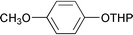
A green chemical method for the direct conversion of alcohol tetrahydropyranyl ethers into the corresponding acetates
Takeshi Oriyama*, Kumiko Kobayashi, Takeshi Suzuki and Mihoko Oda
Department of Environmental Sciences, Faculty of Science, Ibaraki University, 2-1-1 Bunkyo, Mito, 310-8512, Japan. E-mail: tor@mito.ipc.ibaraki.ac.jp
First published on 21st January 2002
Abstract
Alcohol tetrahydropyranyl ethers are readily converted into the corresponding acetates by the reaction with acetyl bromide in acetonitrile. Using various substituted acetyl chlorides with an equimolar amount of sodium iodide, tetrahydropyranyl ethers can be also transformed into the corresponding substituted acetates in high yields. This clean synthesis is a significant method for atom economy.
Green ContextWhile Green Chemistry teaches us to avoid protecting groups whenever possible, they remain important in many areas of organic synthesis. It is important therefore that we develop the most efficient and low-waste methods for protection and de-protection. Here the direct conversion of tetrahydropyranyl ethers to the corresponding acetates in one pot is described. No catalyst is required. The methodology is simple and inexpensive, operated under mild conditions and is very atom efficient.JHC |
Introduction
A great number of protecting groups for the hydroxy function have been extensively explored in organic synthesis1 and in these methods, acetates and substituted acetates are widely used as well as other type protecting groups, such as silyl ether, acetal, and alkyl ether types. In a series of studies2 on the direct transformation of a protecting group of the hydroxy function into another, we have demonstrated that silyl ether, and alkyl ether protecting groups for hydroxy functions can be efficiently converted into the corresponding acetates in good yields under the influence of tin(II) bromide and acetyl bromide in dichloromethane.3 These results suggested a one-step transformation of alcohol THP ethers into acetates. Some useful direct transformations into acetates from tetrahydropyranyl ethers under various reaction conditions, including acidic and basic conditions were reported.4 We considered that a catalyst-free transformation that can be carried out stoichiometrically is very important as an environmentally benign reaction.In this communication, we wish to report a novel and highly efficient and convenient method for the direct conversion of tetrahydropyranyl ethers into the corresponding acetates or substituted acetates in a one-pot procedure.
Results and discussion
Initially, we examined the reaction of the THP ether of 3-phenylpropanol with acetyl chloride in acetonitrile at room temperature. After the reaction mixture was stirred for 3 h at this temperature, usual work-up afforded 3-phenylpropyl acetate in 6% yield (Table 1, entry 1). On the other hand, when using acetyl bromide instead of acetyl chloride, the yield of the corresponding acetate improved dramatically (Table 1, entry 2). Therefore, we used acetyl bromide as the acyl halide. After a screening of various solvents, we found that acetonitrile as a solvent gave the desired acetate in the highest yield (entries 2–6).Representative examples of this direct conversion of various alcohol THP ethers into the corresponding acetates are collected in Table 2. THP ethers of primary and secondary alcohols were transformed into the corresponding acetates in excellent yields (entries 1–5). In the case of the THP ether substrates having other functional groups such as a benzoate and benzyl ether, the THP ether moieties were transformed into the corresponding acetates selectively (entries 6 and 7). The bis-THP ether of an aliphatic 1,6-diol was readily converted into the corresponding bis-acetate in 98% yield (entry 8). Furthermore, phenolic THP ethers were also successfully transformed into the corresponding acetates in good yields (entries 9 and 10).
In order to explore the generality and scope of this reaction, we examined a reaction of the THP ether of 3-phenylpropanol with acetyl chloride. As mentioned above, when a reaction was performed with only acetyl chloride, the desired acetate was hardly obtained (Table 3, entry 1), while the yield was improved when sodium bromide or sodium iodide was used as an additive as shown in Table 3. It was found that an equimolar amount of sodium iodide was an excellent promoter in this one-step conversion into acetate, and that the corresponding acetate was obtained in 91% yield (entry 5). Next, we examined the reaction of the THP ether of 3-phenylpropanol with various substituted acetyl chlorides in the presence of an equimolar amount of sodium iodide. Successful results are shown in Table 4. Various substituted acetyl chlorides tested worked well to afford the desired substituted acetates in high yields.
