Bipolar carrier transport in a lamello-columnar mesophase of a sanidic liquid crystal
Stéphane
Méry
*a,
Denis
Haristoy
a,
Jean-François
Nicoud
a,
Daniel
Guillon
a,
Siegmar
Diele
b,
Hirosato
Monobe
c and
Yo
Shimizu
c
aInstitut de Physique et Chimie des Matériaux de Strasbourg, 23 rue du Lœss, F-67037, Strasbourg, France. Tel: +33 3 88 10 71 65; Fax: +33 3 88 10 72 46; E-mail: mery@ipcms.u-strasbg.fr
bMartin-Luther-Universität, Halle-Wittenberg, Mühlpforte 1, D-06114, Halle/S, Germany
cMesophase Technology Research Group, Special Division of Human Life Technology, National Institute of Advanced Industrial Science and Technology, Midorigaoka 1-8-31, Ikeda, Osaka 563-8577, Japan
First published on 14th November 2001
Abstract
The lamello-columnar mesophase of a [1]benzothieno[3,2-b][1]benzothiophene-2,7-dicarboxylate (BTBT) derivative has been investigated by means of X-rays on an aligned sample. The most probable model of molecular organisation corresponds to an assembly of microcolumns made of alternatively crossed molecules, within the smectic layers. The preliminary investigation by a time-of-flight technique revealed that the mesophase exhibits a bipolar charge transport, with comparable electron and hole mobility values in the order of 2 × 10−3 cm2 V−1 s−1. The high mobility values obtained for this disordered smectic phase can be explained by the improved π-overlap between sandwich-like packed aromatic moieties in the short-range columnar stackings.
Introduction
Liquid crystals are unique materials for studying the effect of molecular order on charge transport properties. Firstly, a microsegregation phenomenon1 which takes place in mesophases is able to lead to a favourable long range π-overlap between the electronically active transport units. Secondly, liquid-like fluctuations2 present in the mesophases act as a “self-repairing” mechanism of the structural defects leading to a reduction of deep traps.3 Finally, the control ability of the macroscopic alignment of the mesophase gives the opportunity to investigate samples of large dimensions4,5 to study the anisotropic conduction. All these characteristics make photoconducting liquid crystals of high interest for fundamental purposes as well as for potential applications in electronic devices.So far, carrier transport properties have mainly been studied in mesophases exhibited by discotic (disk-shaped) and calamitic (rod-shaped) liquid crystals. In columnar mesophases of discotics, the π-stacking of the disk-shaped molecules leads to a molecular self-organisation into columns. Consequently, the electronic charge transport proceeds essentially in the direction of the column long-axes.6 According to the size of the central aromatic part of the molecules and the degree of order in the disk stacking, extremely high values of charge-carrier mobility (up to 1 cm2 V−1 s−1) have recently been attained.7–10 Similarly, in lamellar (smectic) mesophases of calamitics, a stepwise increase of the charge-carrier mobility values was found with upgraded molecular order within the smectic layers.11–15
We have recently reported the synthesis and the preliminary results of the mesomorphic and photoconduction properties of a series of liquid crystals.16 These materials were made of a [1]benzothieno[3,2-b][1]benzothiophene-2,7-dicarboxylate (BTBT) core substituted at both extremities by chains of different nature. A representative of this series with Z-decenyl chains is given in Fig. 1. Because of the flatness of the central aromatic part, the mesogens are regarded as sanidics17
(lath-shaped) rather than usual calamitics. As a result, the compounds were found to exhibit a lamello-columnar mesophase. The local columnar order was deduced by X-ray diffraction analyses from the presence of distances at about 3.5 Å, which are characteristics of intermolecular separation of π-stacking of aromatic cores in columnar
mesophases. The difference of the layer spacing (31.5 Å at 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) and the length of the rod-like molecule in the fully extended shape (37.3 Å) pointed to a tilted alignment of the molecules (or part of the molecules) with respect to the layer normal, despite the optically uniaxial nature of the mesophase (SmA type). From these observations, a model of molecular organisation was initially proposed in which the molecules were crossed and locally packed into microcolumns within the smectic layers.
°C) and the length of the rod-like molecule in the fully extended shape (37.3 Å) pointed to a tilted alignment of the molecules (or part of the molecules) with respect to the layer normal, despite the optically uniaxial nature of the mesophase (SmA type). From these observations, a model of molecular organisation was initially proposed in which the molecules were crossed and locally packed into microcolumns within the smectic layers.
![Chemical structure and transition temperatures of the BTBT derivative di(Z-dec-4-enyl) [1]benzothieno[3,2-b]benzothiophene-2,7-dicarboxylate.](/image/article/2002/JM/b104289k/b104289k-f1.gif) | ||
| Fig. 1 Chemical structure and transition temperatures of the BTBT derivative di(Z-dec-4-enyl) [1]benzothieno[3,2-b]benzothiophene-2,7-dicarboxylate. | ||
The aim of the present paper is firstly to discuss complementary data obtained by X-ray analyses on aligned samples and the structure of the mesophase. The second and main purpose is to report the first results of charge-carrier mobility measurements recorded in these sanidic BTBT liquid crystals. Finally, the last part of the paper is aimed at discussing results on the redox properties of the molecules in solution in an attempt to correlate these with the photoconductive behaviour of the materials. Unless specified, all results presented here have been obtained with the BTBT compound shown in Fig. 1.
Results and discussion
Molecular organisation in the lamello-columnar mesophase
Representative results obtained by X-ray diffraction on the aligned mesophase of the BTBT derivative are given in Fig. 2. In the small-angle region of the pattern presented in Fig. 2(a), one observes the presence of intense Bragg spots located on the meridian, which proves that a well-oriented monodomain could be obtained. The equidistant position of these spots confirms the lamellar structure of the mesophase. In the wide-angle region of the pattern shown in Fig 2(a), three scattering diffuse halos (I, II and III) are observable which correspond to repetitive distances (ca. 3.5, 4.7 and ∼8 Å) taking place at short range. For the two more distinguishable halos I and II, the intensity profiles of diffraction signals as a function of χ angle are given respectively in Figs. 2(b) and 2(c) in order to discuss the origin of the corresponding interactions.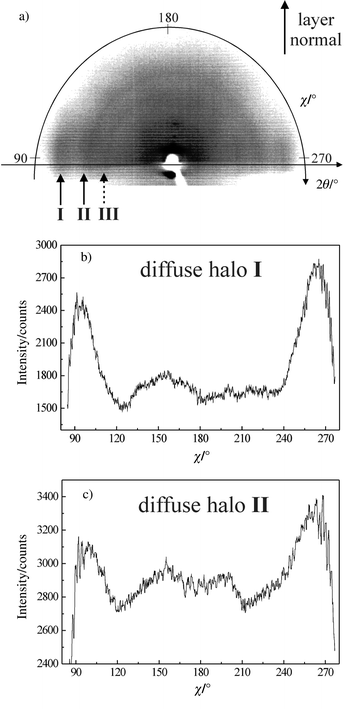 | ||
Fig. 2
X-Ray pattern of an oriented sample of the Z-decenyl BTBT derivative recorded in the mesophase at 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (a). χ-intensity profiles of the wide-angle diffuse halos I
(b) and II
(c), corresponding to distances of ca. 3.5 and 4.7 Å, respectively. °C (a). χ-intensity profiles of the wide-angle diffuse halos I
(b) and II
(c), corresponding to distances of ca. 3.5 and 4.7 Å, respectively.
| ||
Halo I gives evidence of a close stacking of the aromatic cores at a distance of 3.5 Å where π-orbitals overlap. Such interactions, characteristic of the presence of a columnar order, are possible in BTBT mesogens because of their central lath-shaped aromatic moieties. It has to be mentioned that these interactions completely and reversibly disappear in the isotropic state. The presence of stronger interactions than the usual van der Waals ones is evidenced by the value of the enthalpy ΔH of 4.3 kJ mol−1 observed at the clearing temperature, when a regular transition from a disordered smectic to isotropic shows typically a ΔH value of about 1 kJ mol−1. Examination of the χ intensity profile on Fig. 2(b) shows that two maxima are observed for χ values centred at about 90 and 270°. The equatorial location of halo I indicates that the columnar order is localised within the smectic layers. Moreover, the width of halo I demonstrates this columnar order is of short range. From the width at half maximum, it has been possible to calculate that the corresponding microcolumns were composed of about ten molecules.
Halo II corresponds to a repetitive distance of about 4.7 Å, which represents the typical average distance between molten paraffinic chains. As for halo I, the χ intensity profile shown in Fig. 2(c) gives an equatorial position of the scattering, implying that the chains are positioned, on average, parallel to the layer normal. It is worth mentioning the strongly disordered character of the chains, as illustrated from the wide angular distribution of halo II.
Careful examination of the pattern presented in Fig. 2(a) reveals the presence of an additional diffuse halo III on the equator. It indicates an additional period in the short range order perpendicular to the layer normal. The distance of about 8 Å is nearly twice that of the average distance of the single molecules and should most probably be related to the lateral distance between “sandwich-like packed” molecules (see the discussion below).
As previously mentioned, the direct comparison between layer spacings and molecular length points to a tilting of the molecules within the smectic layers. The calculation of the core tilt angle (see experimental section) did show that the rigid aromatic moiety of the Z-decenyl BTBT derivative was highly tilted, with a core tilt angle value of 40° ± 5° with respect to the layer normal. Note that the angle value was found to decrease with the shortening of the paraffinic chain length, to reach down to a value of about 22° for instance, in the case of a terminal ethyl chain.
From all the results presented above, it is possible to envisage different molecular organisations depending on whether the stacks are made of alternatively crossed molecules or molecules that are co-parallel facing each other, as illustrated in Figs. 3(a) and (b), respectively. Note that in both packing modes, contact between sulfur atoms in adjacent molecules are allowed to occur. This type of intermolecular S–S contact is known to give rise to strong cohesive interactions, and is usually observed in flat, π-extended thioaromatic systems such as in TTF derivatives.18–20
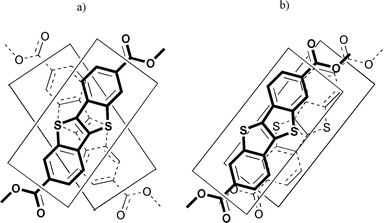 | ||
| Fig. 3 Core stacking in the column formation, when the molecules are (a) crossed over the top of each other, or (b) stacked in a parallel manner. The cores schematized in (b) have been drawn slightly shifted from each others for clarity. | ||
To begin with co-parallel packing (Fig. 3(b)), a uniaxial mesophase can be obtained if there is an alternation of the tilt direction in successive layers (i.e. as in anticlinic-like SmC phase)21,22 or else, if there is no long range correlation of the tilt direction in successive layers and within the layers (i.e. as in de Vries-like SmA phase).23 Such organisations, however, are incompatible with the oriented X-ray pattern obtained. A splitting of the wide-angle scattering (corresponding to π-stacking at ca. 3.5 Å) should be observed out of the equator, which is not found experimentally (see halo I in Figs. 2(a) and (b)). Steric arguments are not in favour of co-parallel stacking either. Actually, it requires to superpose on one hand the flat aromatic cores via π–π interactions (at ca. 3.5 Å) and on the other hand, the more spatially demanding paraffinic chains (at ca. 4.7 Å). Such a steric constraint is absent when the stacks are made of alternatively crossed molecules (compare Figs. 3(a) and (b)).
