Esterification of carboxylic acids with alcohols catalyzed by polyaniline salts
Srinivasan Palaniappan* and Malladi Sai Ram
Organic Coatings and Polymers, Indian Institute of Chemical Technology, Hyderabad, 500 007, India. E-mail: palaniappan5@iict.ap.nic.in
First published on 30th January 2002
Abstract
Polyaniline salts such as polyaniline hydrochloride, sulfate, nitrate, phosphate and p-toluenesulfonate are used as catalysts in the esterification of carboxylic acids with alcohols. The activity, recovery, reusability and handling of the catalysts are found to be good. This process is being reported for the first time.
Green ContextEsterifications are important reaction types which often rely on the use of homogeneous acids. This contribution describes the use of a polymeric salt of a strong acid and a weak base which is shown to be an easily recoverable and effective catalyst for the esterification of lauric acid. While reaction times are moderate, the catalyst can be recovered and reused readily, making product isolation and catalyst reuse simple.DJM |
Introduction
In view of environmental mandates, there is a global effort to replace conventional catalysts by eco-friendly catalysts. The replacement of current chemical processing with more environmentally benign alternatives is an increasingly attractive subject.1 Esterification is one of the most fundamental and important reactions in organic synthesis (see ref. 2 and references cited therein). Esters are useful in a wide variety of industrial applications, such as coatings, adhesives, resins, fragrances, perfumes, plasticizers etc. Although several methods have been explored and developed,2 most of them are not suitable to meet the stringent specifications which are being applied in the chemical industry. The most acceptable method of preparing an ester is to react an acid with an alcohol in the presence of a catalyst.The catalysts employed in the esterification reaction of carboxylic acids with alcohols include mineral acids,3,4 anhydrous magnesium sulfate and catalytic amounts of sulfuric acid,5 tosyl chloride, pyridine,6 boron trifluoride etherate alcohol,7 titanium salts,8 tin salts,9 hafnium salts10,11 aluminium phosphate molecular sieve,12 NaX and NaY zeolites,13 polymer protected reagent,14 graphite bisulfite,15 diorgano tin dichloride,16 Filtrol-24, Amberlyst-15, sulfated zirconia,17 and ion exchange resin.18,19
Results and discussion
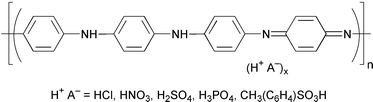
Recent advances in the field of electrically conducting polymers have led to a variety of materials with significant potential applications. Electrically conducting polymers form a unique class of materials, offering the possibility of controlled electrical conductivity combined with their good processing characteristics, low cost and stability. Among them, polyaniline is one of the most interesting materials because of its moderately high conductivity upon doping with acids, well behaved electrochemistry, easy preparation, possible processability and good environmental stability (see ref. 20 and references cited therein). In this work, the application of polyaniline in catalysis was attempted. Polyaniline salts such as polyaniline hydrochloride, sulfate, nitrate, phosphate and p-toluenesulfonate were prepared by chemical oxidative polymerization of aniline by ammonium persulfate in the presence of acid.21 The structure of polyaniline salt is generally represented as shown below.We first investigated the esterification of lauric acid with different amounts of methanol. The esterification of lauric acid (1 g) and different amounts of methanol (1, 2, 3, 4 and 5 ml) was carried out in a 10 ml round bottom flask with 150 mg of polyaniline sulfate powder. The reaction mixture was refluxed at 70 °C for 20 h. The reaction mixture was filtered and washed with chloroform to recover the catalyst. The chloroform solvent and unreacted methanol were evaporated off. The compound was loaded onto a column containing silica gel of finer than 200 mesh and eluted with 20∶80 chloroform–hexane (400 ml). The solvent mixture was recovered to obtain the pure ester (Table 1). More than 3 ml of methanol gave the ester quantitatively, but the use of 1 ml of methanol gave the corresponding ester in low yield.
