KF/natural phosphate as an efficient catalyst for synthesis of 2′-hydroxychalcones and flavanones
D. J. Macquarrie*a, R. Nazihb and S. Sebtib
aDepartment of Chemistry, University of York, Heslington, York, UK YO10 5DD. E-mail: djm13@york.ac.uk
bLaboratoire de Chimie Organique Appliquée et Catalyse, Faculté des Sciences Ben M′Sik, Université Hassan II, Casablanca, Morocco
First published on 31st January 2002
Abstract
Potassium fluoride supported on natural phosphate has a strong basic activity which can be efficiently used to promote the Claisen–Schmidt reaction of 2′-hydroxyacetophenones with benzaldehydes.
Green ContextSolvent, used for reaction mixtures and purification, is often the largest contributor to chemical waste for a given process. Eliminating solvent is therefore a frequent goal in green chemistry, but it is a goal that is not easily achieved. Here, the authors describe a solvent-free system for the condensation of 2′-hydroxyacetophenones with aromatic aldehydes to give chalcones and flavonones, using a solid catalyst derived from KF and the mineral ‘natural phosphate’. The reactions products are of interest, for example as antioxidants. Yields and conversion are excellent, and catalyst recycle is demonstrated.JKB |
Introduction
The use of heterogeneous catalysts under solvent-free conditions represents a potentially valuable and clean route to a range of organic products.1,2 The use of naturally-occurring minerals as catalysts or catalyst supports has been a particularly useful area of research; in particular, many catalysts based on clay minerals have found use in such systems, especially Montmorillonite K10, and related acid treated clays.3 Few basic catalysts have been utilised in such a way, one example being KF/alumina,4 which has been used to catalyse a number of reactions, with the Henry reaction being catalysed by hydrotalcites in the absence of solvent, but in an excess of nitroalkane.5We now report results based on the naturally occurring material natural phosphate (NP). This mineral, brought from the region of Khouribgra in Morocco, belongs to the mineralogical family of phosphocalcic apatite. NP has been exploited in many synthetic applications and both basic and acid activity has been demonstrated. Thus NP alone, or doped with mineral salts, has been used to promote many reactions: Knoevenagel reaction,6 nitrile hydration,7 α-hydroxyphosphonate synthesis,8 Michael addition,9 dipolar cycloaddition,10 and acyclonucleoside synthesis.11
The preparation of the KF/NP material involves a simple evaporation of potassium fluoride solution in the presence of NP. Such a process has been used for the production of KF/Al2O3,12 as well as KF/ZnO and KF/AlPO4.13 The latter two are useful catalysts for the Michael reaction13 and low temperature butene isomerisation,14 respectively. While their structures have not been thoroughly investigated, Zhu and Xu indicate that there is an interaction of KF with the AlPO4 support.14 KF/Al2O3 is the most thoroughly studied and it is well established that the structure is a complex mixture of various tetra- and hexa-fluoroaluminates, as a result of extensive reaction between the components.12
We now present results on the synthesis and use of KF/NP, which indicate that some structural reorganisation does take place in the preparation of KF/NP, and that the resultant material is an efficient base catalyst.
The catalyst is evaluated in the condensation reaction of 2’-hydroxyacetophenones 1 with aromatic aldehydes 2, leading to chalcones 3 and flavanones 4 (Scheme 1), compounds of interest pharmacologically, especially in the areas related to anti-oxidant activity.15–19 Such a reaction has been investigated using heterogeneous catalysts before, with interesting results.20–24
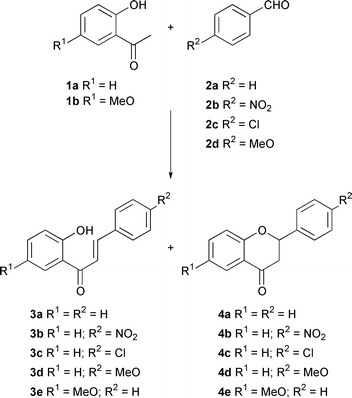 | ||
| Scheme 1 Condensation of aldehydes and 2′-hydroxyacetophenones, giving chalcones and flavanones. | ||
Results and discussion
Preparation and characterisation of KF/NP catalysts
The catalysts were prepared by addition of NP to a solution of KF in water. Ratios of NP∶KF were varied on a mass basis from 2∶1 to 16∶1. Evaporation of water gave the catalysts, designated by the codes KF/NP-r where r = 2–16, and represents the mass ratio of NP∶KF. The catalysts were all grey powders, the colour of NP itself.The analysis of the material is illustrated by KF/NP-8, the most active of the catalysts. X-Ray diffraction of KF/NP-8 gives a diffraction pattern almost identical to that of NP itself. Peak positions and intensities are essentially unaltered, except for a minor reduction in intensity. This result indicates that the crystalline structure of the support remains essentially unaltered in the catalyst, and also indicates that no crystalline KF phase is present. Thus the KF is well dispersed throughout the material. Interestingly, the surface areas of NP and KF/NP-8, as measured by nitrogen adsorption, indicate an increase in surface area and pore volume on supporting KF. The surface area of NP is measured as 1.4 m2 g−1 (i.e. below the 2 m2 g−1 limit of the instrument) and the pore volume is 0.05 cm3 g−1, values which are consistent with a crystalline solid, something which is confirmed by the typical type II isotherm (according to the BDDT classification) displayed by the material. On supporting the KF, the surface area increases to 8.9 m2 g−1, and the pore volume to 0.128 cm3 g−1. Again the isotherm is of the same form. Supporting metal salts on solids will typically reduce the surface area of the material, whereas in this case the surface area has increased significantly, although it is still very low compared to solids such as alumina, clay and silica, which typically have surface areas of >100 m2 g−1. Changes in the IR spectrum of the two materials are relatively minor, the major change being the presence of some additional H-bonded water at 3250 cm−1 and at 1636–1675 cm−1 for the supported material. Scanning electron micrograph images indicate that the morphology of the particles has altered significantly as a consequence of supporting KF onto NP, indicating that some significant change has taken place. Specifically, the particle size has reduced somewhat, but the shape has remained similar.
From the above it appears that supporting KF on NP causes the formation of a more open structure which incorporates KF in such a way that crystalline KF is not formed in significant quantities. However, it appears that the basic structure of the NP is not destroyed, indicating a less profound interaction of KF with NP than is the case with alumina. Further investigations into these reactions are planned.
The use of KF/NP as catalyst for the condensation of 2′-hydroxyacetophenones with benzaldehydes was investigated. This reaction produces chalcones and flavanones, both of which are of interest in a variety of applications, including their wide range of biological activities. Reactions were carried out under a variety of conditions, which were designed to optimise the system in a general way. The results generated indicate that KF/NP is a highly effective, general catalyst, based on inexpensive raw materials, which can work under solvent free conditions.
The reaction of 2′-hydroxyacetophenone 1a with benzaldehyde 2a was chosen to optimise the reaction conditions. 10 mmol of each reactant was added to a flask, heated to reaction temperature and the catalyst then added. Initially, the effect of temperature was analysed, with reactions being sluggish at 150 °C, but significantly quicker at 180 °C. Further optimisation was thus carried out at the latter temperature, in the absence of solvent. The influence of the mass of catalyst was investigated, and it was found that for 10 mmol of reactants, 1 g of KF/NP-4 was optimal (Table 1). Conversion increased up to this amount and then decreased significantly. While the increase is not dramatic from 0.25 g to 1 g catalyst, it was decided to continue optimisation with 1 g of catalyst.
| Conversion of 2′-hydroxyacetophenone 1a (%) | ||||
|---|---|---|---|---|
| Time/h | 0.25 g KF/NP-4 | 0.5 g KF/NP-4 | 1 g KF/NP-4 | 2 g KF/NP-4 |
| 1 | 21 | 20 | 29 | 15 |
| 2 | 25 | 30 | 40 | 28 |
| 4 | 40 | 46 | 56 | 32 |
During these reactions, it was noted that the degree of conversion of benzaldehyde was significantly higher than that of the acetophenone component, and that the formation of benzoic acid was noted, probably due to simple oxidation of benzaldehyde under the basic conditions of the reaction. Thus, prolonged reaction times (up to 12 h) failed to improve yields and conversion of the acetophenone. Such behaviour was also noted by Climent et al.20 in this reaction using hydrotalcite catalysts. It was thus decided to add the benzaldehyde stepwise in an attempt to limit this side reaction. Thus half the benzaldehyde was added at the start of the reaction, and half after 55 min. Results are shown in Table 2.
| Conversion of 1a (%) | ||
|---|---|---|
| Time/h | Two-stage addition | One-stage addition |
| 0.5 | 20 | — |
| 1 | 50 | 29 |
| 2 | 87 | 40 |
| 3 | 88 | — |
| 4 | 88 | 56 |
It is clear that the stepwise addition of benzaldehyde dramatically enhances the conversion and reduces substantially the amount of oxidation to benzoic acid. Addition of benzaldehyde after 30 min resulted in a lower performance enhancement (76 rather than 88% conversion). Separate experiments show that, perhaps surprisingly, the addition of benzoic acid at the start of reaction does not reduce the rate of reaction, and thus appears not to be a catalyst poison.
