Adsorption and separation of cations on silica gel chemically modified by homogeneous and heterogeneous routes with the ethylenimine anchored on thiol modified silica gel
Alexandre G. S. Prado, Luiza N. H. Arakaki and Claudio Airoldi*
Instituto de Química, Universidade Estadual de Campinas, Caixa Postal 6154, 13083-970 Campinas, São Paulo, Brasil. E-mail: airoldi@iqm.unicamp.br
First published on 4th February 2002
Abstract
Ethyleneimine (etn) has been covalently bonded onto silica gel via homogeneous (SiSN1) and heterogeneous (SiSN2) routes. Both synthesised silica gel surfaces have been applied to adsorb divalent cations from aqueous solution at room temperature. The series of isotherms of adsorption were adjusted to a modified Langmuir equation, after collecting the data from the solid/MCl2 solution (M = Co, Ni, Cu, Pb and Hg) interfaces. The maximum adsorption were 1.08, 1.20, 1.70, 1.34 and 4.02 mmol g−1 for SiSN1 and 0.72, 1.74, 1.91, 2.19 and 2.89 mmol g−1 for SiSN2, for a the sequence of divalent cations: Co, Ni, Cu, Pb and Hg, respectively. Columns loaded with immobilised silica show resolutions (R) for separating metal ion couples: RCo–Ni = 0.22, RCo–Pb = 0.76, RCo–Cu = 1.12, : RCo–Hg = 2.06, RNi–Pb = 0.50, RNi–Cu = 1.17, RNi–Hg = 2.38, RPb–Cu = 0.33, RPb–Hg = 1.83 and RCu–Hg = 1.60 for the SiSN1 surface. The sequence: RCo–Ni = 1.10, RCo–Pb = 1.44, RCo–Cu = 1.63, : RCo–Hg = 2.26, RNi–Pb = 0.08, RNi–Cu = 0.33, RNi–Hg = 1.40, RPb–Cu = 0.38, RPb–Hg = 1.82 and RCu–Hg = 1.55 was determined for SiSN2.
Green ContextOrganically modified porous inorganic oxides are attracting considerable interest as hybrid materials for catalysis and reagents. High site loadings, adsorption capacities and good material stabilities are valuable properties that can extend the range of applications for such materials. Here we see the utilisation of organically modified porous silicas for analytical determinations and metal trapping. The materials reported have excellent capacity for mercury and other metals. They are significantly more efficient than other materials.JHC |
Introduction
Inorganic oxides such as silica gel have received great attention not only due to their surface reactivity, but also to their ability in immobilising organic molecules onto the surface.1 The importance of the evolution of this field of investigation in the last three decades reflects in significant review articles.2,3 Thus, the active silanol groups dispersed on silica gel, has a great ability for attaching with a myriad of different pendant functional organosilyl groups, to give some organic characteristics to the precursor inorganic support.4–6 Another feature related to these surfaces is the property of the functional groups already anchored onto silica for reacting with an active molecule to enlarge the original organic chain. On the other hand, these organofuntionalized surfaces are resistant to removal from the surface by organic solvents or water, as well as having high thermal resistance.3,5 Once the compounds are immobilised, the resultant new silica can be applied in many academic and technological areas, such as catalysis for enzymatic reactions,7,8 for heterogeneous catalyst,9,10 biotechnology processes,11–13 cation preconcentration,14,15 agrochemical preconcentration,16 cation separation processes17,18 and development of agrochemicals with controlled released properties.19,20The use of chemically modified silica gel containing a variety of chelating groups on a pendant organic chain can be designed for the adsorption and preconcentration of metal ions from aqueous and non-aqueous solutions. In a simple process, a packed column with organofuntionalized material can be effectively employed in a reliable process for preconcentration of the metal ions before analysis21,22
Sulfur donor atoms covalently attached on pendant chains anchored on a silica surface showed a particularly high chemical selectivity for mercury ions. This procedure can be used to remove and/or preconcentrate this species and, in such an operation, the main objective is to determine this high toxic contaminant. For direct determination of heavy metals in aquatic environments, a number of well-developed sensitive methods are available, including atomic spectroscopy. However, this technique shows systematic interference from the presence of other simple water constituents. For this reason, a preconcentration and/or separation step is often necessary before quantifying the desired analytes.23 The preparation of solid adsorbents, to be applied for removal of these contaminants from waste effluent streams, is one of the major goals of green chemistry.24
Applications designed to improve analytical determinations and to improve the environment by metal removal have recently emerged. For instance, some of these are employed to eliminate and/or separate traces of toxic metals from waste waters,23–26 and relevant aspects of interest appear for monitoring river water and a diversity of other sources of natural waters.24
This present investigation deals with cyclic ethyleneimine covalently attached onto silica gel by exploring homogeneous and heterogeneous synthesis methods. The main interest in these resulting modified silicas is related to their high selectivity toward divalent mercury, which is normally found in combination with lead in some Brazilian rivers.27 Copper, nickel, cobalt and other metals are pollutant components from various sources, mainly from textile industries.28 The adsorption and separation of a range of these cations from aqueous solution are now reported.
