Grignard type reaction via C–H bond activation in water
Chunmei Wei and Chao-Jun Li*
Department of Chemistry, Tulane University, New Orleans, Louisiana 70118, USA. E-mail: cjli@mailhost.tcs.tulane.edu
First published on 25th January 2002
Abstract
A Grignard-type reaction of phenylacetylene with aldehydes was developed via alkynyl C–H activation under aqueous conditions catalyzed by a bimetallic Ru–In system in water.
Green ContextThe idea of carrying out Grignard-like reactions in water seems counterintuitive, but could be a useful addition to synthetic methodology, as it would preclude the use of flammable organic solvents, and also avoid the wasteful process of drying them. This paper describes the development of a system which allows such reaction to occur using bimetallic Ru–In systems.DJM |
Recently, carbon–carbon bond formation based upon C–H bond activation has attracted considerable interest.1 The direct addition of terminal alkynes to aldehydes was reported by using stoichiometric amounts of Sn(II)2 or Zn(II)3 together with amine to generate the acetylenic alcohols. On the other hand, the direct alkynyl C–H activation has been succeeded by using several catalysts,4 among which the use of ruthenium-based catalysts are particularly effective.5 These catalysts have been used successfully in adding terminal alkynes to nucleophiles such as electron deficient alkenes and alkynes.6 Herein we wish to report that by using a bimetallic Ru–In catalytic system, phenylacetylene were added to aldehydes via C–H activation to give Grignard-type nucleophilic addition products in water (Scheme 1). Previously, a Ru–In catalytic system has been used by Trost et al. for addition of allenols to unsaturated compounds using propargyl alcohols as a synthon.7
 | ||
| Scheme 1 | ||
At the beginning of the research, a variety of catalysts (such as Rh, Ru, Pd, and Ni) were examined for such a goal without success. A key observation of those investigations was that while the alkynes were converted into a range of products, the aldehyde did not participate in the reaction. Therefore, it was postulated that a water-tolerant Lewis acid catalyst8 is required to activate the carbonyl. Subsequently, reactions between phenylacetylene and benzaldehyde were carried out by using various bi-catalyst systems: with one to catalyze the overall reaction and the other to activate the carbonyl. Among a variety of bi-catalytic systems, it was found that the desired addition product was formed by using RuCl3–In(NO3)3 in water (Table 1, entry 1),9 although the conversion was very low (<5%). Subsequently, conditions were optimized for forming the desired product (Table 1). The experiments demonstrated that the combination of a catalytic amount of RuCl3 and a catalytic amount of indium salts such as In(OAc)3, In(OTf)3, and In(NO3)3 showed catalytic activity, and the best yield of the desired product was obtained with RuCl3–In(OAc)3. Interestingly enough, no catalytic activity was observed with a combination of RuCl3–InCl3 (entry 6). The combination of RuCl2(PPh3)3–In(OAc)3 was also effective but it provided a decreased yield of the desired product. Similar results were found with the use of a combination of RuCl3–Zn(OAc)2 which was also effective but resulted in a lower conversion. The desired conversion was enhanced by increasing the amount of either RuCl3 or In(OAc)3. Decreasing the amount of the ruthenium or indium catalysts resulted in a longer reaction time to achieve the same conversion. Although it is not essential for the reaction to proceed, the presence of an amine base increases conversion of the addition reaction considerably. A survey of a variety of amine bases revealed that morpholine is the best in providing the highest conversion. The use of 5% aqueous K2CO3 (wt%) instead of water alone further improved the reaction (entry 4).
| Entry | RuCl3 (0.05 equiv.) + additives (equiv.) | Solvent | Conversion (%)a |
|---|---|---|---|
| a Conversion was based on 1H NMR measurement of disappearance of the starting materials (partial polymerization of phenylacetylene was observed in each case); isolated yield in parenthesis.b At 100 °C. | |||
| 1 | In(NO3)3 (0.1)/— | H2O | <5 |
| 2 | In(NO3)3 (0.1)/morpholine (0.5) | H2O | 23 |
| 3 | In(NO3)3 (0.1)/morpholine (0.5) | 5% aq. K2CO3 | 35 |
| 4 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. K2CO3 | 85 (57) |
| 5 | In(OTf)3 (0.1)/morpholine (0.5) | 5% aq. K2CO3 | 60 |
| 6 | InCl3 (0.1)/morpholine (0.5) | 5% aq. K2CO3 | Trace |
| 7 | In(OAc)3 (0.1)/pyrrolidine (0.5) | 5% aq. K2CO3 | 50 |
| 8 | In(OAc)3 (0.1)/piperidine (0.5) | 5% aq. K2CO3 | 45 |
| 9 | In(OAc)3 (0.1)/triethylamine (0.5) | 5% aq. K2CO3 | 35 |
| 10 | In(OAc)3 (0.1)/pyridine (0.5) | 5% aq. K2CO3 | 20 |
| 11 | In(OAc)3 (0.1)/morpholine (0.5) | 1% aq. K2CO3 | 70 |
| 12 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. KHCO3 | 50 |
| 13 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. Cs2CO3 | 60 |
| 14 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. Na2CO3 | 80 (45) |
| 15 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. NaHCO3 | 50 |
| 16 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. KOAc | 55 |
| 17 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. KF | 60 |
| 18 | In(OAc)3 (0.1)/morpholine (0.5) | 5% aq. K2CO3 | 90 (62)b |
A broad range of substituted aromatic aldehydes and aliphatic aldehyde without α-hyrdogen were examined via this catalytic process to afford the corresponding phenylacetyenic alcohol (Table 2). The presence of an electron-withdrawing trifluoromethyl group significantly increased the yield of the reaction whereas a cyano group decreased the yield of the desired products. The moderate yields in most cases were due to the oligamerization and polymerization of phenylacetylene.
| Entry | Aldehyde | Conditions | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 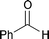 | 80 °C/48 h |  | 57 |
| 2 | 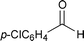 | 95 °C/24 h | 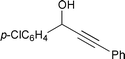 | 52 |
| 3 |  | 95 °C/24 h |  | 40 |
| 4 |  | 95 °C/24 h |  | 60 |
| 5 |  | 95 °C/24 h |  | 51 |
| 6 |  | 80 °C/24 h | 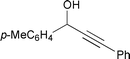 | 46 |
| 7 |  | 95 °C/48 h |  | 41 |
| 8 |  | 70 °C/48 h |  | 94 |
| 9 |  | 60 °C/48 h |  | 27 |
| 10 |  | 95 °C/48 h |  | 42 |
| 11 |  | 60 °C/24 h |  | 38 |
| 12 |  | 85 °C/48 h |  | 47 |
| 13 |  | 85 °C/48 h |  | 62 |
The exact reason behind the success of the reaction is still under investigation. Alkynylmetallic reagents such as Cu(I)10 and Ag(I),11 which are stable in water, are too stable to participate in nucleophilic carbon–oxygen double bond addition reactions. Unlike previous alkyne–aldehyde additions,2 the generation of an alkynyl carbanion is unlikely due to the large pKa difference between the terminal acetylene and the solvent water.12 A tentative mechanism was proposed which involves the simultaneous activation of C–H bond of alkyne by the ruthenium catalyst and the aldehyde carbonyl by indium ion. The ruthenium intermediate then underwent Grignard-type addition followed by an in situ hydrolysis in water to give the desired carbonyl addition product and regenerated the ruthenium and indium catalysts to catalyze further reactions (Scheme 2).
 | ||
| Scheme 2 | ||
In conclusion, the Grignard-type reaction of phenylacetylene with aldehydes via C–H activation has been developed under aqueous conditions. The scope, mechanism, asymmetric, and synthetic applications of this reaction are under investigation.13
Acknowledgment
The research was supported by the NSF-EPA joint program for a Sustainable Environment and the NSF CAREER Award program.References
- For an excellent recent review, see: G. Dyker, Angew. Chem., 1999, 38, 1698 Search PubMed.
- M. Yamaguchi, A. Hayashi and T. Minami, J. Org. Chem., 1991, 56, 4091 CrossRef CAS.
- D. E. Frantz, R. Fassler and E. M. Carreira, J. Am. Chem. Soc., 1999, 121, 11245; CrossRef CAS upon initial submission of the manuscript, Carreira and coworkers reported the catalytic version of the reaction, see: N. K. Anand and E. M. Carreira, J. Am. Chem. Soc., 2001, 123, 9687 Search PubMed.
- B. M. Trost and A. J. Frontier, J. Am. Chem. Soc., 2000, 122, 11727 CrossRef CAS.
- B. M. Trost, F. D. Toste and A. B. Pinkerton, Chem. Rev., 2001, 101, 2067 CrossRef CAS; T. Mitsudo, Y. Nakagawa, K. Watanabe, Y. Hori, H. Misawa, H. Watanabe and Y. Watanabe, J. Org. Chem., 1985, 50, 565 CrossRef CAS.
- B. M. Trost and A. B. Pinkerton, Angew. Chem., Int. Ed., 2000, 39, 360 CrossRef CAS; M. Picquel, C. Bruneau and P. H. Dixneuf, Tetrahedron, 1999, 55, 3937 CrossRef; S. Chang, Y. Na, E. Choi and S. Kim, Org. Lett., 2001, 3, 2089 CrossRef CAS.
- B. M. Trost, L. Krause and M. Portnoy, J. Am. Chem. Soc., 1997, 119, 11319 CrossRef CAS.
- For an excellent review, see: S. Kobayashi, Synlett, 1994, 689 Search PubMed.
- For an example of In-based Lewis acid in water, see: T. P. Loh, J. Pei and M. Lin, Chem. Commun., 1996, 2315 Search PubMed.
- F. Olbrich, J. Kopf and E. Weiss, Angew. Chem., Int. Ed. Engl., 1993, 32, 1077 CrossRef.
- A. M. Sladkov and L. Yu. Ukhin, Usp. Khim, 1968, 37, 1750 Search PubMed.
- W. S. Matthews, J. E. Bares, J. E. Bartmess, F. G. Bordwell, F. J. Cornforth, G. E. Drucker, Z. Margolin, R. J. McCallum, G. J. McCallum and N. R. Vanier, J. Am. Chem. Soc., 1975, 97, 7007.
- Typical experimental procedure: to a mixture of ruthenium trichloride (5 mol%), indium acetate (10 mol%), benzaldehyde (5 mmol), and morpholine (2.5 mmol) in 10 mL of aqueous potassium carbonate (5 wt%), phenylacetylene (15 mmol) was added with stirring under an inert atmosphere of nitrogen. After continuous stirring at room temperature for 10 min, the mixture was heated at 80 °C and stirred. Stirring was continued until no further increase of the reaction product as monitored by 1H NMR. The reaction mixture was poured into water, acidified with dilute HCl, and extracted with diethyl ether or methylene chloride. A usual work-up generated a crude material. After flash column chromatography on silica gel with 1∶20 EtOAc–hexane as eluent, the product was isolated. Characterization of products: most products are known compounds except entries 7, 8, 10, 13. All new compounds have been characterized by 1H and 13C NMR, IR, MS or elemental analysis.
| This journal is © The Royal Society of Chemistry 2002 |