Using this two-step addition protocol, we then studied the influence of the ratio NP∶KF. The ratio was varied from 2 to 16, and the resultant catalysts evaluated. It was found that the catalyst with KF/NP-8 was the best catalyst, giving 95% conversion after 4 h reaction time (Table 3).
| Conversion of 1a (%) | ||||
|---|---|---|---|---|
| Time/h | KF/NP-2 | KF/NP-4 | KF/NP-8 | KF/NP-16 |
| 1 | 40 | 50 | 67 | 20 |
| 2 | 64 | 87 | 90 | 26 |
| 4 | 68 | 88 | 95 | 40 |
Having ascertained the optimum conditions and reaction procedure, we investigated the scope of the reaction with a range of reactants, and the results are shown in Table 4 below.
| Yield (%) | ||||||
|---|---|---|---|---|---|---|
| R1 | R2 | Run | Time/h | Conversion of 1 (%) | 3 | 4 |
| H | H | a | 1 | 67 | 30 | 35 |
| 2 | 90 | 30 | 58 | |||
| H | NO2 | b | 1 | 77 | 37 | 39 |
| 2 | 98 | 44 | 54 | |||
| H | Cl | c | 1 | 66 | 26 | 30 |
| 2 | 91 | 23 | 54 | |||
| H | OCH3 | d | 1 | 65 | 29 | 36 |
| 2 | 91 | 35 | 52 | |||
| OCH3 | H | e | 1 | 50 | 16 | 34 |
| 2 | 54 | 16 | 36 |
It can be seen from Table 4 that for all aldehydes, the conversion is excellent. The only example where conversion is lower is for the 4-methoxyacetophenone, where the early stages of reaction proceed relatively rapidly, but the reaction does not proceed much beyond 50% conversion. The ratio of chalcone∶flavanone varies from 0.42 in example c to 0.81 in example b after 2 h reaction time. While the exact reasons for this behaviour are not clear, studies of the formation of the chalcone,25 its conversion to flavanone26 and the adsorptive behaviours of both flavanones and chalcones on different solids27 indicate that there are many subtle inter-relationships in the detailed mechanism, even in solution, which are further complicated by the presence of a solid catalyst.
Several solid catalysts have been investigated in the Claisen–Schmidt reaction. Barium oxides have shown some promise23,28 but are relatively toxic, and are thus unattractive for environmentally benign processing. Quaternary ammonium hydroxides supported on MCM-41 have also shown promise29 in this reaction, giving 65% conversion after 8 hours at 130 °C for the unsubstituted benzaldehyde and acetophenone. The combination of natural phosphate, water and a phase transfer catalyst, benzyltriethylammonium chloride has been shown to be very efficient for the same reaction of unsubstituted benzaldehyde and acetophenone.30 Neither of the two catalyst systems above have been applied to the 2′-hydroxyacetophenone system. The main work which has been published on the 2′-hydroxyacetophenone/benzaldehydes system was carried out by Climent et al.20 They compared a range of catalysts and several differently substituted reactants. A comparison of their results with those reported in this study is given in Table 5.
As can be seen, the KF/NP system gives a somewhat lower conversion than hydrotalcite C-13 after 1 h reaction time, but significantly outperforms all the other catalysts studied. Indeed, the final conversion achieved by the hydrotalcite C-13 reaches 84% after 2 h reaction time, while KF/NP-8 has achieved 90% by this stage, and 95% after 4 h. Thus, KF/NP-8 appears to be the most efficient catalyst for this reaction.
Reuse studies were performed by filtering the catalyst from the reaction mixture after 2 h, washing briefly with dichloromethane, and allowing to dry at room temperature. The catalyst was then added to a fresh batch of reagents. It was found that a slightly higher yield of products (95%) was obtained upon first reuse, then activity dropped somewhat (70% after 2 h, but 90% after 4 h). One more reuse led to a low conversion (30% after 4 h). At this stage, a thorough wash with acetone (until washings were colourless) and reactivation at 150 °C for 4 h was sufficient to restore activity to that of the fresh catalyst. Selectivity was not affected.
Extending the comparison to a wider range of substrates illustrates that the KF/NP-8 catalyst is consistently efficient, and is less affected by substituent effects than the hydrotalcite20 (Table 6).
| Yield (%)a | ||||||
|---|---|---|---|---|---|---|
| Conversion of 1 (%) | 3 | 4 | ||||
| C-13 | KF/NP-8 | Runb | C-13 | KF/NP-8 | C13 | KF/NP-8 |
| a Figures in parenthesis indicate conversion or yield respectively after 2 h (where available).b See Table 4 | ||||||
| 78(84) | 67(90) | a | 50 | 30(30) | 28 | 35(58) |
| 61 | 77(98) | b | 16 | 37(44) | 45 | 39(54) |
| 35 | 66(91) | c | 27 | 26(23) | 8 | 20(54) |
| 17 | 65(91) | d | 7 | 29(35) | 10 | 36(52) |
| 23 | 50(54) | e | 16 | 16(16) | 7 | 34(36) |
Thus it can be seen that KF/NP catalysts represent a very active and versatile class of catalysts for the Claisen–Schmidt reaction of 2′-substituted acetophenones.
Experimental
Natural phosphate was prepared using the following procedure: Natural phosphate (NP) comes from an extracted ore in the region of Khouribga (Morocco). The fraction of 100–400 μm is isolated, washed with water, calcined at 900 °C for 2 h, sieved (63–125 μm) and conserved at 150 °C or in a desiccator.Deionised water was used throughout. Potassium fluoride, the aldehydes and acetophenones were all obtained from Aldrich. The aldehydes were purified by distillation under reduced pressure before use. The other chemicals were used as received.
GC analysis was carried out using a Hewlett–Packard 6890 GC with autosampler, fitted with a DB-5 capillary column, using nitrogen as carrier gas.
Surface area and pore size analysis were carried out on a Micromeritics ASAP2010 instrument using nitrogen as adsorbent. Surface areas were calculated using the BET equation. SEM images were taken on a Hitachi S-2400 microscope. X-Ray diffraction was carried out on a Philips 1050 using Cu-Kα radiation. IR spectra were obtained using a diffuse reflectance accessory on a Bruker Equinox 55 instrument. Samples were prepared by dilution (10%) in KBr and preheated to 150 °C under vacuum before analysis.
Preparation of KF/NP-8
Natural phosphate (8 g) was added to a solution of KF (1.00 g) in water (100 ml). The mixture is stirred at room temperature for 30 min, then water is removed on a rotary evaporator under reduced pressure at a bath temperature of 60 °C. The solid obtained is then dried under vacuum at 150 °C for 2 h.Condensation of acetophenones with benzaldehydes
A mixture of the acetophenone (10 mmol), the aldehyde (5 mmol) and n-tetradecane (0.60 g) was heated to 180 °C on an oil-bath. After the reaction temperature was reached, the catalyst (1 g) was added. After 55 min, a further quantity of aldehyde (5 mmol) was added and the reaction continued. Samples were taken at appropriate intervals for GC analysis using n-tetradecane as internal standard. Isolation of products is achieved by washing with small quantities of dichloromethane. Confirmation of product identity was achieved by GC-MS, and comparison with authentic samples.Conclusions
The preparation of a series of catalysts based on natural phosphate and potassium fluoride has been described. Supporting KF on natural phosphate leads to an increased surface area and a more open structure, but does not appear to change the crystalline structure of the support significantly. The catalysts are shown to be very active in the Claisen–Schmidt reaction of various substituted benzaldehydes with substituted acetophenones. The conversions and yield of product obtained are excellent for a range of substrates, and demonstrate that the catalystsare both highly active and versatile.Acknowledgement
D. J. M. thanks the Royal Society for a University Research Fellowship. Financial assistance of the Ministry of Education of Morocco (PROTARS, P2T3/59) and the ‘Centre d′Etudes et de Recherches sur les Phosphates Minéraux (CERPHOS), groupe Office Chérifien des Phosphates (OCP)’ is gratefully acknowledged.References
- A. Loupy, Top. Curr. Chem., 1999, 206, 153 Search PubMed.
- J. H. Clark and D. J. Macquarrie, Chem. Commun., 1998, 853 RSC.
- M. D. Nikalje, P. Phukan and A. Sudalai, Org. Prep. Proc. Int., 2000, 32, 1 Search PubMed.
- D. Villemin, B. Martin and M. Khalid, Synth. Commun., 1998, 28, 3195 CAS.
- B. M. Choudary, M. L. Kantam, C. V. Reddy, K. K Rao and F. Figueras, Green Chem., 1999, 1, 187 RSC.
- S. Sebti, A. Saber and A. Rhihil, Tetrahedron Lett., 1994, 35, 9399 CrossRef CAS.
- S. Sebti, A. Rhihil, A. Saber and N. Hanafi, Tetrahedron Lett., 1996, 37, 6555 CrossRef CAS.
- S. Sebti, A. Rhihil, A. Saber, M. Laghrissi and S. Boulaajaj, Tetrahedron Lett., 1996, 37, 3999 CrossRef CAS.
- S. Sebti, H. Boukhal, N. Hanafi and S. Boulaajaj, Tetrahedron Lett., 1999, 40, 6207 CrossRef CAS.
- H. B. Lazrek, A. Rochdi, Y. Kabbaj, M. Taourirte and S. Sebti, Synth. Commun., 1999, 29, 1057 CAS.
- A. Alahiane, A. Rochdi, M. Taourirte, N. Redwane, S. Sebti and H. B. Lazrek, Tetrahedron Lett., 2001, 42, 3579 CrossRef CAS.
- H. Kabashima, H. Tsuji, S. Nakata, Y. Tanaka and H. Hattori, Appl. Catal. A, 2000, 194, 227 CrossRef.
- J. M. Campelo, M. S. Climent and J. M. Marinas, React. Kinet.-Catal. Lett., 1992, 47, 7 Search PubMed.
- J. H. Zhu and Q. H. Xu, Acta Chim. Sinica, 1997, 55, 474 Search PubMed.
- A. S. Tomcufcik, R. G. Wilkinson and R. G. Child, Ger. Offen., 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502
502![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490, 1975; Chem. Abstr., 1975, 83, 179067n.
490, 1975; Chem. Abstr., 1975, 83, 179067n. - K. Yamaguchi, Y. Sakurai and H. K.Urumi, Jpn. Pat., 72
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016, 1972; Chem. Abstr., 1973, 78, 97330d.
016, 1972; Chem. Abstr., 1973, 78, 97330d. - A. Lespagnol, C. Lespagnol, D. Lesieur, J. P. Bonte and Y. Blain, Olabian, Clin. Ther., 1971, 6, 192 Search PubMed.
- B. Botta, G. D. Monache, M. C. de Rosa, R. Scurria, A. Volali, V. Vinciguerra, P. Menendez and D. Misiti, Heterocycles, 1996, 1415 Search PubMed.
- C. Tournaire, S. Croux, M. T. Maurette, I. Beck, M. Hocquaux, A. M. Braun and E. Oliveros, J. Photochem, Photobiol. B: Biol, 1993, 19, 205 Search PubMed.
- M. J. Climent, A. Corma, S. Iborra and J. Primo, J. Catal., 1995, 151, 60 CrossRef.
- L. Guthrie and N. Rabjohn, J. Org. Chem., 1957, 22, 176 CrossRef.
- S. Sathyanarayana and A. G. Krishnamurthy, Curr. Sci., 1988, 57, 1114 Search PubMed.
- A. Aguilera, A. Alcantara, J. M. Marinas and J. V. Sinisterra, Can. J. Chem., 1987, 65, 1165 CAS.
- A. Fuentes, J. M. Marinas and J. V. Sinisterra, Tetrahedron Lett., 1987, 28, 4541 CrossRef CAS.
- E. J. Gasull, J. J. Silber, S. E. Blanco, F. Tomas and F. H. Ferretti, J. Mol. Struct., 2000, 503, 131 CrossRef CAS.
- L. J. Yamín, S. E. Blanco, J. M. Luco and F. H. Ferretti, J. Mol. Struct., 1997, 390, 209 CAS.
- S. E. Blanco, J. J. Silber, G. E. Narda, L. J. Yamín and F. H. Ferretti, J. Colloid Interface Sci., 1996, 180, 144 CrossRef CAS.
- J. V. Sinisterra, A. Garcia-Raso, J. A. Cabello and J. Marinas, Synthesis, 1984, 502 CrossRef.
- I. Rodriguez, S. Iborra, F. Rey and A. Corma, Appl. Catal. A, 2000, 194–195, 241 CrossRef CAS.
- S. Sebti, A. Saber, A. Rhihil, R. Nazih and R. Tahir, Appl. Catal. A, 2001, 206, 217 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
