High oxide ion conductivity in Bi2MoO6 oxidation catalyst
L. T.
Sim
a,
C. K.
Lee
a and
A. R.
West
b
aDepartment of Chemistry, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
bDepartment of Engineering Materials, University of Sheffield, Mappin Street, Sheffield, UK S1 3JD
First published on 16th November 2001
Abstract
Both the low (γ)- and high (γ′)-temperature polymorphs of Bi2MoO6 are oxide ion conductors. The γ′ conductivity at 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C is comparable to that of yttria-stabilized zirconia (YSZ) while its activation energy, 0.510(2) eV, is much less than that of YSZ, 0.79 eV, and is lower than that of the best low temperature oxide ion conductor, BIMEVOX: Bi2V0.9Cu0.1O5.35, 0.56 eV, thus suggesting that γ′-Bi2MoO6 contains a small number of highly mobile oxide ions. This may account for its usefulness as an oxidation catalyst.
°C is comparable to that of yttria-stabilized zirconia (YSZ) while its activation energy, 0.510(2) eV, is much less than that of YSZ, 0.79 eV, and is lower than that of the best low temperature oxide ion conductor, BIMEVOX: Bi2V0.9Cu0.1O5.35, 0.56 eV, thus suggesting that γ′-Bi2MoO6 contains a small number of highly mobile oxide ions. This may account for its usefulness as an oxidation catalyst.
Bismuth molybdates are useful oxidation catalysts in selective olefin oxidation and ammoxidation processes.1 In the partial oxidation of olefins, superior catalytic activity appeared to be confined to the compounds Bi2MoO6 (γ) and Bi2Mo2O9 (β).2 Bi2Mo2O9 produced the best catalyst in terms of activity and selectivity, but was unstable at temperatures above 400
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.3 Kumar and Ruckenstein4 showed the decomposition products to be Bi2MoO6 and MoO2 and suggested that Bi2MoO6 formed near the surface of the β phase and was responsible for the selective catalytic oxidation. Various studies on the selective oxidation of olefin using an 18O-labelled catalyst have shown that diffusion
of lattice oxygen of Bi2MoO6
(γ) played a major role in the catalytic process.5–7 It was proposed5 that during oxidation, a Bi-bound O2− is removed, replaced by a nearby Mo-bound O2− and the vacancy subsequently filled by an external O2−. Galván et al.3 reported that the high-temperature γ′ form of Bi2MoO6 is a suitable catalyst in CO oxidation reactions, showing the same degree of catalytic activity as cobalt perovskites. The study showed that besides oxygen in the feed, oxygen from the lattice was consumed by the reaction, thus suggesting that a mechanism similar to olefin oxidation, in which reactive oxygen was provided by the catalyst, was in operation.
°C.3 Kumar and Ruckenstein4 showed the decomposition products to be Bi2MoO6 and MoO2 and suggested that Bi2MoO6 formed near the surface of the β phase and was responsible for the selective catalytic oxidation. Various studies on the selective oxidation of olefin using an 18O-labelled catalyst have shown that diffusion
of lattice oxygen of Bi2MoO6
(γ) played a major role in the catalytic process.5–7 It was proposed5 that during oxidation, a Bi-bound O2− is removed, replaced by a nearby Mo-bound O2− and the vacancy subsequently filled by an external O2−. Galván et al.3 reported that the high-temperature γ′ form of Bi2MoO6 is a suitable catalyst in CO oxidation reactions, showing the same degree of catalytic activity as cobalt perovskites. The study showed that besides oxygen in the feed, oxygen from the lattice was consumed by the reaction, thus suggesting that a mechanism similar to olefin oxidation, in which reactive oxygen was provided by the catalyst, was in operation.