Experimental
Chemicals
The silylating agent 3-mercaptopropyltrimethoxysilane (mpts) (Aldrich) was used without previous purification. The silica gel (Aldrich), with particle size 70–230 mesh, average pore diameter 60 Å and pore volume 0.75 m3 g−1, was treated and activated by heating at 423 K for 10 h in vacuum and held under a nitrogen atmosphere, as before.1 Ethylenimine was synthesised following a described procedure, by reacting 2-aminoethyl hydrogen sulfate (Aldrich) in hot alkaline medium.29,30 Solutions of all divalent cations were prepared from reagent grade salts.Synthesis
Ethylenimine (eth) was covalently bonded to the precursor mpts through two distinct routes: The homogeneous method consisted of reacting 5.5 cm3 (29.0 mmol) of mpts with 6.0 cm3 (116.0 mmol) of etn under reflux at 323 K. The resulting compound, 3-trimethoxypropylthioethylamine (mptt), was allowed to react with 5.0 g of activated silica in dry xylene at 343 K. The final product, denoted SiSN1, was washed and dried30 (eqn. (1)). | (1) |
The heterogeneous method consisted of reacting 15.0 cm3 (84.0 mmol) of mpts with 40.0 g of activated silica gel suspended in 15.0 cm3 of dry xylene. A sample of 5.0 g of the obtained product, denoted Sil-SH, was then reacted with 2.0 cm3 (38.8 mmol) of liquid etn, which was added under a nitrogen atmosphere, to form the product SiSN2,30 as shown in eqn. (2).
 | (2) |
Adsorption
Adsorption isotherms were obtained using the batchwise method,31,32 which consisted in suspending a series of 50.0 mg samples of the modified silica in 20.0 cm3 of aqueous solutions, containing each cation at several different concentrations, varying from 0 to 50.0 mmol dm−3. The solutions were mechanically stirred for 4 h at 298 ± 1 K and the solid was separated by centrifugation and dried at 313 K. The metal concentration adsorbed was determined by sampling the supernatant, which was complexometrically titrated with EDTA, using convenient indicators33 for cobalt, nickel and copper. The other cations, lead and mercury, were determined by using ICP-AES with a Perkin Elmer model 3000 DV instrument.Separation
The ability for separating the cations was followed using a glass column with 0.50 cm internal diameter packed with 1.00 g of the immobilised SiSNx (x = 1, 2) silicas. To this column 80.0 cm3 of a mixture of divalent metal chloride solutions 0.50 mmol dm−3 each of copper, nickel, cobalt, lead or mercury was percolated through the solid bed with a flow rate of 8.33 × 10−3 cm3 s−1. Passing 2.0 cm3 aliquots of Clark/Lubs buffer solution at four distinct pH values, 4.0, 3.0, 2.0 and 1.0,14 eluted the adsorbed cations. The amount of metal ions recovered was determined using ICP-AES.The amounts of nitrogen and sulfur anchored on silica were determined through the Kjeldhal method and by using a Perkin Elmer model 2400 Elemental Analyser, respectively.19
Results and dicussion
Although the process of ethylenimine molecule immobilisation onto the silica gel surface leads to the same final product, distinct amounts of anchored pendant groups were obtained through the homogeneous and heterogeneous routes. Based on nitrogen analysis for the SiSN1 silica, the homogeneous method gave 1.74 mmol of ethylenimine anchored onto the surface. However, the SiSN2 silica obtained by the heterogeneous route, showed ethylenimine bonded not only on sulfur groups of Sil-SH precursor, but also on the silanol groups of the surface.30 Sulfur analysis showed the presence of 0.78 mmol of this element on the SiSN2 surface, with an amount of nitrogen of 1.73 mmol in the same surface. These values indicate that 0.95 mmol of ethylenimine reacted directly with silanol groups of the surface.The molecules anchored onto silica, containing sulfur and amino groups on pendant chains, permit the interaction of this support with a great range of cations, due to the presence of distinct basic centres. The sulfur basic centres present an effectiveness in interacting with softer acids than do nitrogen basic centres. This combination increases the interaction with various cations, being favourable for interactions with mercury. However, both basic centres can bond cations, simultaneously. Based on structural features presented by the pendant groups attached to the inorganic backbone, it is expected that sulfur atoms can chelate soft cations. By contrast, hard cations are chelated preferentially by nitrogen atoms, with the possibility of also using both centres, as shown in Fig. 1.
 | ||
| Fig. 1 Proposed scheme for interaction of metal ions with anchored surfaces when chelated with SiSN1 (A) and SiSN2 groups (B). | ||
The ability of these surfaces to bind cations from aqueous solution was evaluated by measuring the sorption isotherms for divalent cations such as copper, nickel, cobalt, lead and mercury. Under equilibrium conditions, the exchange processes in the solid/liquid interface can be characterised by the numbers of moles adsorbed (Nf) per gram of support. This value was calculated from the initial number of moles of cation (ni) and that (ns) at the equilibrium condition for a given mass (m) of the support in grams, by applying eqn. (3):
 | (3) |
Profiles of the adsorption isotherms for all the cations in water are represented in Figs. 2 and 3. The number of moles adsorbed versus the number of moles at equilibrium (ns) per volume of solution is illustrated for all cations. A simple observation is related to the maximum adsorption values, which are distinguishable for all these cations. For the series of isotherms, the data reveal that the adsorption process conforms to the Langmuir model (eqn. (4)), as proposed for a series of systems.1,3
 | (4) |
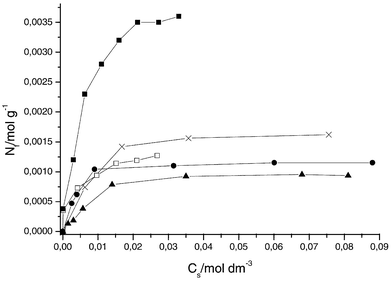 | ||
| Fig. 2 Adsorption isotherms of divalent cations for the SiSN1 surface at 298 ± 1 K: Hg (■), Pb (□), Cu (×), Ni (●), Co (▲). | ||
 | ||
| Fig. 3 Adsorption isotherms of divalent cations for the SiSN2 surface at 298 ± 1 K: Hg(■), Pb (□), Cu (×), Ni (●), Co (▲). | ||
For this expression, Cs is the concentration of the solution in equilibrium (mol dm−3), Nf is concentration of cations adsorbed on the surface (mol g−1), Ns is the maximum cation adsorbed on surface (mol g−1), which depends on the number of adsorption sites, and b is a constant. All these adsorption studies were based on the linearised form of the adsorption isotherm derived from Cs/Nf as a function of Cs. From these data, the maximum retention capacity (Ns) was determined for each cation-matrix interaction through application of the modified Langmuir equation, where Ns and b were obtained from the curved and linear coefficients of the isotherm.
