A positive-working alkaline developable photoresist based on partially tert-Boc-protected calix[4]resorcinarene and a photoacid generator
Kwon Young-Gila, Jin Baek Kima, Tsuyohiko Fujigayab, Yuji Shibasakib and Mitsuru Ueda*b
aDepartment of Chemistry, Korea Advanced Institute of Science & Technology, Yusong-ku Taejon, 305-701, Korea
bDepartment of Organic & Polymeric Materials, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo, 152-8552, Japan. Fax: +81-3-5734-2127; E-mail: mueda@polymer.titech.ac.jp
First published on 20th November 2001
Abstract
A positive working low-molecular-weight photoresist based on partially t-Boc protected tetra-C-methylcalix[4]resorcinarene (t-Boc C-4-R) and a photoacid generator (PAG), diphenyliodonium 9,10-dimethoxyanthracene-2-sulfonate (DIAS) has been developed. t-Boc C-4-Rs were prepared by the reaction of C-4-R with di-tert-butyl dicarbonate in the presence of 4-dimethylaminopyridine (DMAP). A clear film cast from a 20 wt%
t-Boc C-4-R solution in cyclohexanone showed high transparency to UV above 300 nm. The appropriate t-Boc protecting ratio was about 60 mol% in view of adhesion, deprotection temperature and dissolution rate. The photoresist consisting of 60 mol%
t-Boc C-4-R
(95 wt%) and DIAS (5 wt%) showed a sensitivity of
13 mJ cm−2 and a contrast of 12.6 when it was exposed to 365 nm light and postbaked at 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 90 s, followed by developing with a 2.38 wt% aqueous tetramethylammonium hydroxide (TMAH) solution at room temperature. A fine positive image featuring 1.5 µm of minimum line and space patterns was observed on the film of the photoresist exposed to 40 mJ cm−2 of UV-light at 365 nm by the contact mode.
°C for 90 s, followed by developing with a 2.38 wt% aqueous tetramethylammonium hydroxide (TMAH) solution at room temperature. A fine positive image featuring 1.5 µm of minimum line and space patterns was observed on the film of the photoresist exposed to 40 mJ cm−2 of UV-light at 365 nm by the contact mode.
Introduction
Amorphous materials are attractive for their excellent processability, flexibility, transparency, inexistence of grain boundaries, and isotropic properties.1 As amorphous organic materials, many polymers are known and have received attention from both fundamental and practical viewpoints.2 However, little attention has been paid to low-molecular-weight materials that form stable amorphous glasses above room temperature.To meet the upcoming demand of next generation lithography, new chemically amplified resist materials should be developed for fabrication of devices whose critical dimensions are close to the size of a molecule. All commercially available resists so far are based on linear polymers in the formation of chemically amplified resist systems. Low-molecular-weight materials, which have a definite structure and no molecular weight distribution, as is inevitable in the case of polymer, have several advantages over linear polymers as patterning features become smaller.3 The limit of resolution can be expanded to a molecular level since the building block of the image feature shrinks to a small molecular size. When a polymer is used as the building block of patterns, the image size never excels over the size of building block itself and small patterns on the order of a molecule can not be delineated. Furthermore, the non-linear small molecular resist helps to dissolve more uniformly during development. The ability to create an image in a resist using the lithographic process depends on the difference in dissolution rates between the exposed and unexposed regions of the resist. The dissolution rate of polymers depends critically on physical properties such as polydispersity, degree of branching, molecular weights, viscosity, etc.4 However, low-molecular-weight amorphous materials such as star shaped molecules do not exhibit strong intermolecular interactions such as chain entanglement due to the short chain length, the small radius of gyration, and the high density of sterically congested peripheral groups. Resist molecules that are free of intermolecular chain entanglement may have implications in decreasing line edge roughness at very small feature sizes. Also, the small uniform size of molecule can be incorporated in multi-component resist systems where these materials serve as a dissolution inhibitor/promoter with good miscibility. We have been exploiting such small molecular materials in the hope of achieving an improved class of resist materials with the capability of imaging features into molecular scale resolution based on dendrimers and calixarenes as core compounds.5
In previous work,6 we reported a positive working photoresist based on fully t-Boc protected tetra-C-hexylcalix[4]resorcinarene which was prepared from resorcinol and hexan-1-al, followed by successive protection with di-tert-butyl dicarbonate in the presence of 4-dimethylaminopyridine (DMAP), where a long alkyl chain was introduced to prevent crystallization of the fully t-Boc protected tetra-C-methylcalix[4]resorcinarene. This resist, however, has an adhesion problem because of high hydrophobicity. In chemically amplified resists, partially t-Boc protected poly(hydroxystyrene)s are employed to control the dissolution rate.7 Thus, to remedy these problems, we decided to prepare partially t-Boc protected tetra-C-methylcalix[4]resorcinarene (t-Boc C-4-R). This would give a stable amorphous film with good adhesion to silicon wafer because this consists of various molecular weight compounds possessing many hydroxy groups. Herein we report the synthesis of t-Boc C-4-R as a new matrix and a lithographic evaluation of a two-component photoresist consisting of t-Boc C-4-R and diphenyliodonium 9,10-dimethoxyanthracene-2-sulfonate (DIAS) as a photo-acid generator.