In summary, we have described a novel direct conversion of alcohol THP ethers into the corresponding acetates using only acetyl bromide without catalyst. In addition, THP ethers were readily converted into the corresponding substituted acetates in the presence of an equimolar amount of sodium iodide. Although the reaction with only acetyl chloride scarcely proceeded, we found that the corresponding acetate was obtained in good yields in the presence of an equimolar amount of sodium iodide. Hence THP ethers were readily converted into the corresponding substituted acetats. The procedure can be performed without any difficulty employing readily available chemicals, and the reaction proceeded smoothly at room temperature under very mild reaction conditions. Moreover, because the reagent is almost completely consumed, the reaction is expected to be extremely effective from the viewpoint of atom economy.
Experimental
Typical procedure for the direct conversion of alcohol THP ethers into the corresponding acetates
1-Tetrahydropyranyloxy-3-phenylpropane (66.8 mg, 0.30 mmol) was added to acetyl bromide (44.3 mg 0.36 mmol) in CH3CN (1 ml) at room temperature under argon atmosphere. The reaction mixture was stirred for 1 h at room temperature and quenched with phosphate buffer (pH 7). The organic materials were extracted with Et2O and dried over anhydrous magnesium sulfate. The solvent was evaporated and 3-phenylpropyl acetate (52.9 mg, 99%) was isolated by thin-layer chromatography on silica gel (diethyl ether–hexane = 1∶3). The product gave satisfactory 1H NMR and IR spectra. IR: 3025, 2950, 1740, 1365, 1240, 1035, 745, 700 cm−1; 1H NMR (CDCl3): δ 1.96 (m, 2H, CH2), 2.05 (s, 3H, CH3), 2.69 (t, 2H, J 7.6 Hz, CH2), 4.09 (t, 2H, J 6.4 Hz, CH2), 7.17–7.31 (m, 5H, Ph); 13C NMR (CDCl3): δ 20.95 (CH3), 30.15 (CH2), 32.16 (CH2), 63.83 (CH2), 125.98 (Ph), 128.37 (Ph), 128.42 (Ph), 141.18 (Ph), 171.16 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).Acknowledgements
Financial support from the Uehara Memorial Foundation is gratefully acknowledged.References
- (a) T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, New York, 3rd edn., 1999 Search PubMed; (b) P. J. Kocienski, Protecting Groups, Georg Thieme Verlag, Stuttgart, New York, 1994 Search PubMed.
- (a) T. Oriyama, M. Kimura and G. Koga, Bull. Chem. Soc. Jpn., 1994, 67, 885 CAS; (b) T. Oriyama, K. Yatabe, Y. Kawada and G. Koga, Synlett, 1995, 45 CrossRef CAS; (c) T. Oriyama, K. Yatabe, S. Sugawara, Y. Machiguchi and G. Koga, Synlett,, 1996, 523 CrossRef CAS; (d) T. Oriyama, K. Noda and K. Yatabe, Synlett, 1997, 701 CAS; (e) T. Oriyama, K. Noda and S. Sugawara, Synth. Commun., 1999, 29, 2217 CAS; (f) T. Suzuki, K. Ohashi and T. Oriyama, Synthesis,, 1999, 1561 CrossRef CAS; (g) T. Suzuki, K. Kobayashi, K. Noda and T. Oriyama, Synth. Commun., 2001, 31, 2761 CrossRef CAS.
- (a) T. Oriyama, M. Kimura, M. Oda and G. Koga, Synlett, 1993, 437 CrossRef CAS; (b) T. Oriyama, M. Oda, J. Gono and G. Koga, Tetrahedron Lett., 1994, 35, 2027 CrossRef CAS.
- (a) M. Jacobson, R. E. Redferm, W. A. Jones and M. H. Aldridge, Science, 1970, 170, 543; (b) M. Schwarz and R. M. Waters, Synthesis, 1972, 567 CrossRef CAS; (c) S. Kim and W. J. Lee, Synth. Commun., 1986, 16, 659 CAS; (d) T. Bakos and I. Vincze, Synth. Commun., 1989, 19, 523; (e) S. Chandrasekhar, T. Ramachandar, M. V. Reddy and M. Takhi, J. Org. Chem., 2000, 65, 4729 CrossRef CAS; (f) K. L. Chandra, P. Saravanan and V. K. Singh, Tetrahedron Lett., 2001, 42, 5309 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |