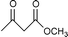
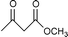
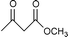
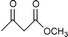
Transesterification of β-ketoesters with alcohols catalyzed by montmorillonite K-10
Tongshou Jin*, Suling Zhang and Tongshuang Li
Department of Chemistry, Hebei University, Baoding, 071002, Hebei Province, P. R. China. E-mail: orgsyn@mail.hbu.edu.cn
First published on 29th January 2002
Abstract
Montmorillonite K-10 is an efficient reusable catalyst for the transesterification of ethyl/methyl β-ketoesters with various alcohols in good yields. The main advantages of the catalyst are easy operation in the workup and a more economical and environmentally benign procedure.
Green ContextTransesterification is an important synthetic transformation used in many sectors of the chemical industry. Traditional methods based on soluble or liquid acids and other reagents cause major problems in separation and product purification leading to relatively large volumes of waste. Here we see how a solid acidic clay can be effectively used instead of conventional acids giving very good product yields in the transesterification of β-keto esters—a particularly important reaction in pharmaceutical, agrochemical, polymer and other chemical industries. Reactions are carried out in a relatively benign hydrocarbon solvent. Separation is easy and the clay can be reused.JHC |
Introduction
Transesterification is one of the established organic reactions that command numerous laboratory uses and industrial applications.1,2 β-Ketoesters are of interest as chemical intermediates in the pharmaceutical, agrichemical, chemical and polymer industries.3 Thus, a number of procedures for transesterification have been reported, which are catalyzed by a variety of protic or Lewis acids, organic or inorganic bases, enzymes or antibodies. However, it is well known that traditional acidic catalysts such as sulfuric acid, phosphoric acid and hydrochloric acid can cause severe environmental problems.2 In addition most of the normal catalysts are not efficient for the transesterification of β-ketoesters so that various other catalysts have been applied to the reaction. Transesterification of β-ketoesters is catalyzed by DMAP4 in good yield, but the application of this catalyst is limited owing to its toxicity, high price and requirement for high temperature. An efficient method of transesterification employing tert-butyl acetoacetate has been reported, but is restricted to tert-butyl esters, thus lacking generality.5 Some other catalysts have been documented, such as sulfated tin oxide,6 zeolites,7 kaolinitic clay,8 Mo-ZrO2,9 FeSO4 and CuSO4,10 an yttria–zirconia based strong Lewis acid11 and Envirocat EPZGR.12 However, they are not very satisfactory due to some drawbacks such as tedious workup, low selectivity, long reaction time, corrosivity, effluent pollution and non-recoverable catalysts.The most important issue in the scientific community of chemistry is to develop economical and practical processes based on the idea of green chemistry. In recent years, solid acidic catalysts13,14 such as montmorillonite clays and MxOy/SO42− superacids have received considerable attention in different areas of organic synthesis because of their environmental compatibility, reusability, high selectivity, operational simplicity, non-corrosiveness and low cost. Among them, the application of montmorillonite clays has been extensively studied in many organic reactions.15
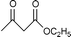
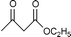
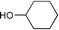
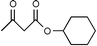
Montmorillonite K-10 is a type of acidic stratified silicate mineral with a three-layer structure with an ideal chemical formula of (Al2−yMgy)Si4O10(OH)2·nH2O. In montmorillonite clay one octahedral aluminate layer is sandwiched between two octahedral silicate layers. The interlayer cations are exchangeable, which allows alteration of the acidic nature of the material by a simple ion-exchange procedure. Both Brönsted and Lewis acidic catalytic sites are available, and its natural occurrence as well as its ion exchange properties make it a useful catalyst. Up to now, montmorillonite and in particular montmorillonite K-10, has been used as an efficient acidic catalyst for many organic transformations, such as substitution,13 acetalization16etc. This prompted us to initiate a systematic investigation on montmorillonite K-10 catalyzed transesterification of β-ketoesters (Scheme 1) and herein we wish to disclose our results.
 | ||
| Scheme 1 | ||
Results and discussion
Montmorillonite K-10 was purchased from Aldrich. The catalyst has surface area of 220–270 m2 g−1 and a bulk density of 300–370 g l−1. The montmorillonite K-10 was activated at 120 °C over night prior to use.In a typical experimental procedure, when the β-ketoesters were treated with alcohols in the presence of a catalytic amount of montmorillonite K-10 catalyst, the corresponding transesterified products were obtained in good to excellent yields. The results of transesterification are summarized in Table 1. After completion of the reaction, the reaction mixture was separated and the wet catalyst was washed with methanol and reused for three cycles after activation without significant loss of activity.
Spectroscopic17 and kinetic17–19 studies have indicated that the transacetoacetylation reaction proceeds via a mechanism in which acetylketene 2 is formed in the rate-limiting nuimolecular step (Scheme 2). One diketene free approach involves the transesterification of the corresponding nucleophile with an appropriate acetoacetate (transacetoacetylation). We can use a variety of alcohols as nucleophiles to react with the acetylketene and the reactions are easily carried out under heating at 110 °C in toluene in the presence of montmorillonite K-10.
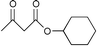
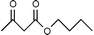
It is clear from Table 1 that the conversion from methyl/ethyl ketoesters to higher homologues appears to be efficient and practical through this procedure. The present procedure is quite general for a wide range of structurally varied alcohols such as open chain, cyclic and aromatic ones (entries 2, 7 and 9). The reaction proceeds smoothly with primary and secondary alcohols in excellent yields. As for tert-butyl alcohol (entry 4), which is often problematic in acid catalyzed reactions, a satisfactory outcome is realized by this reagent with a moderate yield of product.
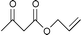
It should be pointed out that transesterification of β-ketoesters with unsaturated alcohols is rather difficult as it is offset by facile decarboxylation rearrangment; however, using this method β-ketoesters underwent smooth reaction. It is importment to mention that the reaction appears to be specific only for the transesterification of β-ketoesters. Other esters such as α-ketoesters, γ-ketoesters as well as normal esters failed to undergo the reaction. The difference in the reactivity between β-ketoesters and other esters in transesterification is probably be due to the formation of acyl ketene intermediates in the former as proposed by Campbell and Lawrie.19
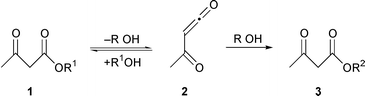 | ||
| Scheme 2 | ||
In summary, we have described a new and highly efficient procedure for the transesterification of β-ketoesters using an environmentally acceptable montmorillonite K-10 clay as catalyst. The obvious advantages of the catalyst are easy and simple operation in the workup in an inexpensive, non-toxic, non-corrosive, recyclable, more economical and environmentally friendly procedure. We believe this will present a better and more practical method to the existing methodologies and should find widespread application in academic and industrial fields.
Experimental
Liquid alcohols were purified by distillation prior to use. IR spectra were recorded on a Bio-Rad FTS-40 spectrometer (KBr). 1H NMR spectra were measured on a Bruker AC-80 (80 MHz), spectrometer using TMS as internal standard and CDCl3 as solvent. MS were determined on a VG-7070E spectrometer (EI, 70 eV). The products were identified by IR, MS,1H NMR spectra.Typical procedure
A 1∶1 mole ratio mixture of β-ketoester (5 mmol) and alcohol (5 mmol) together with the montmorillonite K-10 catalyst (0.1 g) and the solvent toluene was heated at 383 K in a round-bottomed flask with a distillation condensor to remove methanol or ethanol. The reaction was monitored by TLC. After completion, the reaction mixture was cooled, filtered and the filtrate was concentrated and chromatographed on silica gel using light petroleum and diethyl ether as eluent to afford the pure transesterified products.Acknowledgment
The project was supported by the National Natural Science Foundation of China (29872011 and 29572039), Educational Ministry of China, Educational Department of Hebei Province (990104) and Science and Technology Commission of Hebei Province.References
- T. Fujita, M. Tanaka, Y. Norimine and H. Suemune, J. Org. Chem., 1997, 62, 3824 CrossRef; G. Shapiro and M. Marli, J. Org. Chem., 1997, 62, 7096 CrossRef CAS.
- X. Otera, J. Chem. Rev., 1993, 93, 1449 Search PubMed.
- R. Clemens, J. Chem. Rev., 1986, 86, 241 Search PubMed.
- D. F. Taber, J. C. Amedio and Y. K. Patte, J. Org. Chem., 1985, 50, 3618 CrossRef CAS; J. C. Gilbert and T. A. Kelly, J. Org. Chem., 1988, 53, 449 CrossRef CAS.
- J. S. Gilbert and W. D. Nottingham, J. Org. Chem., 1991, 56, 1713 CrossRef CAS.
- S. P. Chavan, P. K. Zubaidha, S. W. Dantale, A. Keshavaraja, A. V. Ramaswamy and T. Ravindranathan, Tetrahedron Lett., 1996, 37, 233 CrossRef CAS.
- B. S. Balaji, M. Sasidharan, R. Kumar and M. Chanda, Chem. Commun., 1996, 707 RSC; B. S. Balaji and M. Chanda, Tetrahedron, 1998, 54, 13237 CrossRef CAS.
- D. E. Ponde, V. H. Deshpande, V. J. Bulbule, A. Sudalai and A. S. Gajare, J. Org. Chem., 1998, 63, 1058 CrossRef.
- B. M. Reddy, V. R. Reddy and B. Manohar, Synth. Commun., 1999, 29, 1235 CAS.
- B. P. Bandgar, V. S. Sadavarte and L. S. Uppalla, Synth. Commun., 2001, 31, 2063 CrossRef CAS.
- P. Kumar and R. K. Pandey, Synlett., 2000, 2, 251.
- B. P. Bandgar, L. S. Uppalla and V. S. Sadavarte, Green Chem., 2001, 3, 39 RSC.
- T. S. Li, L. J. Li, B. Lu and F. Yang, J. Chem. Soc., Perkin Trans. 1, 1998, 3561 RSC.
- T. S. Jin, X. Sun and T. S. Li, J. Chem. Res. (S), 2000, 128 Search PubMed.
- T. S. Li and T. S. Jin, Org. Chem. (Chinese), 1996, 16, 385 Search PubMed.
- T. S. Li, S. H. Li, J. T. Li and H. Z. Li, J. Chem. Res. (S), 1997, 26 RSC.
- J. S. Witzeman, Tetrahedron Lett., 1990, 31, 1401 CrossRef CAS.
- R. J. Clemens and J. S. Witzeman, J. Am. Chem. Soc., 1989, 111, 2186 CrossRef CAS.
- D. S. Campbell and C. W. Lawrie, Chem. Commun., 1971, 355 RSC.
| This journal is © The Royal Society of Chemistry 2002 |