A greener approach to cotton dyeings with excellent wash fastness
Richard S. Blackburn and Stephen M. Burkinshaw
Specialty Chemical Group, School of Textiles & Design, University of Leeds, UK LS2 9JT. E-mail: r.s.blackburn@leeds.ac.uk
First published on 1st February 2002
Abstract
Attempts were made to find a more environmentally friendly method of dyeing cotton as an alternative to standard reactive dyeing processes that require high levels of water, salt and alkali and produce high levels of effluent contamination. It was intended that the new method would not compromise the excellent wash fastness levels typical of reactive dyed cotton. By employing a pre-treatment method, salt and alkali could be completely eliminated from the dyeing process and, in comparison with standard reactive dyeing processes, the time taken for the dyeing process to be completed could be significantly reduced and the volume of water required could be halved. The dyeings secured using the pre-treatment method had wash fastness values equal to those observed for the standard reactive dyeings.
Green ContextA high proportion of textiles used in the world are made of cotton or other cellulose materials. Many textiles are dyed using reactive dyes because of their greater stability on washing. Unfortunately standard reactive dyeing procedures require high levels of water, salt and alkali which lead to very large volumes of effluent. Here it is shown how a fabric pre-treatment method can lead to complete elimination of salt and alkali, lower water volumes and reduced process times. Thus several green chemistry reductions (waste, energy, raw materials) are achieved in this very important area of industrial chemistry.JHC |
Introduction
Cotton and other cellulosics comprise over 40% of world textile consumption. Colour can be imparted to cellulosic fibres using several different classes of dye, with reactive dyes finding greatest use with over 50% of world consumption.1 Reactive dyes are desirable because of their excellent wash fastness, which arises from a covalent bond formed between dye and fibre.However, the reactive dyeing process can be expensive and time-consuming, needing significantly high salt and alkali concentrations. Electrolyte is needed in the dyeing process to overcome the long-range repulsion forces operating between slightly negatively charged fibre and negatively charged dye molecules (as a result of water-solubilising sulfonic acid groups). Without salt addition, adsorption of dye to fibre will not occur, and with dyes such as reactive dyes, which are very soluble in water, a high amount of salt is required in the dyeing process. The salt remains at the end of the dyeing process and is very difficult and expensive to remove from the effluent.
Alkali is required in reactive dyeing to achieve a pH of around 11 in order to generate sufficient cellulosate anions (cell–O−) for fixation; the neutral species (cell–OH) is not sufficiently nucleophilic for reaction between dye and fibre to occur. Alkali must be neutralised at the end of dyeing, the product of which is additional cost and an increased salt concentration in the effluent. In addition, up to 40% of the dyestuff may hydrolyse in the dyeing process. This hydrolysed dye is highly substantive to the fibre, but is unfixed and requires extensive wash-off in order to achieve the characteristic very high fastness to washing (Schemes 1 and 2). This wash-off and subsequent effluent treatment to remove the resultant colour pollution can account for up to 50% of the total cost of reactive dyeing.2
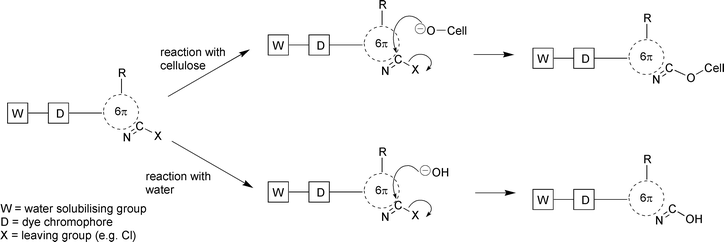 | ||
| Scheme 1 Reaction of reactive dyes via a nucleophilic substitution mechanism. | ||
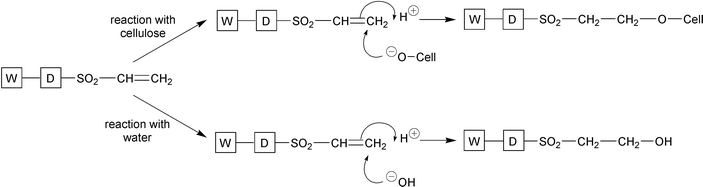 | ||
| Scheme 2 Reaction of reactive dyes via a Michael addition mechanism. | ||
The high level of salt and alkali used in reactive dyeing causes a high level of pollution, particularly to fresh water, the wash-off process requires high volumes of water and energy, and the whole process is very time-consuming. The purpose of this work was to determine whether a greener alternative to reactive dyeing could be used successfully without compromising the desirable attributes of reactive dyes on cotton.