Bi2MoO6 exists in three polymorphic forms: γ, γ″
(or I) and γ′, with transformation temperatures of 604 and 640–670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, respectively for the low to intermediate and intermediate to high temperature polymorphs.8 The γ to I transition is reversible while the I to γ′ transition is not, except when high pressures are applied. The temperature of the γ (or γ″) to γ′ transition depends somewhat on heating conditions; transformation was reported to occur as low as 580
°C, respectively for the low to intermediate and intermediate to high temperature polymorphs.8 The γ to I transition is reversible while the I to γ′ transition is not, except when high pressures are applied. The temperature of the γ (or γ″) to γ′ transition depends somewhat on heating conditions; transformation was reported to occur as low as 580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C;9 in this study we found the transformation occurred on prolonged heating at 600
°C;9 in this study we found the transformation occurred on prolonged heating at 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. γ-Bi2MoO6 has a layered Aurivillius structure consisting of Bi2O22+ sheets alternating with MoO42− perovskite layers;10
in γ′-Bi2MoO6, the cation distribution forms a fluorite-related supercell with infinite channels of Bi polyhedra surrounded by Mo tetrahedra.9 Despite the difference in crystal structure, similar mechanisms involving lattice oxygen appear to operate when these materials are used as oxidation catalysts. The anionic conductivity of the bismuth-rich fluorite-like phases in the Bi2O3–MoO3 system has been reported.11,12 However, there is only one report13 on the conductivity of Bi2MoO6. Fixed frequency (1 kHz) conductivity data on γ and γ′ polymorphs, combined with transport number measurements, led to the conclusion that the γ polymorph was an oxide ion conductor at low temperatures but became increasingly electronic above 300
°C. γ-Bi2MoO6 has a layered Aurivillius structure consisting of Bi2O22+ sheets alternating with MoO42− perovskite layers;10
in γ′-Bi2MoO6, the cation distribution forms a fluorite-related supercell with infinite channels of Bi polyhedra surrounded by Mo tetrahedra.9 Despite the difference in crystal structure, similar mechanisms involving lattice oxygen appear to operate when these materials are used as oxidation catalysts. The anionic conductivity of the bismuth-rich fluorite-like phases in the Bi2O3–MoO3 system has been reported.11,12 However, there is only one report13 on the conductivity of Bi2MoO6. Fixed frequency (1 kHz) conductivity data on γ and γ′ polymorphs, combined with transport number measurements, led to the conclusion that the γ polymorph was an oxide ion conductor at low temperatures but became increasingly electronic above 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, whereas the converse
occurred for the γ′ polymorph which became increasingly electronic for temperatures below 700
°C, whereas the converse
occurred for the γ′ polymorph which became increasingly electronic for temperatures below 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.13 We became aware of this paper only at the conclusion of the present study and there are major discrepancies between the two sets of findings, as discussed below.
°C.13 We became aware of this paper only at the conclusion of the present study and there are major discrepancies between the two sets of findings, as discussed below.
γ- and γ′-Bi2MoO6 were prepared by mixing and heating stoichiometric mixtures of high purity Bi2O3 and MoO3 for 20 hours at 530 and 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, respectively. The identities of the products were confirmed by their respective XRD patterns.
°C, respectively. The identities of the products were confirmed by their respective XRD patterns.
The electrical properties of Bi2MoO6 depended very much on the sample history and polymorph; powder X-ray diffraction, XRD, on crushed pellet fragments was carried out at the end of each set of conductivity measurements in order to establish whether or not any structural changes had occurred during the measurements. An impedance complex plane plot of γ-Bi2MoO6 sintered at 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C shows a broadened semicircle and a low frequency spike, Fig. 1a. The sintering temperature had to be kept below 600
°C shows a broadened semicircle and a low frequency spike, Fig. 1a. The sintering temperature had to be kept below 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C to avoid phase transformation; hence it was difficult to obtain a dense ceramic for conductivity measurements; prolonged heating, 4 days, at 530
°C to avoid phase transformation; hence it was difficult to obtain a dense ceramic for conductivity measurements; prolonged heating, 4 days, at 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, did not lead to any noticeable change in conductivity. Total conductivity values were obtained from the low frequency intercept of the broadened semicircle.
°C, did not lead to any noticeable change in conductivity. Total conductivity values were obtained from the low frequency intercept of the broadened semicircle.
 | ||
Fig. 1
Complex plane plots at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C of (a)
γ-Bi2MoO6 and (b)
γ′-Bi2MoO6 sintered at 530 and 900 °C of (a)
γ-Bi2MoO6 and (b)
γ′-Bi2MoO6 sintered at 530 and 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, respectively. °C, respectively.
| ||
Impedance data of the γ′ polymorph were very dependent on sintering conditions. Impedance complex plane plots ranged from a slightly distorted high frequency semicircle for pellets sintered at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C to an ideal, undistorted semicircle after repeated heat–cool cycles or sintering at higher temperature, Fig. 1b.
°C to an ideal, undistorted semicircle after repeated heat–cool cycles or sintering at higher temperature, Fig. 1b.
Arrhenius conductivity plots of the total pellet conductivity of γ′-Bi2MoO6 were very dependent on the sintering temperature with the highest conductivities observed after sintering at 928![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, a temperature close to melting, Fig. 2. Linearity of the Arrhenius plots appeared to increase either with increasing sintering temperature or after repeated heat–cool cycles. The increases in conductivity on isothermal annealing at high temperature were irreversible and could not be reversed by isothermal annealing at lower temperature, e.g. on reheating overnight at 800
°C, a temperature close to melting, Fig. 2. Linearity of the Arrhenius plots appeared to increase either with increasing sintering temperature or after repeated heat–cool cycles. The increases in conductivity on isothermal annealing at high temperature were irreversible and could not be reversed by isothermal annealing at lower temperature, e.g. on reheating overnight at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. There was also no evidence by either XRD or conductivity measurements that the γ → γ′ transition could be reversed. These effects appear to be similar to those seen in Ca12Al14O33,14 which were attributed to an increase
in grain size and increased area of contact between grains in well-sintered samples, giving rise to an overall increase in pellet conductivity. Consistent with this explanation, scanning electron micrographs (SEM) of pellets of γ′-Bi2MoO6 sintered at 800
°C. There was also no evidence by either XRD or conductivity measurements that the γ → γ′ transition could be reversed. These effects appear to be similar to those seen in Ca12Al14O33,14 which were attributed to an increase
in grain size and increased area of contact between grains in well-sintered samples, giving rise to an overall increase in pellet conductivity. Consistent with this explanation, scanning electron micrographs (SEM) of pellets of γ′-Bi2MoO6 sintered at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, 850
°C, 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 900
°C and 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, Fig. 3, show an increase in grain size, decreased porosity and increased contact between grains with increasing sintering temperature.
°C, Fig. 3, show an increase in grain size, decreased porosity and increased contact between grains with increasing sintering temperature.
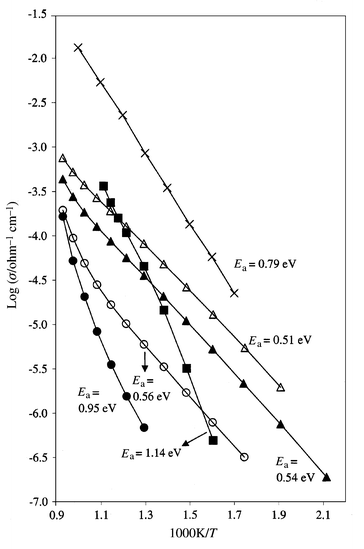 | ||
Fig. 2
Arrhenius plots of γ-Bi2MoO6
(■), γ′-Bi2MoO6 sintered at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (●), 850 °C (●), 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (○), 900 °C (○), 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (▲), 928 °C (▲), 928![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (△) and yttria-stabilized zirconia (×). °C (△) and yttria-stabilized zirconia (×).
| ||
 | ||
Fig. 3
SEM micrographs of γ′-Bi2MoO6 sintered at (a) 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, (b) 850 °C, (b) 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and (c) 900 °C and (c) 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. °C.
| ||
The conductivity Arrhenius plot of γ-Bi2MoO6 was reversible on cooling, with an activation energy of ∼1.14 eV for temperatures up to 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, Fig. 2. At higher temperatures (not shown), a sharp drop in the conductivity of γ-Bi2MoO6 was observed, within the temperature range 625–675
°C, Fig. 2. At higher temperatures (not shown), a sharp drop in the conductivity of γ-Bi2MoO6 was observed, within the temperature range 625–675![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, as the sample transformed from the γ to the γ′ polymorph; the conductivity of the resulting γ′ polymorph was comparable to that of the γ′ sample sintered at 800
°C, as the sample transformed from the γ to the γ′ polymorph; the conductivity of the resulting γ′ polymorph was comparable to that of the γ′ sample sintered at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The conductivity–temperature dependence of γ-Bi2MoO6 is, therefore, very different to that of well-sintered γ′-Bi2MoO6, with much higher activation energy and lower conductivity, especially at lower temperatures.