The crossed molecular stacking corresponds to the model initially proposed.16 In this sandwich-like packing model, the building unit may be approximated by a toast-like body of paired molecules, as shown in Fig. 4. These “toasts” form small blocks within the layers, each block has the same direction of the short axes of the “toasts”, but from block to block the short axes are statistically distributed. This model remains in perfect agreement with the complementary results obtained by X-ray analyses on aligned samples. Regarding the case of crossed molecular stacking, one may add the following comment. From the visualisation of the packing of two optimised molecules (MOPAC-6/AM1), we noticed that the maximum face-to-face S–S distance is obtained when the molecular long-axes between two crossed molecules are rotated from about 66°. Experimentally, the rotation angle between two directions of molecular orientations (equal to twice the core tilt angle) was found to vary in the range 44 to 80°, depending of the chain length. This observation shows that the sulfur atoms are not perfectly sitting on top of each other, as is most commonly found in stacks of TTF derivatives.20
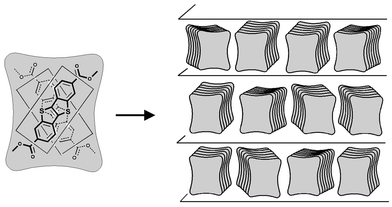 | ||
| Fig. 4 Sandwich-like arrangement of crossed molecules within the smectic layers represented as an assembly of paired molecule subunits. | ||
To conclude, it has to be noted that a similarly oriented X-ray pattern has recently been observed in a mixed system of a metallomesogen and 2,4,7-trinitrofluorenone, for which it was concluded that the mesophase was a biaxial SmA phase.24 This assignment was made from the presence of a schlieren texture observable under a polarising microscope, that is different from the case investigated in the present study.
Charge transport property
Time-of-flight technique was used to investigate the charge transport mobility of the material. For this study, transient photocurrent measurements have been performed at different temperatures from the isotropic to the crystalline phase.In the isotropic, non-dispersive charge transports have been observed, which were characterised by long transit times. The corresponding mobility values were low and were found to decrease with temperature from 5 × 10−5 cm2 V−1 s−1 at 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C to about 10−6 cm2 V−1 s−1 at the transition to the mesophase. According to Walden's rule,25,26 such low mobility values in a fluid medium can easily be attributed to ionic transport. The temperature dependence of the mobility also strongly supports this assumption. It has to be mentioned that both positive and negative charge transport were observed and that the values of the mobility were found to be similar in both cases. The presence of ions might possibly be charged BTBT molecules, or ionic species coming from
the ITO electrodes.
°C to about 10−6 cm2 V−1 s−1 at the transition to the mesophase. According to Walden's rule,25,26 such low mobility values in a fluid medium can easily be attributed to ionic transport. The temperature dependence of the mobility also strongly supports this assumption. It has to be mentioned that both positive and negative charge transport were observed and that the values of the mobility were found to be similar in both cases. The presence of ions might possibly be charged BTBT molecules, or ionic species coming from
the ITO electrodes.
The transition to the mesophase has been made throughout a very slow cooling rate from the isotrope. Observed by polarising optical microscopy, the sample exhibited a typical fan-shaped texture, with a polydomain structure. The size of the polydomain texture was larger than 50 µm in width. According to the recent works by Hanna et al.,14,27,28 domain boundaries in liquid crystalline systems essentially do not affect the charge migration efficiency when the size of the domain structure is larger than the cell thickness, and it is proposed to be a characteristic property of charge transportation in liquid crystals.
The analysis of the transient photocurrents in the mesophase revealed the presence of two ranges of transit times corresponding to mobility values in the order of about 2 × 10−3 and 10−6–10−7 cm2 V−1 s−1. More unexpectedly, very similar transient photocurrent traces are observed both for positive and negative charges, as can be seen in Fig. 5. The lower range of mobility values is attributed to ionic transport while the other range of values is too high to be owing to anything other than to an electronic process.25,26 These results thus clearly show the presence in the mesophase of both types of electronic charge-carriers, i.e. electrons and holes, along with the presence of both negative and positive ions. Careful examination of traces in Fig. 5 shows that higher relative photocurrent intensity is obtained for positive carriers, which confirms a previous observation that holes are in the majority.16 It has also to be mentioned that both electrons and holes have comparable mobilities.
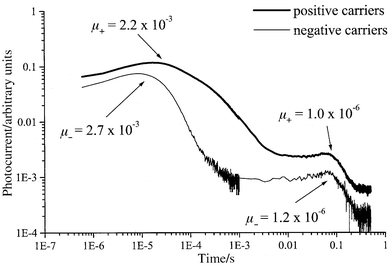 | ||
Fig. 5
Double logarithmic plots of transient photocurrent as a function of time recorded in the mesophase at 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C under an applied electric field of 3 × 104 V cm−1. Top trace (thick): positive carriers; bottom trace (thin): negative carriers. The calculated mobility values for positive (μ+) and negative (μ−) carriers are reported without the units (cm2 V−1 s−1) for clarity. °C under an applied electric field of 3 × 104 V cm−1. Top trace (thick): positive carriers; bottom trace (thin): negative carriers. The calculated mobility values for positive (μ+) and negative (μ−) carriers are reported without the units (cm2 V−1 s−1) for clarity.
| ||
By further cooling down the temperature throughout the supercooled mesophase, similar transient photocurrent traces are observed until the crystallisation is reached (e.g. 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C). In the crystalline state, no carrier mobility could be determined due to the transit photocurrents that drop off dispersely to very small values that are not measurable. This result shows that no significant charge transport is observable in the crystal most probably because of the presence of a polydomain structure with many structural defects at boundaries of the domains of microscopic size.
°C). In the crystalline state, no carrier mobility could be determined due to the transit photocurrents that drop off dispersely to very small values that are not measurable. This result shows that no significant charge transport is observable in the crystal most probably because of the presence of a polydomain structure with many structural defects at boundaries of the domains of microscopic size.
The values of the mobilities as a function of temperature are given in Fig. 6. This graph shows the temperature dependence of the two ranges of mobility from the isotrope to the crystalline phase. It also clearly shows that similar values are systematically obtained both for positive and negative carriers. The lowest mobility range is found to decrease from the isotrope to the low temperature supercooled mesophase. This decrease of mobility with increasing viscosity is in perfect agreement with ionic transport. Note that a change of slope is observable at the isotrope–mesophase transition temperature for both carriers. The highest mobility range (about 2 × 10−3 cm2 V−1 s−1) is present only in the mesophase and is independent of temperature. The latter behaviour is usually given as a characteristic of the presence of an electronic transport process in the mesophase. It has to be mentioned that the mobility values in the order of 2 to 3 × 10−3 cm2 V−1 s−1 are high when compared to previously reported values for usual disordered smectic phases (i.e. smectic A or C phase).11,14,26,28 This effect most probably arises from the increased contact time between sandwich-like packed aromatic moieties in the columnar stackings. Actually, the presence of columnar stacking is known to considerably enhance the charge transport properties, as demonstrated by recent reports on discotic coronene derivatives8 and in sanidic perylene derivatives.15
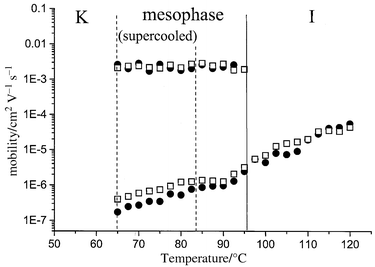 | ||
| Fig. 6 Mobility of positive (●) and negative carriers (□) as a function of temperature. The upper trace corresponds to electronic charge transport (electrons and holes), and the lower trace to ionic transport (anions and cations). | ||
To conclude with charge transport property, it is worth pointing out that the presence of both hole and electron transport with similar mobilities is not so common, although it has been recently reported in a few other liquid crystals (in lamellar mesophases of phenylnaphthalene28 and terthiophene14 derivatives and in a columnar mesophase of a porphyrin-based compound29). Such ambipolar carrier transport however, remains unusual in the most general case of single component organic materials. In principle, both photogeneration and transport properties imply redox processes and therefore should be related to the molecular electronic property of the material. Actually, it is reported in the literature that a high electron affinity EA (or LUMO) is required for electron transport and inversely, a low first ionisation potential Ip (or HOMO) is favourable for hole transport.30-33 Analysed by cyclic voltammetry, the BTBT compound led to a value of EA = 2.73 eV, which can be considered as a high value when compared to data on efficient electron transporting materials.34,35 The value of Ip however, was not determined by cyclic voltammetry because of the lack of an oxidation peak in the potential window investigated, but was estimated from the onset of the HOMO–LUMO optical bandgap (≈3.22 eV). The value of Ip thus obtained (Ip ≈ 6.05 eV) appears as an unfavourable high value on looking at other values corresponding to known efficient hole transporting materials.35,36 This result clearly indicates that a direct comparison between ionization potentials (calculated from electrochemical measurements in solution) and charge tranport property appears not so obvious and should therefore be taken with much precaution. In fact, the presence of a bipolar charge transport might simply and mainly be related to the absence of deep traps in the mesophase of BTBT mesogens.37 Similarly, the high mobility values obtained should rather be explained from this absence of deep traps and more importantly, from the improved molecular organisation of the BTBT mesogens in their lamello-columnar mesophase.
Conclusion
The presence of a lamello-columnar mesophase was confirmed in a [1]benzothieno[3,2-b][1]benzothiophene-2,7-dicarboxylate (BTBT) liquid crystal derivative by means of X-ray experiments on aligned samples. It was deduced that the column formation should be induced by π-stacking of the lath-shaped aromatic moieties and reinforced by sulfur–sulfur interactions. The proposed molecular organisations correspond to small packs made of alternatively crossed molecules within the smectic layers.BTBT was also subjected to a preliminary investigation by time-of-flight experiments which revealed the presence of both electron and hole transport with comparable mobility. The mobility values were found in the order of 2 × 10−3 cm2 V−1 s−1 in the mesophase. The high mobility values observed, despite its disordered character, should be related to the presence of the columnar stacking which increases the contact time between flat aromatic moieties.
In summary, the results obtained in this present work indicate that lath-shaped liquid crystals represent a promising class of materials for photoconduction because they combine the advantages of both the columnar and lamellar systems. Charge transport properties are considerably enhanced by columnar stacking and macroscopic alignment is in general more easily achievable in lamellar mesophases. Investigation of transport properties of other lath-like compounds, macroscopically aligned, are currently under progress.
Experimental
Materials
BTBT derivatives have been synthesised and the purity was characterised as previously described.16 Particular care was taken in the purification steps to minimise the presence of ionic impurities responsible for dark ionic current. The materials were thus recrystallised several times from high-quality grade filtered (0.5 µm) hexane prior to use.Structure investigation
X-Ray diffraction experiments on aligned mesophases were performed by means of a simple preparation technique currently used by G. Pelzl and co-workers at the University of Halle, Germany. A small drop of the sample was heated on a cleaned glass plate up to the isotropic state and cooled down slowly to the temperature of investigation. In this way, a well-aligned monodomain of the BTBT derivative could be obtained by surface interaction. The incident X-ray beam was parallel to the glass plate and the scattered intensity was collected on a two-dimensional detector (HI-STAR, Siemens AG, Germany). The core tilt angle of the mesogens within the smectic layers was calculated from knowing the end-to-end core length, the molecular volumes and the layer spacings.38 The core length was deduced from molecular modelling (MOPAC-6/AM1) using Insight II software from MSI, the molecular volumes were calculated from data of known compounds and the layer spacings was determined by X-ray diffraction.Charge-carrier mobility measurements
Carrier mobility was measured by a conventional time-of-flight method, in which the liquid crystal cell was irradiated by N2 pulse laser (337 nm, 800 ps in pulse width). The induced transient photocurrent was monitored by a digital oscilloscope after its amplification. No particular surface treatment of the ITO electrode surface of the cell was done in this study. The cell thickness was estimated to be 34.6 µm by ellipsometry. The applied voltage was ±90 V. The transient photocurrent curves were measured in the two time ranges of 1 ms and 500 ms, to observe two transportation processes in good time resolution. The measurement was divided into two time ranges due to the limitation of the sampling point of the oscilloscope.The authors gratefully acknowledge Drs B. Heinrich and B. Donnio for their help in X-ray experiments as well as Professor R. Ziessel and Dr A. Khatyr for their kind assistance in cyclic voltammetry measurements.
References
- A. Skoulios and D. Guillon, Mol. Cryst. Liq. Cryst., 1988, 165, 317 Search PubMed.
- C. Schmidt and H. W. Spiess, in Handbook of Liquid Crystals, Vol 1: Fundamentals, ed. D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, 1998, chapter VIII Search PubMed.
- I. G. Voigt-Martin, R. W. Garbella and M. Schumacher, Liq. Cryst., 1994, 17, 775 CAS.
- J. Cognard, Mol. Cryst. Liq. Cryst. Suppl. 1, 1982, 1 Search PubMed.
- B. Jerôme, Rep. Prog. Phys., 1991, 54, 391 CrossRef CAS.
- N. Boden, R. J. Bushby, J. Clements, B. Movaghar, K. J. Donovan and T. Kreouzis, Phys. Rev. B, 1995, 52, 13274 CrossRef CAS.
- J. Simmerer, B. Glüsen, W. Paulus, A. Kettner, P. Schumacher, D. Adam, K.-H. Etzbach, K. Siemensmeyer, J. H. Wendorff, H. Ringsdorf and D. Haarer, Adv. Mater., 1996, 8, 815 CrossRef CAS.
- A. M. van de Craats, J. M. Warman, A. Fechtenkötter, J. D. Brand, M. A. Harbison and K. Müllen, Adv. Mater., 1999, 11, 1469 CrossRef CAS.
- T. Kreouzis, K. Scott, K. J. Donovan, N. Boden, R. J. Bushby, O. R. Lozman and Q. Liu, Chem. Phys., 2000, 262, 489 CrossRef.
- A. M. van de Craats and J. M. Warman, Adv. Mater., 2001, 13, 130 CrossRef CAS.
- H. Tokuhisa, M. Era and T. Tsutsui, Adv. Mater., 1998, 10, 404 CrossRef CAS.
- M. Funahashi and J.-I. Hanna, Appl. Phys. Lett., 1997, 71, 602 CrossRef CAS.
- K. Kurotaki and J.-I. Hanna, J. Imag. Sci. Technol., 1999, 43, 237 Search PubMed.
- M. Funahashi and J-I. Hanna, Appl. Phys. Lett., 2000, 76, 2574 CrossRef CAS.
- C. W. Struijk, A. B. Sieval, J. E. J. Dakhorst, M. van Dijk, P. Kimbes, R. B. M. Koehorst, H. Donker, T. J. Schaafsma, S. J. Picken, A. M. van de Craats, J. M. Warman, H. Zuilhof and E. J. R. Sudhölter, J. Am. Chem. Soc., 2000, 122, 11057 CrossRef.
- D. Haristoy, S. Méry, B. Heinrich, L. Mager, J. F. Nicoud and D. Guillon, Liq. Cryst., 2000, 27, 321 CrossRef CAS.
- The term sanidic was first introduced in the group of H. Ringsdorf to name the mesophases made of board-like (macro)molecules stacked parallel on top each other: (a) M. Ebert, O. Herrmann-Schonherr, J. H. Wendorff, H. Ringsdorf and P. Tschirner, Liq. Cryst., 1990, 7, 63 CAS; (b) See also: I. G. Voigt-Martin, P. Simon, S. Bauer and H. Ringsdorf, Macromolecules, 1995, 28, 236 Search PubMed; (c) I. G. Voigt-Martin, P. Simon, D. Yan, A. Yakimansky, S. Bauer and H. Ringsdorf, Macromolecules, 1995, 28, 243 CrossRef CAS.
- C. Polycarpe, N. B. Chanh, M. Cottrait, J. Gaultier and Y. Haget, Mol. Cryst. Liq. Cryst., 1983, 101, 143 Search PubMed.
- M. Adam and K. Müllen, Adv. Mater., 1994, 6, 439 CrossRef.
- J. M. Williams, H. H. Wang, T. J. Emge, U. Geiser, M. A. Beno, P. C. W. Leung, K. D. Carlson, R. J. Thorn, A. J. Schultz and M.-H. Whangbo, Prog. Inorg. Chem., 1987, 35, 51 Search PubMed.
- Y. Galerne and L. Liebert, Phys. Rev. Lett., 1990, 64, 906 CrossRef CAS.
- J. W. Goodby, in Handbook of Liquid Crystals, Vol 2A: Low Molecular Weight Liquid Crystals I, ed. D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, 1998, p. 14 Search PubMed.
- A. de Vries, A. Ekachai and N. Spielberg, Mol. Cryst. Liq. Cryst. Lett., 1979, 49, 143 Search PubMed.
- T. Hegmann, J. Kain, S. Diele, G. Pelzl and C. Tchierske, Angew. Chem., Int. Ed., 2001, 40, 887 CrossRef CAS.
- P. W. Atkins, in Physical Chemistry 4th Ed., Oxford University press, Oxford, 1992, p 755 and 766 Search PubMed.
- M. Funahashi and J.-I. Hanna, Phys. Rev. Lett., 1997, 11, 2184 CrossRef CAS.
- H. Maeda, M. Funahashi and J.-I. Hanna, Mol. Cryst. Liq. Cryst., 2000, 346, 183 Search PubMed.
- M. Funahashi and J.-I. Hanna, Appl. Phys. Lett., 1998, 73, 3733 CrossRef CAS.
- Y. Yuan, B. A. Gregg and M. F. Lawrence, J. Mater. Res., 2000, 15, 2494 CAS.
- D. M. Pai, J. F. Yanus and M. Stolka, J. Phys. Chem., 1984, 88, 4714 CrossRef CAS.
- M. Stolka, J. F. Yanus and D. M. Pai, J. Phys. Chem., 1984, 88, 4707 CrossRef CAS.
- A. V. Vannikov, A. D. Grishina and S. V. Novikov, Russ. Chem. Rev., 1994, 63, 103 Search PubMed.
- J. L. Segura, Acta Polym., 1998, 49, 319 CrossRef CAS.
- R. Fink, C. Frenz, M. Thelakkat and H.-W. Schmidt, Macromolecules, 1997, 30, 8177 CrossRef CAS.
- Y. Shirota, J. Mater. Chem., 2000, 10, 1 RSC.
- F. Cacialli, R. H. Friend, N. Haylett, R. Daik, W. J. Feast, D. Santos and J. L. Brédas, Appl. Phys. Lett., 1996, 69, 3794 CrossRef CAS.
- L. Zuppiroli, Private communication.
- (a) D. Guillon and A. Skoulios, J. Phys. Lett., 1977, 38, 79 Search PubMed; (b) M. Ibn-Elhaj, A. Skoulios, D. Guillon, J. Newton, P. Hodge and H. J. Coles, J. Phys. II, (France), 1996, 6, 271 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