Experimental
General structure of pre-treatment agent
It was postulated that incorporation of nucleophilic sites within a polymer, which could then be applied to cotton, could enable reactive dyeing to be carried out in a more efficient manner at neutral pH values.The pre-treatment agent employed is a developmental polymer and as such specific details of its structure cannot be disclosed. In general, it has a plurality of cationic centres and a plurality of nucleophilic centres and is a copolymer of at least one cationic monomer and another monomer including a nucleophilic group. The nucleophilic centres are strong nucleophiles and as such have high reactivity with the dye. Generally, the degree of cationicity is at least one cationic, particularly quaternary ammonium, centre per 500 g mol−1, with a maximum of one per 150 g mol−1. The polymer has at least one nucleophilic centre per five monomer residues with an upper limit of one nucleophilic centre per monomer residue. The polymer has a molecular weight in the region of 10000–30000 g mol−1.
The pre-treatment agent is highly substantive to cellulosic fibre. Once the agent has been adsorbed onto the cotton reactive dye is introduced, the anionic dye molecules are attracted to the cationic sites in the polymer through ionic association, enabling adsorption of the dye without the need for adding salt. Free primary amino groups in the polymer provide extremely reactive nucleophilic sites for reaction with reactive dyes, they are much more reactive than the hydroxyl groups in cotton, so no addition of alkali is required as fixation occurs at neutral pH. The mechanism of how the pre-treatment agent works is shown schematically in Scheme 3. In addition, it is proposed that at neutral pH that hydrolysis of the reactive dye will be minimised, thus only minimal wash-off should be required as most of the dye applied is fixed to the fibre.
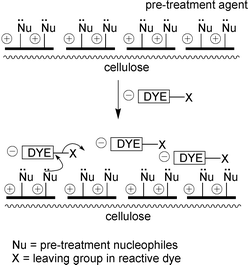 | ||
| Scheme 3 Pre-treatment of cotton and dyeing with reactive dyes. | ||
Materials
The dyes used in the study, their Colour Index generic name, maker and chemical constitution are shown in Table 1. The respective dye maker generously supplied samples of each of the dyes. Bleached, scoured, fluorescent brightener free, woven cotton (150 g m−2) was supplied by Whaley’s, Bradford, UK. Uniqema supplied the development pre-treatment agent. All other chemicals were of general laboratory grade.| Commercial name | C. I. Generic name | Dye maker | Reactive group |
|---|---|---|---|
| Procion Yellow H-EXL | C. I. Reactive Yellow 138:1 | DyStar | Monochlorotriazine |