| Entry | Amount of methanol/ml | Yield (%) |
|---|---|---|
| 1 | 1 | 55 |
| 2 | 2 | 90 |
| 3 | 3 | 92 |
| 4 | 4 | 99 |
| 5 | 5 | 99 |
We investigated the catalytic activities of various polyaniline salts which promote the reaction of lauric acid with methanol at reflux. The esterification of lauric acid (1 g, 5 mmol) and methanol (4 ml, 98.7 mmol) was carried out with 150 mg (15 wt% based on the amount of the acid) of polyaniline salt powder. The reaction mixture was refluxed at 70 °C for 20 h. Five different catalysts were employed in esterification. Polyaniline hydrochloride, sulfate, nitrate, p-toluene sulfonate gave esters in good yield, whereas the polyaniline phosphate gave the corresponding ester in low yield (Table 2). This is due the low strength of phosphoric acid when compared to the other acids.
| Entry | Polyaniline salt | Yield of ester (%) |
|---|---|---|
| 1 | Polyaniline hydrochloride | 99 |
| 2 | Polyaniline sulfate | 99 |
| 3 | Polyaniline nitrate | 99 |
| 4 | Polyaniline phosphate | 75 |
| 5 | Polyaniline p-toluene sulfonate | 99 |
To explore the generality and scope of the polyaniline salt catalyzed esterification, we examined the reaction of various structurally diverse alcohols with carboxylic acids (Table 3). The esterification of carboxylic acid (1 g) and alcohol (4 ml) was carried out with 200 mg of polyaniline sulfate salt as catalyst. The reaction mixture was refluxed at 70 °C for 24 h. The esters of aliphatic carboxylic acids with aliphatic alcohols gave quantitative yields. The yield of the esters was found to decrease (99, 26 and 11%) in going from primary, to secondary to tertiary alcohols, respectively. Phenoxyacetic acid (α-substituted acid) gave the ester in 85% yield while cinnamic acid (α,β-unsaturated acid) gave a 99% yield of ester. Menthyl acetate (57%) was prepared in the same configuration using menthol (chiral alcohol) and acetic acid. The methyl ester of Naproxen (81%) was obtained in the same configuration from Naproxen (chiral acid) and methanol.
| Entry | Acid | Alcohol | Yield of ester (%) |
|---|---|---|---|
| a The products were analyzed by 1H NMR spectra and authenticity of the products was established. | |||
| 1 | Lauric acid | Methanol | 99 |
| 2 | Lauric acid | Ethanol | 98 |
| 3 | Lauric acid | Propanol | 98 |
| 4 | Lauric acid | Butanol | 98 |
| 5 | Lauric acid | 1-Decanol | 98 |
| 6 | Lauric acid | Isopropyl alcohol | 26 |
| 7 | Lauric acid | tert-Butyl alcohol | 11 |
| 8 | Caprylic acid | Methanol | 95 |
| 9 | Caproic acid | Methanol | 99 |
| 10 | Myristic acid | Methanol | 98 |
| 11 | Stearic acid | Methanol | 99 |
| 12 | 11-Bromoundecanoic acid | Methanol | 99 |
| 13 | Cinnamic acid | Methanol | 99 |
| 14 | Phenoxyacetic acid | Methanol | 85 |
| 15 | Naproxen | Methanol | 81 |
| 16 | Acetic acid | Menthol | 57 |
Reusability of catalyst was checked by the esterification of lauric acid with methanol catalyzed by polyaniline sulfate salt and resulted in a stoichiometric yield (99%). The experiment was repeated nine times with the same filtered catalyst and it gave the corresponding ester in the same high yield (99%). After completing ten cycles, the catalyst, polyaniline sulfate salt was recovered and characterized. The results for the used polyaniline sulfate salt catalyst were found to be essentially the same as that of the polyaniline sulfate salt prepared originally (Table 4).
The esterification reaction has been carried out using monoaliphatic carboxylic acids, α-substituted acid, α,β-unsaturated acid; primary, secondary and tertiary alcohols; chiral alcohols and chiral acids. The advantages of polyaniline salt catalysts over the conventional catalyst are: (i) preparation of polyaniline salts through a simple synthetic route, (ii) various types of polyaniline salts can be synthesized, (iii) the amount of acid in the polyaniline chain can be varied, (iv) quick regeneration of the catalyst, and (vi) better reusability of the catalyst.