°C. The conductivity–temperature dependence of γ-Bi2MoO6 is, therefore, very different to that of well-sintered γ′-Bi2MoO6, with much higher activation energy and lower conductivity, especially at lower temperatures.
The decrease in conductivity associated with the γ → γ′ transition and the subsequent increase on sintering γ′ above ∼800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C are believed to have a common origin. From published data,9,10 the unit cell volumes (densities) of γ′-Bi2MoO6 and γ-Bi2MoO6 are 2162.35 (7.493) and 489.23 Å3
(8.28 g cm−3), respectively. As the density of γ is ∼9% greater than that of γ′, the γ → γ′ transition is likely to be accompanied by significant expansion of all the grains in the ceramics and a reduction in the area of the grain–grain contacts. Consequently, the conductivity of the resulting γ′ pellet is comparable to that of the γ′ sample sintered at 800
°C are believed to have a common origin. From published data,9,10 the unit cell volumes (densities) of γ′-Bi2MoO6 and γ-Bi2MoO6 are 2162.35 (7.493) and 489.23 Å3
(8.28 g cm−3), respectively. As the density of γ is ∼9% greater than that of γ′, the γ → γ′ transition is likely to be accompanied by significant expansion of all the grains in the ceramics and a reduction in the area of the grain–grain contacts. Consequently, the conductivity of the resulting γ′ pellet is comparable to that of the γ′ sample sintered at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
and whose density, and conductivity, had not been enhanced by heating >800
°C
and whose density, and conductivity, had not been enhanced by heating >800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
°C.
The conducting species appear to be oxide ions, for two reasons. First, the conductivity data (Z* plots) were completely unaffected by changing the atmosphere during measurement from dry to wet air, and so there was no evidence of protonic conduction. Second, the impedance data show evidence at low frequencies of an inclined spike, seen clearly for γ′-Bi2MoO6 in Fig. 1b, at an angle of ∼45° to the Z′ axis. Such a spike is characteristic of a diffusion-limited process represented by a Warburg impedance; in the present case, the origin is likely to be the diffusion of oxygen molecules towards/away from the electrode/sample contact and is a very strong indicator that the present materials are oxide ion conductors and not electronic semiconductors; in order to confirm this, emf measurements of an oxygen concentration cell containing Bi2MoO6 as the membrane are needed.
In conclusion, a well-sintered sample of γ′-Bi2MoO6 is a better oxide ion conductor than the low temperature γ polymorph at temperatures below 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. It has an activation energy of 0.510(2) eV (average of three conductivity sweeps on a pellet sintered at 928
°C. It has an activation energy of 0.510(2) eV (average of three conductivity sweeps on a pellet sintered at 928![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) and conductivity of 7.3 × 10−6 ohm−1 cm−1 at 300
°C) and conductivity of 7.3 × 10−6 ohm−1 cm−1 at 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Its conductivity at 300
°C. Its conductivity at 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C is comparable to that of yttria-stabilized zirconia (YSZ), Fig. 2, while its activation energy is much less than that of YSZ, 0.79 eV. The activation energy of γ′-Bi2MoO6 is, in fact, less than that of the BIMEVOX family, which contains materials with the highest known oxide ion conductivity for temperatures below ∼500
°C is comparable to that of yttria-stabilized zirconia (YSZ), Fig. 2, while its activation energy is much less than that of YSZ, 0.79 eV. The activation energy of γ′-Bi2MoO6 is, in fact, less than that of the BIMEVOX family, which contains materials with the highest known oxide ion conductivity for temperatures below ∼500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, e.g. 0.56 eV for
Bi2V0.9Cu0.1O5.3515 and 0.57 eV for Bi4V1.5Sb0.5O11.16 This indicates that γ′-Bi2MoO6 contains a small number of mobile oxide ions. The relatively high oxide ion conductivity of γ-Bi2MoO6 at low temperatures and, especially, the low activation energy, may account for its usefulness as an oxidation catalyst where migration of lattice oxygen from the bulk to the surface appears to be of paramount importance.
°C, e.g. 0.56 eV for
Bi2V0.9Cu0.1O5.3515 and 0.57 eV for Bi4V1.5Sb0.5O11.16 This indicates that γ′-Bi2MoO6 contains a small number of mobile oxide ions. The relatively high oxide ion conductivity of γ-Bi2MoO6 at low temperatures and, especially, the low activation energy, may account for its usefulness as an oxidation catalyst where migration of lattice oxygen from the bulk to the surface appears to be of paramount importance.
The results presented here contrast significantly with those in ref. 13, which showed a low conductivity for both γ and γ′ polymorphs which was comparable to that of our data for γ′-Bi2MoO6 sintered at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C; however, the data in ref 13 which were fixed frequency data, may not have represented true bulk conductivities and were not presented in conventional Arrhenius format, thus making comparison difficult. It appears that those data were for poorly sintered samples, and therefore the conductivities were 1 to 2 orders of magnitude lower than those of our well-sintered samples.
°C; however, the data in ref 13 which were fixed frequency data, may not have represented true bulk conductivities and were not presented in conventional Arrhenius format, thus making comparison difficult. It appears that those data were for poorly sintered samples, and therefore the conductivities were 1 to 2 orders of magnitude lower than those of our well-sintered samples.
C. K. L. thanks the RSC for a Journals Grant for International Authors.
References
- Y. Moro-Oka and W. Ueda, Adv. Catal., 1994, 40, 233 Search PubMed.
- Ph. A. Baptist, A. H. W. M. Der Kinderen, Y. Leeuwenburgh, F. A. M. G. Metz and G. C. A. Schuit, J. Catal., 1968, 12, 45 CrossRef.
- D. H. Galván, S. Fuentes, M. Avalos-Borja and L. Cota-Araiza, Catal. Lett., 1993, 18, 273 Search PubMed.
- J. Kumar and E. Ruckenstein, J. Solid State Chem., 1980, 31, 41 CrossRef CAS.
- D. B. Dadyburjor and E. Ruckenstein, J. Phys. Chem., 1978, 82, 1563 CrossRef CAS.
- H. Miura, T. O. Otsubo, T. Shirasaki and Y. Morikawa, J. Catal., 1979, 56, 81 CrossRef CAS.
- L. C. Glaeser, J. F. Brazdil, M. A. Hazle, M. Mehicic and R. K. Grasselli, J. Chem. Soc., Faraday Trans. 1, 1985, 81, 2903 RSC.
- H. Kodama and A. Watanabe, J. Solid State Chem., 1985, 56, 225 CrossRef CAS.
- D. J. Buttrey, T. Vogt, U. Wildgruber and W. R. Robinson, J. Solid State Chem., 1994, 111, 118 CrossRef CAS.
- R. G. Teller, J. F. Brazdil, R. K. Grasselli and J. D. Jorgensen, Acta Crystallogr., Sect. C, 1984, 40, 2001 CrossRef.
- T. Takahashi, T. Esaka and H. Iwahara, J. Appl. Electrochem., 1977, 7, 31 CrossRef CAS.
- G. Chiodelli, A. Magistris, G. Spinolo, C. Tomasi, V. Antonucci and N. Giordano, Solid State Ionics, 1994, 74, 37 Search PubMed.
- V. I. Utkin, Yu. E. Roginskaya, R. P. Kayumov and Yu. N. Venevtsev, Zh. Fiz. Khim., 1980, 54, 2953 Search PubMed.
- M. Lacerda, J. T. S. Irvine, E. E. Lachowski, F. P. Glasser and A. R. West, Br. Ceram. Trans. J., 1988, 87, 191 Search PubMed.
- F. Abraham, J. C. Boivin, G. Mairesse and G. Nowogrocki, Solid State Ionics, 1990, 40, 934 Search PubMed.
- O. Joubert, A. Jouanneaux, M. Ganne, R. N. Vannier and G. Mairesse, Solid State Ionics, 1994, 73, 309 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