| Procion Brilliant Red H-EGXL | C. I. Reactive Red 231 | ||
| Procion Blue H-EXL | C. I. Reactive Blue 198 | ||
| Remazol Yellow RR | None ascribed | DyStar | Sulfatoethylsulfone |
| Remazol Red RR | None ascribed | ||
| Remazol Blue RR | None ascribed | ||
| Cibacron Yellow F-3R | C. I. Reactive Orange 91 | Ciba | Monofluorotriazine |
| Cibacron Red F-B | C. I. Reactive Red 184 | ||
| Cibacron Blue F-R | C. I. Reactive Blue 182 |
Dyeing by pre-treatment method
Cotton samples were pre-treated, dyed and washed-off in sealed stainless steel dyepots of 300 cm3 capacity, housed in a laboratory-scale Roaches Pyrotec S dyeing machine. At the end of dyeing the samples were removed and dried in the open air. The method used for each of the dyes shown in Table 1 is displayed graphically in Fig. 1.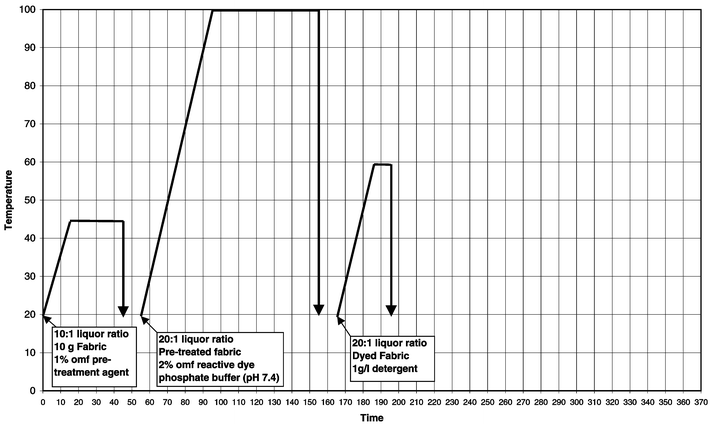 | ||
| Fig. 1 Method of pre-treatment and application of reactive dyes to cotton. | ||
Dyeing by standard method
For comparison purposes, standard dyeings were carried out on cotton that had not been pre-treated following the dye manufacturer’s recommendations.Cotton samples were dyed and washed-off in sealed stainless steel dyepots of 300 cm3 capacity, housed in a laboratory-scale Roaches Pyrotec S dyeing machine. At the end of dyeing the samples were removed and dried in the open air. The method used for Remazol RR reactive dyes3 is displayed graphically in Fig. 2, the method used for Procion H-EXL reactive dyes4 is displayed graphically in Fig. 3 and the method used for Cibacron F reactive dyes5 is displayed graphically in Fig. 4.
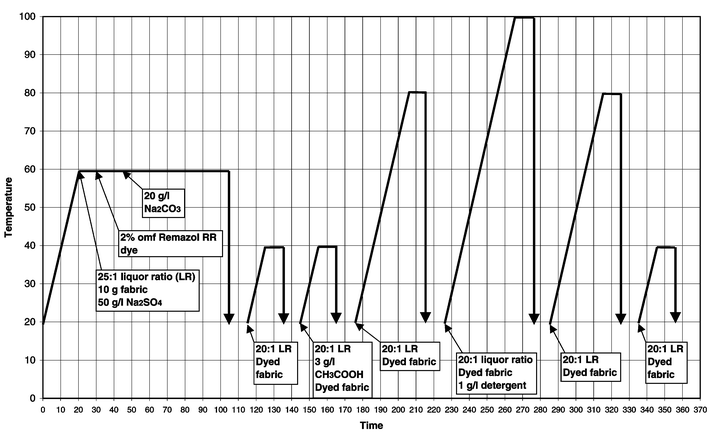 | ||
| Fig. 2 Method of application of Remazol RR reactive dyes to cotton (Michael addition fixation mechanism). | ||
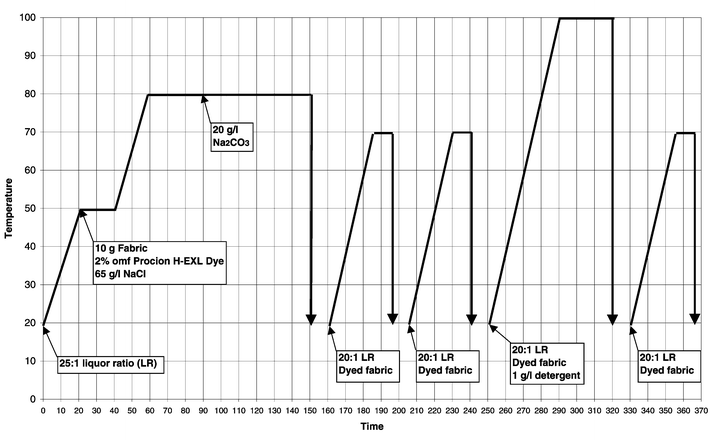 | ||
| Fig. 3 Method of application of Procion H-EXL reactive dyes to cotton (nucleophilic substitution fixation mechanism). | ||
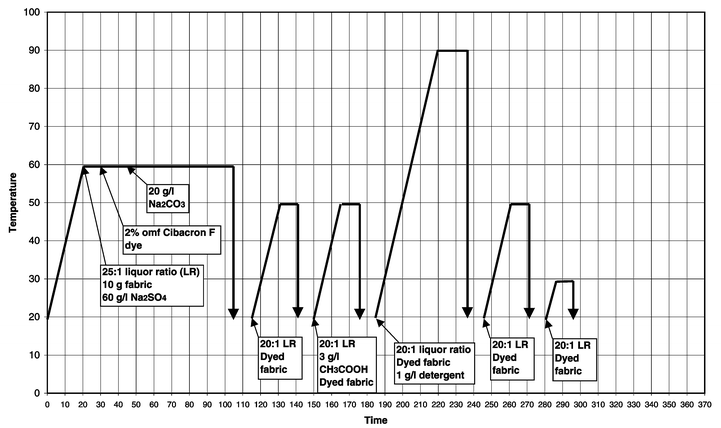 | ||
| Fig. 4 Method of application of Cibacron F reactive dyes to cotton (nucleophilic substitution fixation mechanism). | ||
Wash fastness testing
Samples were subjected to the ISO 105∶C06/C2S wash test (60 °C) using SDC multifibre fabric as adjacent.6 The samples were washed five times using the same piece of multifibre strip for each washing. After washing the samples were visually assessed using grey scales according to the ISO 105∶A02 and ISO 105∶A03 test protocols6 to determine the degree of washdown and cross staining, respectively. The grey scale ranges from 5 for no shade change (or no stain on the adjacent fibres) down to 1 for a severe shade change (or staining), with half points in between.Colour measurement