Conclusion
Polyaniline salts are used as catalysts in the esterification of carboxylic acids with alcohols. Preparation and handling of the catalyst are easy. This catalyst can be easily separated and reused, there is no acidic waste and catalyst life time can be maximized. Polyaniline salts are environmental-friendly. However, esterification reactions take longer (20 h) and also require higher amount of catalyst (15 wt%) to obtain the ester in quantitative yield. Work is in progress to modify the polyaniline system and thereby increase the efficiency of the catalyst. The use of polyaniline salts as catalysts in organic transformations may open up future work in the catalysis field.Experimental
In a typical experiment, esterification of lauric acid (1 g) and methanol (4 ml) was carried out in a 10 ml round bottom flask with 200 mg of polyaniline sulfate powder. The reaction mixture was refluxed at 70 °C for 24 h. The reaction mixture was filtered and washed with chloroform to recover the catalyst. The chloroform solvent and unreacted methanol were evaporated off. The compound was loaded a column containing silica gel of finer than 200 mesh and eluted with 20∶80 chloroform–hexane (400 ml). The solvent mixture was recovered to obtain the pure ester.References
- P. T. Anastas and J. C. Warner,Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- R. C. Larock, Comprehensive Organic Transformations,VCH, New York, 1989, p. 966 Search PubMed.
- A. Vogel, Text book of Practical Organic Chemistry, Longman Group Ltd., England, Vth edn., 1996 Search PubMed.
- Kirk-Othmer Encyclopedia of Chemical Technology, ed. M. H. Grant, Wiley Interscience Publications, 4th edn., vol. 9, p. 755 Search PubMed.
- S. W. Wright, D. L. Hageman, A. S. Wright and L. D. Mc Clure, Tetrahedron Lett., 1997, 38, 7345 CrossRef CAS.
- J. H. Brewster and C. J. Ciotti, J. Am. Chem. Soc., 1955, 77, 6214 CrossRef CAS.
- J. L. Marshall, K. C. Erickson and T. K. Folsom, Tetrahedron Lett., 1970, 46, 4011 CrossRef.
- J. F. White, Jpn. Pat., 52-75684, 1977 Search PubMed.
- J. Otera, N. Dan-oh and H. Nozaki, J. Org. Chem., 1991, 56, 5307 CrossRef CAS.
- K. Ishihara, S. Ohara and H. Yamamoto, Science, 1990, 290, 1140 CrossRef CAS.
- K. Takahashi, M. Shibagaki and H. Matsushita, Bull. Chem. Soc. Jpn., 1989, 62, 2353 CAS.
- Z H. Zhao, J. Mol. Catal. A: Chem., 2000, 154, 131 CrossRef.
- N. N. Peeran and M. Prasad, React. Kinet.-Catal. Lett., 1997, 61, 155 Search PubMed.
- E. C. Blossey, L. M. Turuer and D. C. Neckers, Tetrahedron Lett., 1973, 21, 1823 CrossRef.
- J. Bertin, H. B. Kagan and J. L. Luche, J. Am. Chem. Soc., 1974, 96, 8113 CrossRef CAS.
- A. K. Kumar and T. K. Chattopadhyay, Tetrahedron Lett., 1987, 28, 3713 CrossRef CAS.
- G. D. Yadav and P. H. Mehta, Ind. Eng. Chem. Res., 1994, 33, 2198 CrossRef CAS.
- S. M. Mahajani, React. Funct. Polym., 2000, 44, 253 CrossRef.
- B. Saha, S. P. Chopade and S. M. Mahajani, Catal. Today, 2000, 60, 147 CrossRef CAS.
- Handbook of Organic Conductive Molecules and Polymers, ed. H. S. Nalwa, John Wiley & Sons, England, 1997, vol. 1–4 Search PubMed.
- S. Palaniappan, Polym. Adv. Technol., 1994, 5, 225 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |