three-dimensionally stacked silver carboxylate layers.2–4 Due to the specific arrangement of Ag atoms in a layer, AgCO2R materials have been proposed to possess 2D conductivity in solid state.5 AgCO2R materials are also known to decompose into alkane carboxylate-derivatized silver nanoparticles.6 Considering the current interest in patterned micro/nano-molecular devices, it would be desirable to assemble such organic–inorganic hybrid materials in two-dimensionally ordered ways on a solid substrate. In this communication, we demonstrate that a prototype AgCO2R, silver stearate, can be grown site-selectively on a solid substrate on to which organic monolayers have previously been assembled in a designed pattern.
Fig. 1 schematically describes the experimental procedure for patterned growth of layered silver stearates on carboxylic-group terminated organic monolayers. Gold substrates were prepared by resistive evaporation of titanium (5 nm) and gold (100 nm) on pre-cleaned glass slides. Microcontact printing (μCP) for the fabrication of patterned monolayers on Au was performed following the published procedure.7 Layered silver stearates were constructed on the patterned monolayers as follows: the patterned substrate was immersed in a mixture of an aqueous AgNO3 solution (2 mM) and a toluene solution of stearic acid (2 mM). After vigorous stirring for 2 h, the substrate was thoroughly washed successively with water, toluene and ethanol, and then dried under a stream of nitrogen. Evidence of the patterned growth of the silver stearate on gold in a layered structure was obtained using atomic force microscopy (AFM) and IR spectroscopy.†
 | ||
| Fig. 1 Flowchart showing the strategy for growing patterned silver stearate on Au; PDMS = poly(dimethylsiloxane). | ||
As can be seen from a typical AFM image (Fig. 2(a)), layered silver stearates are formed exclusively on the hydrophilic regions of the patterned monolayers. The height difference between the reacted and the unreacted regions is estimated to be ∼150 Å. Recalling the interlayer spacing of silver stearate,3 the measured height difference corresponds to the formation of more or less three layers of silver stearate on gold. A molecular resolution image (Fig. 2(b)) indicates further that the silver stearate grown on gold assumes triclinic subcell packing. The methyl groups are distributed on the outermost surface since the patterned silver stearate shows hydrophobic behavior to water droplets. As can be evidenced from the inset in Fig. 2(b), the nearest distances between the lattice points are about 4.3 and 4.8 Å (a × b). The latter values are in good agreement with the triclinic unit cell parameters measured by Tolochko et al.,4 determined using Ag K-EXAFS.
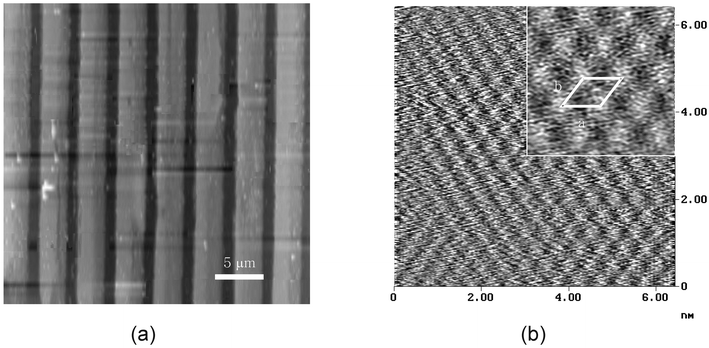 | ||
| Fig. 2 (a) Tapping mode AFM image of the patterned silver stearate on Au. (b) Enlarged molecular resolution image of the area where silver stearates are formed (a: 4.3 Å, b: 4.8 Å). | ||
Fig. 3(a) shows the IR spectrum of the silver stearate formed on the patterned substrate. Characteristic IR bands of stearate are observed not only in the high frequency region (2954, 2916, 2871 and 2848 cm−1) but also in the low frequency region (1517, 1470, 1419 and 717 cm−1). Two strong peaks at 2848 and 2916 cm−1 can be assigned to the symmetric (νs(CH2)) and the antisymmetric (νas(CH2)) stretching vibrations of the methylene groups, respectively. These modes usually lie in the narrow ranges of 2846–2850 and 2915–2918 cm−1, respectively, for all-trans extended chains,8 and in the distinctly different ranges of 2854–2856 and 2924–2928 cm−1 for disordered chains;9 the alkyl chains in silver stearate thus appear to be fully extended. The scissoring and rocking modes of the methylene groups observed at 1470 and 717 cm−1, respectively, clearly suggest that silver stearate adopts triclinic packing with two chains per unit cell,10 in agreement with the AFM measurement. Other bands at 2954, 2871, 1517 and 1419 cm−1 can be attributed to the νas(CH3), νs(CH3), νas(COO−) and νs(COO−) modes of silver stearate, respectively.11
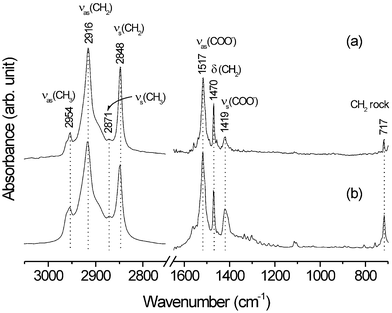 | ||
| Fig. 3 IR spectra of silver stearate (a) grown on Au and (b) synthesized via a two-phase (water/toluene) method. | ||
All of the IR bands observed for patterned silver stearate are exactly the same as those of layered silver stearate prepared in solution via a two-phase method12 (see Fig. 3(b)).‡ Hence, we surmise that the silver stearate is actually grown on a patterned substrate with single-crystalline features rather than with polycrystalline features.