The samples were measured using a Match-Rite™ colour spectrophotometer attached to a personal computer. From the reflectance values at the λmax of the dyeings (R), the colour strength (K/S) of the sample was calculated using the Kubelka–Munk equation [eqn. (1)].7| K/S = (1 − R)2/2R | (1) |
Results and discussion
Colorimetric analysis of dyed samples
The K/S values calculated from the colorimetric data for the reactive dyes applied using the standard method and for the reactive dyes applied using the pre-treatment method are shown in Table 2. It was observed that the K/S values secured using the pre-treatment method were higher for all dyes used in comparison with the standard dyeings. It was not an intention of the work to achieve colour strength values in excess of the standard, only to equal. Nevertheless, this is advantageous, as less dye would need to be applied using the pre-treatment system to achieve a specific K/S value, hence reducing the cost and reducing the concentration of dye in the effluent. It is proposed that this result was achieved because less dye is hydrolysed and more of the dye applied fixes to the cotton. In theory if up to 40% of the dye applied using a standard method is hydrolysed, then a 2% omf application of dye could result in a 1.2% omf dyeing, whereas using the pre-treatment method a 2% omf dyeing is theoretically possible if no hydrolysis occurs.| Dye | % omf | Standard /PT | K/S |
|---|---|---|---|
| Remazol Yellow RR | 2 | Standard | 3.81 |
| 2 | PT | 4.73 | |
| Remazol Red RR | 2 | Standard | 8.55 |
| 2 | PT | 8.73 | |
| Remazol Blue RR | 2 | Standard | 6.53 |
| 2 | PT | 6.75 | |
| Procion Yellow H-EXL | 2 | Standard | 10.23 |
| 2 | PT | 10.49 | |
| Procion Brilliant Red H-EGXL | 2 | Standard | 8.30 |
| 2 | PT | 8.79 | |
| Procion Blue H-EXL | 2 | Standard | 6.20 |
| 2 | PT | 6.78 | |
| Cibacron Yellow F-3R | 2 | Standard | 5.18 |
| 2 | PT | 5.26 | |
| Cibacron Red F-B | 2 | Standard | 5.47 |
| 2 | PT | 6.33 | |
| Cibacron Blue F-R | 2 | Standard | 4.38 |
| 2 | PT | 4.67 |
Visual inspection of the samples showed only very slight variations in shade between dye type when comparing colour differences between standard and pre-treated dyeings. Remazol RR and Cibacron F pre-treated dyeings were effectively identical in shade to the standard dyeings (taking into account the differences in strength). The Procion H-EXL dyeings were a little duller than the standard.
Wash fastness assessment
The wash fastness results for the standard and pre-treated dyeings are given in Table 3. It was observed after one wash that all the dyeings produced using the various processes displayed excellent wash fastness, the dyeings exhibited similar levels of colour loss, inferring that the pre-treatment process secured dyeings for which the dye was adequately fixed. A lack of staining of adjacent fabric, particularly in the first wash, indicated that extensive wash-off of unfixed dye was not required with the pre-treatment method.| Dye | Standard /PT | Washes | S | D | C | N | P | A | W |
|---|---|---|---|---|---|---|---|---|---|
| a S Shade change, staining to D diacetate, C cotton, N nylon, P polyester, A acrylic, W wool. | |||||||||
| Remazol Yellow RR | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Remazol Red RR | Standard | 1 | 4/5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4 | 5 | 4 | 4/5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Remazol Blue RR | Standard | 1 | 4/5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4 | 5 | 4 | 4/5 | 5 | 5 | 5 | ||
| PT | 1 | 4/5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Procion Yellow H-EXL | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Procion Brilliant Red H-EGXL | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Procion Blue H-EXL | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 4/5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Cibacron Yellow F-3R | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| Cibacron Red F-B | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 4/5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Cibacron Blue F-R | Standard | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5 | 4/5 | 5 | 4/5 | 5 | 5 | 5 | 5 | ||
| PT | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
After five washes, although the wash fastness levels in general were very good to excellent for all dyeings, in some cases the wash fastness of the dyeings using the pre-treatment method were slightly superior to those secured with the conventional systems. In the conventional dyeing processes a long wash-off procedure was used and it is proposed that this contributed to the inferior wash fastness. It is suggested that, in the case of conventionally dyed cotton, a small amount of dye-fibre bond cleavage occurs as a result of the wash-off stages and, particularly, the five-wash fastness tests. It is known that the dye–fibre bond formed using vinylsulfone reactive dyes on cotton is more alkali-sensitive than that derived from monohalotriazine dyes,8 and this would explain the Remazol RR dyeings having slightly inferior wash fastness to the Procion H-EXL and Cibacron F dyeings. The cotton adjacent picks up the small amount of loose colour that is generated, the stain becoming quite noticeable after five washes; in addition, the colour strength of the dyeing is reduced. With the pre-treatment method, the long wash-off method is negated, thus slightly reducing the cumulative dye-fibre bond cleavage.
Comparison of the different dyeing processes
A summary of the differences in the dyeing processes used is given in Table 4. It was observed that the pre-treatment method vastly reduced the total time of the dyeing operation. The standard reactive dyeing processes were between 295 and 365 min in duration, whereas the pre-treatment method was completed in 195 min.| Procedure | Time/min | Water/l (kg fabric)−1 | NaCl/g (kg fabric)−1 | Na2SO4/g (kg fabric)−1 | Na2CO3/g (kg fabric)−1 | Other chemicals/g (kg fabric)−1 |
|---|---|---|---|---|---|---|
| Remazol RR | 355 | 145 | 0 | 1250 | 500 | Acetic acid (60), detergent (20) |
| Procion H-EXL | 365 | 105 | 1625 | 0 | 500 | Detergent (20) |
| Cibacron F | 295 | 125 | 0 | 1500 | 500 | Acetic acid (60), detergent (20) |
| Pre-treatment | 195 | 50 | 0 | 0 | 0 | Pre-treatment (10), phosphate buffer (120), detergent (20) |
Water consumption is highly significant from an environmental and cost point of view and dyeing processes that can reduce the volume of water used are desirable. The amount of water used is calculated using eqn. (2) by a summation of the water used in each separate process; where the liquor ratio (LR) is given as a ratio of liquor to fibre, e.g. a 25∶1 liquor ratio is 25 l of water per kg of fibre used.
| volume water used (l (kg fibre)−1) = LR (dyeing) + LR(pretreatment) + LR(washoff 1) + LR(washoff 2) + ... | (2) |
A high amount of salt (up to 1.6 kg per kg of fabric dyed) and alkali (0.5 kg Na2CO3 per kg of fabric) was consumed in the standard reactive dyeing processes, but by employing the pre-treatment method both salt and alkali were completely eliminated from the dyeing process.
Considering the other chemicals used in the dyeing processes, the pre-treatment agent was applied in relatively low concentration and it is suggested that it would have high exhaustion values, hence, its presence in the effluent would be expected to be in extremely low concentrations. In addition, the agent is polymeric and as such poses minimal environmental impact. It is noted that in the pre-treatment method 120 g of phosphate buffer per kg of fabric is used. This was for a laboratory situation and it is hoped that the process can be developed so that on a larger scale the neutral pH of tap water would negate the need for a buffer, certainly, it is hoped that the system would be as efficient in a pH range of 6–8.
Conclusions
It has been demonstrated that dyeings on cotton with excellent wash fastness can be achieved using alternative methods to the standard reactive dyeing processes. Employing the pre-treatment method may significantly reduce the time taken for the dyeing process to be completed and halve the volume of water required. In addition, the salt and alkali used in the dyeing process is negated.Dyeings secured using the pre-treatment method had higher colour strength values than the standard dyeings and it was proposed that this was because more dye fixed to the cotton as hydrolysis was minimal. In addition, only minimal wash-off was required. Visual inspection of the samples showed only very slight variations in shade between dye type when comparing colour difference between standard and pre-treated dyeings.
The pre-treatment method did not negatively affect the wash fastness of the dyeings over initial and repeated washing; the results being equal, if not slightly superior, to those secured using the standard methods.
It could be concluded that the pre-treatment system provided a much ‘greener’ alternative to achieving wash fast dyeings on cotton.
Acknowledgements
We would like to thank Uniqema for their work in the development of the pre-treatment agent and the associated application methods, which are patent protected by Uniqema.References
- D. A. S. Phillips, J. Soc. Dyers Colour., 1996, 114, 183 Search PubMed
.
- J. Shore, in Cellulosics Dyeing, ed. J. Shore, Society of Dyers & Colourists, Bradford, UK, 1995 Search PubMed
.
- Remazol Automet, Pat. card, DF 1004 E, 1987 Search PubMed
.
- Procion H-EXL, Pat. card, PL 284 Search PubMed
.
- Cibacron F, Pat. card, 3170-N Search PubMed
.
- Standard Methods for the Determination of the Colour Fastness of Textiles and Leather, 5th edn., Amendment No. 1, Society of Dyers and Colourists, Bradford, UK, 1992.
- R. McDonald, J. Soc. Dyers Colour., 1980, 96, 486 CrossRef
.
- J. Benz, J. Soc. Dyers Colour., 1961, 77, 734 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2002 |