Experimental
Materials
DIAS was synthesized from diphenyliodonium chloride with sodium 9,10-dimethoxyanthracene-2-sulfate obtained by the reduction of sodium anthraquinone-2-sulfonate with zinc in aqueous sodium hydroxide solution followed by methylation with dimethyl sulfate.8 Solvents were dried and purified in the usual manner. Other reagents were obtained commercially and used as received.Tetra-C-methylcalix[4]resorcinarene (C-4-R)9
A solution of 33.0 g (0.3 mol) of resorcinol and 16.8 mL (0.3 mol) of acetaldehyde in 300 mL methanol was heated at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. To the solution, 75 mL of concentrated hydrochloric acid was added over 0.5 h. The reaction solution was stirred at 75
°C. To the solution, 75 mL of concentrated hydrochloric acid was added over 0.5 h. The reaction solution was stirred at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 1 h and a yellow solid began to precipitate from the homogeneous mixture. After additional refluxing for 2 h, the mixture was cooled with an ice bath. A precipitate was collected with a glass filter and dried under reduced pressure. The product was recrystallized from methanol. Yield: 23.0 g (57%).
°C for 1 h and a yellow solid began to precipitate from the homogeneous mixture. After additional refluxing for 2 h, the mixture was cooled with an ice bath. A precipitate was collected with a glass filter and dried under reduced pressure. The product was recrystallized from methanol. Yield: 23.0 g (57%).1H-NMR (DMSO-d6): δ (ppm) 1.36 (d, CH3, 12H), 4.52 (q, CH, 4H), 6.18 (s, ArH, 4H), 6.83 (s, ArH, 4H); 13C-NMR (DMSO-d6): δ (ppm) 20.3 (CH3), 28.7 (CH), 103.6 (ArC), 125.2, 126.1, 152.3.
Octa-O-tert-butyl carbonated C-methylcalix[4]resorcinarene (fully t-Boc C-4-R)
To a solution of 0.55 g (1.0 mol) of C-4-R and 0.012 g (0.1 mmol) of DMAP in 5 mL of acetone, 2.2 mL (9.6 mmol) of di-tert-butyl dicarbonate was added dropwise at room temperature. A vigorous bubbling occurred immediately. The reaction mixture was stirred for 20 min at room temperature and the solvent was evaporated. The obtained white solid was recrystallized from propan-2-ol–acetone (90∶10 in volume ratio). Yield: 1.0 g (75%).IR: ν 1758 cm−1
(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (CDCl3): δ
(ppm) 1.42 (d, CH3, 12H), 1.46 (s, CH3, 72H), 4.43 (q, CH, 4H), 6.89 (br, ArH, 8H); 13C-NMR (CDCl3): δ
(ppm) 20.5, 27.7 (CH3) 31.4 (CH), 82.8 (tert-C), 116.3 (ArC), 125.7, 134.0, 147.0, 151.5 (C
O). 1H-NMR (CDCl3): δ
(ppm) 1.42 (d, CH3, 12H), 1.46 (s, CH3, 72H), 4.43 (q, CH, 4H), 6.89 (br, ArH, 8H); 13C-NMR (CDCl3): δ
(ppm) 20.5, 27.7 (CH3) 31.4 (CH), 82.8 (tert-C), 116.3 (ArC), 125.7, 134.0, 147.0, 151.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal.Calcd. for C72H96O24: C, 64.27; H, 7.19. Found: C, 63.56; H, 7.00%.
O). Anal.Calcd. for C72H96O24: C, 64.27; H, 7.19. Found: C, 63.56; H, 7.00%.
Average 60 mol% t-Boc C-4-R
To a solution of 2.7 g (5.0 mmol) of C-4-R and 0.06 g (0.5 mmol) of DMAP in 15 mL of acetone, 8 mL (36.0 mmol) of di-tert-butyl dicarbonate was added dropwise at room temperature. A vigorous bubbling occurred immediately. The reaction mixture was stirred for 20 min at room temperature and the solvent was evaporated. Purification was performed by flash column chromatography (hexane–ethyl acetate = 1∶1 in volume ratio). The product was obtained as a white powder. Yield: 6.3 g (99%). IR: ν 1758 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (CDCl3); δ
(ppm) 1.40–1.42 (m, CH3, 12H), 1.46–1.62 (m, CH3, 43.2H), 4.10–4.60 (m, CH, 4H), 6.02–7.42 (m, ArH, 8H).
O). 1H-NMR (CDCl3); δ
(ppm) 1.40–1.42 (m, CH3, 12H), 1.46–1.62 (m, CH3, 43.2H), 4.10–4.60 (m, CH, 4H), 6.02–7.42 (m, ArH, 8H).Dissolution rate
t-Boc C-4-Rs with various protecting ratios (95 wt%) and DIAS (5 wt%) were dissolved in cyclohexanone. The solution was spin-coated onto a silicon wafer that was pre-treated with 1,1,1,3,3,3-hexamethyldisilazane (HMDS) and exposed to 365 nm UV-light, followed by heating at 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 90 s. The sample was developed in a 2.38 wt% aqueous TMAH solution at room temperature, and rinsed with water. The changes in the film thickness against exposure energy were measured by a Dectak3 system (Vecco Instruments Inc.).
°C for 90 s. The sample was developed in a 2.38 wt% aqueous TMAH solution at room temperature, and rinsed with water. The changes in the film thickness against exposure energy were measured by a Dectak3 system (Vecco Instruments Inc.).Lithographic evaluation
Average 60 mol% protected t-Boc C-4-R and DIAS were dissolved at 20 wt% in cyclohexanone at room temperature. Films spin-cast on silicon wafers pre-treated with HMDS were pre-baked at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 3 min and exposed through a filtered super-high-pressure mercury lamp (Ushio USH-200DP). The exposed wafer was baked again and developed by dipping in a 2.38 wt% aqueous TMAH solution. Imagewise exposure through a mask was carried out in a contact-printing mode.
°C for 3 min and exposed through a filtered super-high-pressure mercury lamp (Ushio USH-200DP). The exposed wafer was baked again and developed by dipping in a 2.38 wt% aqueous TMAH solution. Imagewise exposure through a mask was carried out in a contact-printing mode.Measurements
FT-IR spectra were measured on a Horiba FT-720 spectrophotometer. 1H and 13C NMR spectra were recorded on a BRUKER DPX-300 spectrometer. Thermal analyses were performed on a Seiko SSS 5000 TG-DTA 220 thermal analyzer at a heating rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 for thermogravimetry (TG) and 5
°C min−1 for thermogravimetry (TG) and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 for differential scanning calorimetry (DSC) under a nitrogen atmosphere, respectively. The temperature on a silicon wafer was measured by Anritsu HFT-40 digital surface thermometer. To examine surface properties of t-Boc C-4-Rs obtained, the contact angles of water and diiodomethane were measured by placing drops of distilled water (H2O) and diiodomethane (CH2I2) on the prepared t-Boc C-4-R films using a microscope goniometer (Kyowa Interface Science CA-A) at 25
°C min−1 for differential scanning calorimetry (DSC) under a nitrogen atmosphere, respectively. The temperature on a silicon wafer was measured by Anritsu HFT-40 digital surface thermometer. To examine surface properties of t-Boc C-4-Rs obtained, the contact angles of water and diiodomethane were measured by placing drops of distilled water (H2O) and diiodomethane (CH2I2) on the prepared t-Boc C-4-R films using a microscope goniometer (Kyowa Interface Science CA-A) at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The scanning electron
micrograph image was taken by a Hitachi S-800 microscope.
°C. The scanning electron
micrograph image was taken by a Hitachi S-800 microscope.Results and discussion
C-4-R was prepared by condensation of resorcinol and acetaldehyde in aqueous hydrochloric acid at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and was recrystallized from methanol (Scheme 1). C-4-R can in principle exist in the four possible configurations formulated cis-cis-cis, cis-cis-trans, cis-trans-trans, and trans-cis-trans. The obtained C-4-R was confirmed to have an all-cis configuration by a 1H NMR spectrum on the basis of the previous report.9
°C and was recrystallized from methanol (Scheme 1). C-4-R can in principle exist in the four possible configurations formulated cis-cis-cis, cis-cis-trans, cis-trans-trans, and trans-cis-trans. The obtained C-4-R was confirmed to have an all-cis configuration by a 1H NMR spectrum on the basis of the previous report.9 | ||
| Scheme 1 | ||
t-Boc C-4-Rs with various protecting ratios were synthesized by reaction of C-4-R with di-tert-butyl dicarbonate in the presence of DMAP in acetone. t-Boc C-4-Rs were characterized by IR and 1H NMR spectroscopies. The IR spectra showed characteristic absorptions at 3450 and 1758 cm−1 due to OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretchings, respectively. The 1H NMR spectrum for fully t-Boc C-4-R exhibited a single peak at 1.46 ppm due to characteristic tert-butyl protons. On the other hand, complex peaks were observed at 1.46–1.62 ppm for t-Boc C-4-Rs because of a mixture of several compounds.
O stretchings, respectively. The 1H NMR spectrum for fully t-Boc C-4-R exhibited a single peak at 1.46 ppm due to characteristic tert-butyl protons. On the other hand, complex peaks were observed at 1.46–1.62 ppm for t-Boc C-4-Rs because of a mixture of several compounds.
The actual protecting ratio of partially t-Boc C-4-Rs were estimated by TG (Fig. 1). The results are summarized in Table 1. The tert-butyl carbonate releases a quantitative amount of carbon monoxide and isobutylene at about 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C upon heating at 5
°C upon heating at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1. The observed weight-losses are in good agreement with the calculated ones.
°C min−1. The observed weight-losses are in good agreement with the calculated ones.
 | ||
| Fig. 1 TG curves of t-Boc C-4-R having various protecting ratios. | ||
| t-Boc (mol%) | 50 | 60 | 70 | 90 | 100 | |
|---|---|---|---|---|---|---|
| Weight loss (%) | Calcd. | 42.7 | 47.3 | 51.1 | 57.8 | 59.9 |
| Found | 43 | 48 | 51 | 57 | 59 |
t-Boc C-4-Rs showed good solubility in organic solvents such as propylene glycol methyl ether acetate (PGMEA), ethyl lactate, cyclohexanone, and 2-methoxyethanol at room temperature.
The effects of protecting ratios on the dissolution rate to a 2.38 wt% aqueous TMAH solution and the adhesion to a silicon wafer were investigated (Fig. 2). The dissolution rate was estimated by measuring the film thickness after development, and the adhesion properties of polymers on the silicon substrate were evaluated by calculating work of adhesion (Wad) on the basis of the following eqn. (1) which can be derived from Owens–Wendt's equation.10
| Wad = 2{(γPd·γSd)1/2 + (γPh·γSh)1/2} | (1) |
| cosθ·γL = 2{(γPorSd·γLd)1/2 + (γPorSh·γLh)1/2} | (2) |
 | ||
| Fig. 2 Effect of protection ratios on the dissolution rate to a 2.38 wt% aqueous TMAH solution and the adhesion to a silicon wafer. | ||
| Protecting ratio/mol% | Contact angle/° | Wad/dyne cm−1 | |
|---|---|---|---|
| H2O | CH2I2 | ||
| 50 | 34.0 | 50.0 | 70.3 |
| 60 | 38.0 | 46.0 | 65.5 |
| 70 | 92.0 | 41.0 | 60.8 |
| 90 | 102.4 | 31.6 | 47.0 |
The difference of dissolution rate between the exposed and unexposed areas increased with increasing the ratio of protection since the polarity between exposed and unexposed area changes greatly. On the other hand, the adhesion property decreased with increasing the protecting ratios; clearly, the hydrophilic surface properties of t-Boc C-4-Rs are governed by the content of phenol units. The 50 mol% protected one can be cast without priming HMDS, but in the cases of 70 mol% and 90 mol% protected ones, the films were peeled off during development.
From these data, the appropriate ratio of protection turned out to be an average of 60 mol% in view of adhesion to wafer and dissolution rate contrast.
DSC analysis of 60 mol% protected t-Boc C-4-R showed no peak due to the high glass transition temperature (Tg) up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, nearly deprotection temperature. This result indicates Tg is above 100
°C, nearly deprotection temperature. This result indicates Tg is above 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and this matrix has a good thermal property as a photoresist.
°C and this matrix has a good thermal property as a photoresist.
Lithographic evaluation
Based on these findings, we formulated a resist consisting of t-Boc C-4-R and DIAS (5 wt%) as a PAG in cyclohexanone. The UV spectrum of 1.0 µm thick t-Boc C-4-R film indicates that it is very transparent around 250 nm and above 300 nm, and this spectrum is very similar to that of poly(hydroxystyrene).To investigate the dissolution behavior of exposed and unexposed areas, the effects of the post-exposure bake (PEB) temperature and time on the dissolution rate were studied. The results in the case of the resist formulated by mixing average 60 mol%
t-Boc C-4-R
(95 wt%) and DIAS (5 wt%) in cyclohexanone are shown in Fig. 3 and 4, respectively, where the film was exposed to 365 nm UV-light of 30 mJ cm−2 intensity, postbaked at various temperatures for various times and developed with a 2.38 wt% aqueous TMAH solution. The difference of dissolution rate between exposed and unexposed areas increased rapidly at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 15 s, respectively. This indicates that deprotection of t-Boc groups occurs mainly at this temperature and time in the presence of acid.
°C and 15 s, respectively. This indicates that deprotection of t-Boc groups occurs mainly at this temperature and time in the presence of acid.
 | ||
| Fig. 3 Effect of PEB temperature on the dissolution rate of average 60 mol% t-Boc C-4-R film containing DIAS. Exposure 30 mJ cm−2 (365 nm); PEB time 3 min; developer 2.38 wt% aqueous TMAH solution. | ||
 | ||
Fig. 4 Effect of PEB time on the dissolution rate of average 60 mol%
t-Boc C-4-R film containing DIAS. Exposure 30 mJ cm−2
(365 nm); PEB temperature 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C; developer 2.38 wt% aqueous TMAH solution. °C; developer 2.38 wt% aqueous TMAH solution. | ||
After these preliminary optimization studies involving PEB temperature and time, adhesion, and dissolution rate contrast, we formulated a resist system consisting of average 60 mol%
t-Boc C-4-R
(95 wt%) and DIAS (5 wt%) in cyclohexanone. The film spin-cast on HMDS treated silicon wafer was prebaked at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 3 min (thickness of 1.8 µm), exposed to 365 nm UV irradiation, postbaked at 105
°C for 3 min (thickness of 1.8 µm), exposed to 365 nm UV irradiation, postbaked at 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 90 s, and developed in positive mode with the 2.38 wt% aqueous TMAH solution at room temperature. The sensitivity curve shown in Fig. 5 indicates that the sensitivity and contrast are 13 mJ cm−2 and 12.6, respectively. This good sensitivity and contrast is thought to be due to the ease of changing polarity in the whole molecule, stemming from the small size of the resist
molecule and good permeation of developer into the resist film.
°C for 90 s, and developed in positive mode with the 2.38 wt% aqueous TMAH solution at room temperature. The sensitivity curve shown in Fig. 5 indicates that the sensitivity and contrast are 13 mJ cm−2 and 12.6, respectively. This good sensitivity and contrast is thought to be due to the ease of changing polarity in the whole molecule, stemming from the small size of the resist
molecule and good permeation of developer into the resist film.
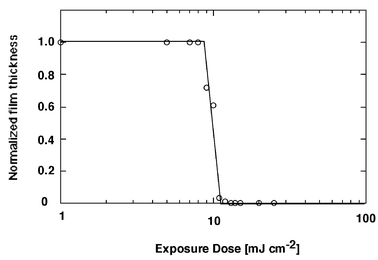 | ||
| Fig. 5 Exposure characteristic curves for average 60 mol% t-BocC-4-R film containing DIAS. | ||
Fig. 6 presents a scanning electron micrograph of the contact-printed image that was obtained using the resist described above after exposure to 20 mJ cm−2, post-exposure baked at 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 90 s, and developed with the 2.38 wt% aqueous TMAH solution. The clear positive pattern with 1.5 µm resolution, which is the limitation of our exposure system, was obtained.
°C for 90 s, and developed with the 2.38 wt% aqueous TMAH solution. The clear positive pattern with 1.5 µm resolution, which is the limitation of our exposure system, was obtained.
 | ||
| Fig. 6 Scanning electron micrograph of the contact-printed positive image obtained from the average 60 mol% t-Boc C-4-R film containing 5 wt% of DIAS. | ||
Conclusions
A positive working low-molecular-weight photoresist based on t-Boc C-4-R and DIAS as a PAG has been developed. t-Boc C-4-Rs with various protecting ratios were synthesized and evaluated as a chemically amplified resist. The appropriate t-Boc protecting ratio was an average of 60 mol% in view of adhesion and dissolution rate contrast. The photoresist consisting of average 60 mol% t-Boc C-4-R (95 wt%), and DIAS (5 wt%) showed a sensitivity of 13 mJ cm−2 and a contrast of 12.6 when it was exposed to 365 nm light and postbaked at 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 90 s, followed by developing with the 2.38 wt% aqueous TMAH solution at room temperature.
°C for 90 s, followed by developing with the 2.38 wt% aqueous TMAH solution at room temperature.References
- D. C. Trully, K. Wilder, J. M. J. Fréchet, A. R. Trimble and C. F. Quate, Adv. Mater., 1999, 11, 31 CrossRef; D. C. Trully, A. R. Trimble, J. M. J. Fréchet, K. Wilder and C. F. Quote, Chem. Mater., 1999, 11, 2892 CrossRef CAS.
- J. M. J. Fréchet and C. G. Willson, Proc. Microcircuit Eng., 1982, 260 Search PubMed; J. M. J. Fréchet, E. Eichler, H. Ito and C. G. Willson, Polymer, 1983, 24, 995 CrossRef CAS.
- G. G. Barclay, M. A. King, Z. Mao, C. Szmanda, D. Benoit, E. Malmstrom, H. Ito and C. J. Hawker, Polym. Prepr., 1999, 40, 448 Search PubMed; H. Y. Shih and A. Reiser, Macromolecules, 1996, 29, 2082 CrossRef CAS.
- P. C. Tsiartas, L. W. Flanagin, C. L. Henderson, W. D. Hinsberg, I. C. Sanchez, R. T. Bonnecaze and C. G. Willson, Macromolecules, 1997, 30, 4656 CrossRef CAS; L. W. Flanagin, V. K. Singh and C. G. Willson, J. Vac. Sci. Technol. B, 1999, 17, 1371 CrossRef CAS; G. G. Barclay, C. J. Hawker, H. Ito, A. Orellana, P. R. L. Malenfant and R. F. Sinta, Macromolecules, 1998, 31, 1024 CrossRef CAS; L. W. Flanagin, V. K. Singh and C. G. Willson, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 213 Search PubMed.
- M. Ueda, D. Takahashi, T. Nakayama and O. Haba, Chem. Mater., 1998, 10, 2230 CrossRef CAS; T. Fujigaya and M. Ueda, J. Photopolym. Sci. Technol., 2000, 13, 339 Search PubMed; T. Nakayama and M. Ueda, J. Mater. Chem., 1999, 9, 697 RSC; T. Nakayama, K. Haga, O. Haba and M. Ueda, Chem. Lett., 1997, 265; T. Nakayama, M. Nomura, K. Haga and M. Ueda, Bull. Chem. Soc. Jpn., 1998, 71, 2979 CAS; O. Haba, K. Haga and M. Ueda, Chem. Mater., 1999, 11, 427 CrossRef CAS.
- K. Takeshi, R. Nakayama and M. Ueda, Chem. Lett., 1998, 865 CrossRef CAS.
- T. Kumada, S. Kubota, H. Koezuka, T. Hanawa, S. Kishimura and H. Nagata, J. Photopolym. Sci. Technol., 1991, 4, 469 Search PubMed; Y. Kawai, A. Tanaka and T. Matsuda, Jpn. J. Appl. Phys., 1992, 31, 4316 CAS; F. Houlihan, F. Bouchard, J. M. J. Fréchet and C. G. Willson, Can. J. Chem., 1985, 63, 153 CAS.
- K. Nitoh, T. Yamaoka and A. Umehara, Chem. Lett., 1991, 1869.
- L. M. Tunstad, J. A. Tucker, E. Dalcanale, J. Weiser, J. A. Bryant, J. C. Sherman, R. C. Helgeson, C. B. Knobler and D. J. Cram, J. Org. Chem., 1989, 54, 1305 CrossRef CAS.
- D. K. Owens and R. C. Wendt, J. Appl. Polym. Sci., 1969, 13, 1741 CrossRef CAS; J. B. Kim, J. Y. Kim and M. H. Jung, Polym. Commun., 1998, 40, 273 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