In summary, we shown that layered silver stearate can be grown in a controlled way on patterned organic monolayers. A more detailed study on the structure and thermal behavior of the patterned silver stearate is in progress. Nonetheless, we hope the present method to be applicable to the fabrication of microelectronic devices, incorporating, for instance, the 2D-conductivity properties of AgCO2R materials. We suppose further that, if the terminal functionality of the precursor organic monolayers is judiciously chosen, other classes of organic–inorganic hybrid materials can also be grown via the current method. The present scheme for the patterned growth of organic–inorganic multilayers is, in fact, a soft-lithographic process, which can be applied to a variety of substrates including non-flat and non-metallic surfaces. This cannot be achieved easily by using a conventional method such as the Langmuir–Blodgett technique.
Acknowledgements
This work was supported in part by the Korea Research Foundation (KRF, 042-D00073) and the Korea Science and Engineering Foundation (KOSEF, 1999-2-121-001-5). K. K. also acknowledges KOSEF for providing a leading-scientist grant (KOSEF, R03-2001-00021). S. W. H was supported by KOSEF through the Center for Molecular Catalysis at Seoul National University. S. J. L. is a recipient of the BK21 fellowship.Notes and references
- I. A. Aksay, M. Trau, S. Manne, I. Honma, N. Yao, L. Zhou, P. Fenter, P. M. Eisenberger and S. M. Gruner, Science, 1996, 273, 892 CrossRef CAS; P. G. Lacroix, R. Clement, K. Nakatani, J. Zyss and I. Ledoux, Science, 1994, 263, 658 CrossRef CAS; L. A. Vermeulen and M. E. Thompson, Nature, 1992, 358, 656 CrossRef CAS; V. Laget, C. Hornick, P. Rabu, M. Drillon, P. Turek and R. N. Ziessel, Adv. Mater., 1998, 10, 1024 CrossRef CAS; A. N. Shipway, E. Katz and I. Willner, ChemPhysChem, 2000, 1, 18 CrossRef CAS; P. C. Hidber, E. Kim and G. M. Whitesides, Langmuir, 1996, 12, 1375 CrossRef CAS.
- A. Vand, A. Atkins and R. K. Cambell, Acta Crystallogr., 1949, 2, 398 CrossRef.
- F. W. Matthews, G. G. Waren and J. H. Michell, Anal. Chem., 1950, 22, 514 CrossRef CAS.
- B. P. Tolochko, S. V. Chernov, S. G. Nikitenko and D. R. Whitcomb, Nucl. Instrum. Methods A, 1998, 405, 428 CrossRef CAS.
- N. F. Uvarov, L. P. Burleva, M. B. Mizen, D. R. Whitcomb and C. Zou, Solid State Ionics, 1998, 107, 31 Search PubMed.
- K. Abe, T. Hanada, Y. Yoshida, N. Tanigaki, H. Takiguchi, H. Nagasawa, M. Nakamoto, T. Yamaguchi and K. Yase, Thin Solid Films, 1998, 524, 327; S. J. Lee, S. W. Han, H. J. Choi and K. Kim, Eur. Phys. J. D, 2001, 16, 293 Search PubMed.
- Y. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550 CrossRef CAS.
- R. A. MacPhail, H. L. Strauss, R. G. Snyder and C. A. Elliger, J. Phys. Chem., 1984, 88, 334 CrossRef CAS.
- R. G. Snyder, H. L. Strauss and C. A. Elliger, J. Phys. Chem., 1982, 86, 5145 CrossRef CAS.
- C. Almirante, G. Minoni and G. Zerbi, J. Phys. Chem., 1986, 90, 852 CrossRef.
- S. J. Lee and Kwan Kim, Vib. Spectrosc., 1998, 18, 187 CrossRef CAS.
- F. Bensebaa, T. H. Ellis, E. Kruus, R. Voicu and Y. Zhou, Langmuir, 1998, 14, 6579 CrossRef CAS.
Footnotes |
| † Infrared spectra were measured using a Bruker IFS 113v FT-IR spectrometer equipped with a globar light source and a liquid N2-cooled wide-band mercury cadmium telluride detector, in diffuse reflectance and external reflection modes for patterned and powdered samples, respectively. The morphology of the patterned silver stearate was examined with a scanning probe microscope (Digital Instruments, Model Nanoscope IIIa). |
| ‡ Silver stearate was prepared by the two-phase method. An aqueous solution of AgNO3 was added dropwise to a stearic acid solution dissolved in toluene. After 3 h of vigorous stirring, the resulting yellowish-white precipitates were filtered off, washed successively with ethanol, toluene and cold water, and finally dried in vacuo. |
| This journal is © The Royal Society of Chemistry 2002 |