Data calculated by applying the Langmuir equation (eqn. (4)) are listed in Table 1 and show that the adsorption followed the sequence Hg ≫ Cu > Pb > Ni > Co and Hg > Pb > Cu > Ni > Co, for SiSN1 and SiSN2, respectively.
| M2+ | N1/mmol g−1 | N2/mmol g−1 |
|---|---|---|
| Hg | 4.02 | 2.89 |
| Pb | 1.34 | 2.19 |
| Cu | 1.70 | 1.91 |
| Ni | 1.20 | 1.74 |
| Co | 1.08 | 0.72 |
The interactive effect of mercury on the SiNS1 surface is much more effective than for other cations. A similar order was observed for the other anchored surface, SiNS2, but with weaker interactions. This fact reflects the lower amount of sulfur in SiSN2 than in SiSN1, even though, in both cases, the binding with mercury is significant, confirming the high affinity between sulfur molecules and mercury. This process clearly reveals the high affinity soft acid–soft base interaction, with the preference for sulfur–mercury interactions.
By comparing both anchored surfaces in relation to the cation interaction process, the data showed that the presence of a higher sulfur content on the SiSN1 surface caused an increase in affinity for cobalt and mercury. This same behaviour was not observed for lead, in spite of its soft acid characteristics. This fact could probably be associated with steric hindrance due to the increase in the number of pendant chains on the SiSN1 surface. On the other hand, the results obtained with nickel and copper cations showed a decrease in binding on the SiSN1 surface, as a consequence of an enhancement in hard acid properties. Moreover, as the presence of a hard amine base on SiSN2 increases, then the pendant chains on the surface favour the interaction with copper and nickel.
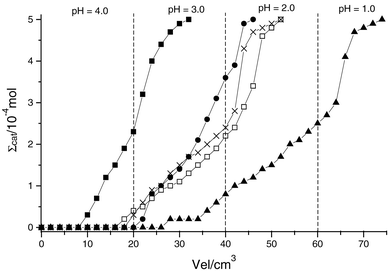
The separation ability of these surfaces was obtained for five cations through the percolation of solutions containing mixtures of two of them in separate experiments. The pH is one of the most important factors in controlling the extraction of these cations at the surface.15,18 The ability to separate the cations at different pH values is illustrated in Figs. 4–7 for both surfaces. When a column is loaded, the cations can be removed under distinct conditions: (i) cobalt at pH 3, (ii) lead, nickel and copper at pH 2 and (iii) mercury at pH 1 for the SiSN1 surface. The same behaviour was observed for the SiSN2 surface in removing cobalt at pH 3, nickel and lead at pH 2 and mercury at pH 1.
 | ||
| Fig. 4 Extraction of divalent cations in a column packed with SiSN1: Hg (■), Pb (□), Cu (×), Ni (●), Co (▲). The amount (Σcat) was obtained by elution with variable amounts (Vel) of aqueous solutions varying in pH. | ||
 | ||
| Fig. 5 Extraction of divalent cations in a column packed with SiSN2: Hg (■), Pb (□), Cu (×), Ni (●), Co (▲). The amount (Σcat) was obtained by elution with variable amounts (Vel) of aqueous solutions varying in pH. | ||
 | ||
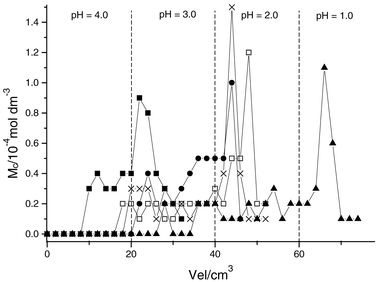
| Fig. 6 Separation of divalent cations in a column packed with SiSN1: Hg (■), Pb (□), Cu (×), Ni (●), Co (▲). The uptake cation concentration (Mc) was obtained by elution with variable amounts (Vel) of aqueous solutions with decreasing pH values for the homogeneous (Hom) and heterogeneous (Het) immobilization routes. | ||
 | ||
| Fig. 7 Separation of divalent cations in a column packed with SiSN2: Hg (■), Pb (□), Cu (×), Ni (●), Co (▲). The uptake cation concentration (Mc) was obtained by elution with variable amounts (Vel) of aqueous solutions with decreasing pH values. | ||
To confirm the ability of these surfaces to separate cations, the resolution was calculated by considering the separation of the column, using eqn. (5),
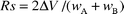 | (5) |
| Ma–Mb | R1 | R2 |
|---|---|---|
| Co–Ni | 0.22 | 1.10 |
| Co–Pb | 0.76 | 1.44 |
| Co–Cu | 1.12 | 1.63 |
| Co–Hg | 2.06 | 2.26 |
| Ni–Pb | 0.50 | 0.08 |
| Ni–Cu | 1.17 | 0.33 |
| Ni–Hg | 2.38 | 1.40 |
| Pb–Cu | 0.33 | 0.38 |
| Pb–Hg | 1.83 | 1.82 |
| Cu–Hg | 1.60 | 1.55 |
The results with SiSN1 showed that cobalt is quickly eluted while mercury is more strongly retained, eluting at low pH. The other cations were almost eluted together. However, mercury was completely isolated from the mixture of these cations. Lead, copper, nickel and cobalt cations did not present an effective separation. Cobalt and nickel are eluted together, but this couple is well separated from other cations. Lead and copper are eluted together as well, although these cations also show an effective separation from the other cations.
The SiSN2 surface data showed that cobalt was quickly eluted while mercury needs a large amount of the eluent at low pH, similar to the SiSN1 surface. There are differences between the surfaces with respect to the separations. Mercury and cobalt are well separated from mixtures, however, the remaining three cations did not present an effective separation on SiSN2.
In summary, both surfaces present a high affinity for mercury and low affinity for cobalt. This fact permits an excellent separation of mercury from other cations, and these surfaces may be useful in separating divalent cations, mainly mercury. The characteristics of these matrices showed their ability to adsorb and separate toxic elements. These abilities can be explored for applications in clean technologies, reinforcing the importance of developments in basic sciences for green chemistry.
Conclusion
Surfaces chemically modified with ethyleneimine by either homogeneous or heterogeneous processes present a high affinity for divalent mercury, due to presence of one basic sulfur centre in the pendant chains anchored on the silica surface. These surfaces also present useful adsorption behavior for copper, lead, nickel and cobalt due to the presence of a basic nitrogen atom centre. Both surfaces presented a high ability for separating mercury from cation mixtures. SiNS1 and SiNS2 are good separating matrices for copper and lead, and cobalt from other cations, respectively. Nickel did not show good separation on the SiSN1 surface, but was eluted together with copper and lead. For the SiSN2 surface nickel was eluted very closely with cobalt. The high selectivity in bonding mercury suggests that these materials may be useful for removal of this toxic heavy metal ion from waste waters.Acknowledgements
The authors thank FAPESP for a fellowship to A. G. S. P. and for financial support, and CNPq for fellowships to C. A. and L. N. H. A.References
- C. Airoldi and E. F. C. Alcantara, Thermochim. Acta, 1995, 259, 95 CrossRef CAS.
- B. Buszewki, M. Jezierska, M. Welniak and D. Berek, J. High Resolut. Chromatogr., 1998, 21, 267 CrossRef.
- V. Z. Bermudez, L. D. Carlos and L. Alcacer, Chem. Mater., 1999, 11, 569 CrossRef.
- M. R. M. C. Santos and C. Airoldi, J. Colloid Interface Sci., 1996, 183, 416 CrossRef CAS.
- V. I. Lygin, Kinet. Catal., 1994, 35, 480 Search PubMed.
- L. Arakaki and C. Airoldi, Quim. Nova, 1999, 22, 246 Search PubMed.
- T. N. Shekhovtsova, S. V. Chernetskaya and I. F. Dolmonova, J. Anal. Chem., 1993, 48, 94 Search PubMed.
- G. Schwedt, D. O. Waldheim, K. D. Neumann and K. Stein, Fresenius J. Anal. Chem., 1993, 346, 659 CrossRef CAS.
- H. E. Fisher, A. S. King, J. B. Miller, J. Y. Ying and J. B. Benzinger, Inorg. Chem., 1991, 30, 4403 CrossRef.
- Y. Kurusu, J. Macromol. Sci. Chem., 1990, 27, 1389 Search PubMed.
- K. M. R. Kallury, W. E. Lee and M. Thompson, Anal. Chem., 1993, 65, 2459 CAS.
- S. Caroli, A. Alimont, F. Petrucci and Z. Horvarth, Anal. Chim. Acta, 1991, 248, 716 CrossRef CAS.
- V. Porta, C. Sarzanini, O. Abolino, E. Mentasti and E. Cartini, J. Anal. Spectrom., 1992, 7, 19 RSC.
- L. A. M. Gomes, P. M. Padilha, J. C. Moreira, N. L. Dias Filho and Y. Gushikem, J. Braz. Chem. Soc., 1998, 9, 494 Search PubMed.
- A. G. S. Prado and C. Airoldi, Anal. Chim. Acta, 2001, 432, 201 CrossRef CAS.
- A. G. S. Prado and C. Airoldi, Fresenius J. Anal. Chem., 2001, 371, 1028 Search PubMed.
- A. A. El Nasser and R. V. Parish, J. Chem. Soc., Dalton Trans., 1999, 3463 RSC.
- A. G. S. Prado and C. Airoldi, J. Chem. Soc., Dalton Trans., 2001, 2206 RSC.
- A. G. S. Prado and C. Airoldi, Pest Manag. Sci., 2000, 56, 419 CrossRef CAS.
- A. G. S. Prado and C. Airoldi, J. Colloid Interface Sci., 2001, 236, 161 CrossRef CAS.
- N. L. Dias Filho, Y. Gushikem, J. C. Moreira, W. L. Polito and E. Rodrigues, J. Braz. Chem. Soc., 1994, 5, 53 Search PubMed.
- N. L. Dias Filho, Y. Gushikem, W. L. Polito, J. C. Moreira and E. O. Ehrim, Talanta, 1995, 42, 1625 CrossRef.
- P. M. Padilha, J. C. Rocha, J. C. Moreira, J. T. S. Campos and C. C. Frederici, Talanta, 1997, 45, 317 CrossRef.
- P. M. Price, J. H. Clark and D. J. Macquaire, J. Chem. Soc., Dalton Trans., 2000, 101 RSC.
- P. M. Padilha, L. A. de Melo Gomes, C. C. F. Padilha, J. C. Moreira and N. L. Dias Filho, Anal. Lett., 1999, 32, 1807 CrossRef CAS.
- L. N. H. Arakaki and C. Airoldi, Polyhedron, 2001, 20, 929 CrossRef.
- J. A. G. Neto, L. F. Zara, J. C. Rocha, A. Sanrtos, C. S. Dakuzaku and J. A. Nóbrega, Talanta, 2000, 51, 587 CrossRef.
- S. Lacar, J. C. Bollinger, B. Serpard, P. Chantan and R. Arcor, Anal. Chim. Acta, 2001, 428, 121 CrossRef.
- L. N. H. Arakaki, L. M. Nunes, J. A. Simoni and C. Airoldi, J. Colloid Interface Sci., 2000, 228, 46 CrossRef CAS.
- L. N. H. Arakaki and C. Airoldi, Polyhedron, 2000, 19, 367 CrossRef CAS.
- E. M. Vieira, A. G. S. Prado, M. D. Landgraf and M. O. O. Rezende, Quim. Nova, 1999, 22, 305 Search PubMed.
- A. G. S. Prado, E. M. Vieira and M. O. O. Rezende, J. Braz. Chem. Soc., 2001, 12, 485 Search PubMed.
- H. A. Flaschka, EDTA Titration, An Introdution to Theory and Practice, Pergamon, Oxford, 2nd edn., 1967 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
