A highly stereoselective synthesis of β-olivosides by glycosidations of 2-iodo-olivosyl fluoride using montmorillonite K-10 as an environmentally benign solid acid
Kazunobu Toshima*, Keizou Uehara, Hideyuki Nagai and Shuichi Matsumura
Department of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama, 223-8522, Japan. E-mail: toshima@applc.keio.ac.jp
First published on 14th January 2002
Abstract
The novel and highly β-stereoselective glycosidations of a 2-iodo-olivosyl fluoride and alcohols using a heterogeneous and environmentally benign solid acid, montmorillonite K-10, have been developed to afford the corresponding 2-iodo-β-olivosides in high yields with high stereoselectivities.
Green Contextβ-Olivosides are representative of a versatile class of compounds with applications in biological systems, and in materials chemistry. Synthesis of these by displacement of a fluoride leaving group by an incoming alcohol has been found to proceed very well in the presence of a cheap and simple clay catalyst. The reaction requires the presence of molecular sieves to avoid hydrolysis. Solvent effects on the reaction are dificult to understand, and currently the best system involves dichloromethane.DJM |
Introduction
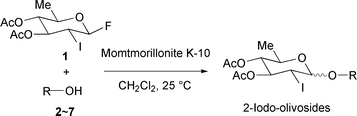
Highly effective, simple and environmentally benign glycosidations have attracted considerable attention in current synthetic organic chemistry related to both biomolecules and functional materials.1 In this context, some of the challenges for the greening of the chemical glycosidations may include the use of a heterogeneous and reusable solid acid as the activator.2 On the other hand, deoxy sugars frequently appear in the sugar components of bioactive substances.3,4 Among them, olivoside, a typical 2-deoxy (2,6-dideoxy) glycoside, is one of the most common and important, and found in many biologically important natural products such as aureolic acids, angucyclines, avilamycins, concanamycins, venturicidins, etc.3,4 However, the β-stereoselective glycosidation of 2-deoxy sugars has proved particularly difficult to achieve due to the anomeric effect and the lack of stereodirecting anchimeric assistance from the C-2 position.1c,5,6 The most extensively developed strategy for the synthesis of 2-deoxy-β-glycosides utilizes donors with equatorial C-2 heteroatom substituents that are easily and reductively removed after the glycosidation event. However, most of these methods use a homogeneous Lewis acid as the activator which contaminates the reaction solvent and can not be reused. Furthermore, these homogeneous Lewis acids are volatile, corrosive and have an odor. In contrast, heterogeneous catalysts are easily handled due to their nonvolatile, noncorrosive and odorless properties.We now report the new and highly stereoselective synthesis of β-olivosides by the glycosidations of 2-iodo-olivosyl fluoride and alcohols using a heterogeneous and environmentally friendly solid acid, montmorillonite K-10, as illustrated in Fig. 1.
 | ||
| Fig. 1 | ||
Results and discussion
During our initial attempts of searching for a new activator, we examined the glycosidations of the 3,4-di-O-acetyl-2-iodo-β-D-olivosyl fluoride (1)7,8 and cyclohexylmethanol (2) in CH2Cl2 using several heterogeneous solid acids such as montmorillonite K-10 (K-10),9 Nafion-H®10 and SO4/ZrO2,11 all of which could be recovered from the reaction mixture by simple filtration and then reused. These results are summarized in Table 1. It was found that montmorillonite K-10, Nafion-H® and SO4/ZrO2 could activate the anomeric C–F bond of 1,12 and that montmorillonite K-10 was superior to the other solid acids with respect to both the chemical yield and stereoselectivity to give the corresponding 2-iodo-β-olivoside in moderate yield (entries 1–3 in Table 1).† Our attention next turned to the solvent and the dehydrating agent effect in this glycosidation. Therefore, we tested the glycosidations of 1 and 2 using montmorillonite K-10 in several solvents, CH2Cl2, CH3CN or Et2O, in the presence or absence of molecular sieves 5A (MS 5A).13 Although CH3CN was found to be superior to CH2Cl2 and Et2O in the absence of MS 5A, a considerable amount of the corresponding 1-hydroxyolivose was generated as a result of the hydrolysis of 1 (entry 5 in Table 1). On the other hand, the glycosidation was best affected in the presence of MS 5A in CH2Cl2 (entry 6 in Table 1). Surprisingly, the glycosidations in the presence of MS 5A in CH3CN and Et2O did not proceed at all (entries 7 and 8 in Table 1). Furthermore, the use of 100 wt% montmorillonite K-10 and 100 wt% MS 5A significantly increased the chemical yield (entry 10 in Table 1). Thus, the glycosidation of 1 and 2 using 100 wt% montmorillonite K-10 in the presence of 100 wt% MS 5A in CH2Cl2 at 25 °C for 5 h proceeded effectively to afford the corresponding 2-iodo-olivoside, the precursor of olivoside, in 98% yield with 92∶8 β-stereoselectivity. In addition, when half the amounts of montmorillonite K-10 and MS 5A were employed, a longer reaction time gave a similar result (entry 11 in Table 1). Thus, the glycosidation of 1 and 2 using 50 wt% montmorillonite K-10 in the presence of 50 wt% MS 5A in CH2Cl2 at 25 °C for 20 h yielded the corresponding 2-iodo-olivoside in 95% yield with 91∶9 β-stereoselectivity.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Solid acid (wt%) | MS (wt%) | Solvent | Time/h | Yield (%)b | α/β Ratioc |
| a All reactions were carried out by use of 2.0 equiv. of 2 to 1.b Isolated yields after purification by column chromatography.c α∶β Ratios were determined by 1H NMR (270 MHz) spectroscopy and/or isolation of pure isomers. K-10: montmorillonite K-10. | ||||||
| 1 | Nafion-H® (20) | — | CH2Cl2 | 5 | 56 | 10∶90 |
| 2 | SO4/ZrO2 (20) | — | CH2Cl2 | 5 | 19 | 10∶90 |
| 3 | K-10 (20) | — | CH2Cl2 | 5 | 65 | 5∶95 |
| 4 | K-10 (20) | — | Et2O | 5 | 11 | 31∶69 |
| 5 | K-10 (20) | — | MeCN | 5 | 88 | 10∶90 |
| 6 | K-10 (20) | MS 5A (100) | CH2Cl2 | 5 | 90 | 8∶92 |
| 7 | K-10 (20) | MS 5A (100) | Et2O | 5 | 0 | — |
| 8 | K-10 (20) | MS 5A (100) | MeCN | 5 | 0 | — |
| 9 | K-10 (50) | MS 5A (100) | CH2Cl2 | 5 | 93 | 9∶91 |
| 10 | K-10 (100) | MS 5A (100) | CH2Cl2 | 5 | 98 | 8∶92 |
| 11 | K-10 (50) | MS 5A (50) | CH2Cl2 | 20 | 95 | 9∶91 |
To enhance the synthetic utility of this novel and simple reaction, glycosidations using other primary and secondary alcohols 3–7 were next examined. Based on the results shown in Table 2, all the glycosidations of 1 and 3–7, as well as that of 2, effectively proceeded under similar conditions to give the corresponding 2-iodo-β-olivosides in high yields with good to high stereoselectivities. It was noted that even the hindered carbohydrate glycosyl acceptor 7 smoothly reacted with 1 to produce the corresponding disaccharide in high yield with good β-stereoselectivity. Furthermore, a low reaction temperature was not required for obtaining good to high β-stereoselectivities. It was also confirmed that no epimerization at the anomeric center occurred during the glycosidation due to the mild reaction conditions. Finally, we tested the catalyst recycling. After filtration, washing with methanol and heating at 100 °C/1 mmHg for 12 h, a mixture of montmorillonite K-10 and MS 5A was reused two times showing moderate yields. Since the ease and effective conversion of the 2-iodo substituent in the glycosides into hydrogen using H2 in the presence of Pd-C or Bu3SnH is well known,1c,5,6,8 the present novel method provides a significantly new way to stereoselectively synthesize β-olivosides.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Alcohol (ROH) | Wt% of K-10 | Wt% of MS 5A | Time/h | Yield (%)b | α/β Ratioc |
| a All reactions were carried out by use of 2.0 equiv. of the alcohol to 1.b Isolated yields after purification by column chromatography.c α∶β Ratios were determined by 1H NMR (270 MHz) spectroscopy and/or isolation of pure isomers. | ||||||
| 1 | 2 | 100 | 100 | 5 | 98 | 8∶92 |
| 2 | 2 | 50 | 50 | 20 | 95 | 9∶91 |
| 3 | 3 | 100 | 100 | 5 | 95 | 8∶92 |
| 4 | 3 | 50 | 50 | 20 | 93 | 9∶91 |
| 5 | 4 | 100 | 100 | 5 | 98 | 7∶93 |
| 6 | 4 | 50 | 50 | 20 | 97 | 7∶93 |
| 7 | 5 | 100 | 100 | 5 | 86 | 9∶91 |
| 8 | 5 | 50 | 50 | 20 | 97 | 9∶91 |
| 9 | 6 | 100 | 100 | 5 | 93 | 17∶83 |
| 10 | 6 | 50 | 50 | 20 | 90 | 18∶82 |
| 11 | 7 | 100 | 100 | 5 | 88 | 19∶81 |
| 12 | 7 | 50 | 50 | 20 | 88 | 17∶83 |
Conclusions
In conclusion, we have presented a new and highly stereoselective synthesis of β-olivosides by the glycosidations of 2-iodo-olivosyl fluoride and alcohols using a heterogeneous and environmentally acceptable solid acid, montmorillonite K-10. The results including the simple and environmentally friendly protocol, high yield and stereoselectivity should find widespread application for the synthesis of biologically important natural products which possess β-olivoside as a sugar component.Experimental
Typical procedure
To a stirred solution of 3,4-di-O-acetyl-2-iodo-β-D-olivosyl fluoride (1) (0.5 mmol) and an alcohol (1.0 mmol) in dry CH2Cl2 (5.0 ml) were added MS 5A (100 wt% to the glycosyl donor 1) and montmorillonite K-10 (100 wt% to the glycosyl donor 1). After stirring at 25 °C for 5 h, the mixture was filtered and the filtrate was concentrated in vacuo. Purification of the residue by flash column chromatography gave the corresponding 2-iodo-olivosides which predominately contained the β-anomer. All 2-iodo-olivosides were purified by silica gel column chromatography and were fully characterized by spectroscopic means. The configurations of the anomeric centers were clearly confirmed by the coupling constants between H-1 and H-2 in the 1H NMR analyses.Acknowledgments
We thank the New Energy and Industrial Technology Development Organization (NEDO) and the Research Institute of Innovative Technology for the Earth (RITE) for financial support.Notes and references
- For some reviews on O-glycosidations, see : R. R. Schmidt, Angew. Chem., Int. Ed. Engl., 1986, 25, 212 Search PubMed; P. Sinaÿ, Pure Appl. Chem., 1991, 63, 519 CrossRef; K. Toshima and K. Tatsuta, Chem. Rev., 1993, 93, 1503 CrossRef CAS; G.-J. Boons, Tetrahedron, 1996, 52, 1095 CrossRef CAS; Preparative Carbohydrate Chemistry, ed. S. Hanessian, Marcel Dekker, New York, 1977, ch. 12–22 CrossRef CAS; B. G. Davis, J. Chem. Soc., Perkin Trans. 1, 2000, 2137 CrossRef CAS.
- For glycosidations using a reusable solid acid, see: (a) J.-C. Florent and C. Monneret, J. Chem. Soc., Chem. Commun., 1987, 1171 RSC; (b) K. Fukase, H. Winarno and S. Kusumoto, Chem. Express, 1993, 8, 409 Search PubMed; (c) K. Toshima, T. Ishizuka, G. Matsuo and M. Nakata, Synlett, 1995, 306 CrossRef CAS; (d) K. Toshima, N. Miyamoto, G. Matsuo, M. Nakata and S. Matsumura, Chem. Commun., 1996, 1379 RSC; (e) K. Toshima, Y. Ushiki, G. Matsuo and S. Matsumura, Tetrahedron Lett., 1997, 38, 7375 CrossRef CAS; (f) K. Toshima, K. Kasumi and S. Matsumura, Synlett, 1998, 643 CAS; (g) K. Toshima, K. Kasumi and S. Matsumura, Synlett, 1999, 813 CAS; (h) T. Jyojima, N. Miyamoto, Y. Ogawa, S. Matsumura and K. Toshima, Tetrahedron Lett., 1999, 40, 5023 CrossRef CAS; (i) H. Nagai, K. Kawahara, S. Matsumura and K. Toshima, Tetrahedron Lett., 2001, 42, 4159 CrossRef CAS.
- P. Jütten and R. Greven, in Polysaccharides in Medicinal Applications, ed. S. Dumitriu, Marcel Dekker, New York, 1996, p. 339 Search PubMed.
- A. Kirschning, A. F.-W. Bechtold and J. Rohr, Top. Curr. Chem., 1997, 188, 1 Search PubMed.
- J. Thiem and W. Klaffke, Top. Curr. Chem., 1990, 154, 285 Search PubMed.
- C. H. Marzabadi and R. W. Frank, Tetrahedron, 2000, 56, 8385 CrossRef CAS.
- 2-Iodo-olivosyl fluoride (1) was stereoselectively prepared from 2-iodo-olivosyl acetate in two steps in 63% overall yield (i, NH2NH2 (aq.), MeOH–Et2O, 25 °C, 0.5 h; ii. DAST, THF, −30 to 0 °C, 0.5 h). Also see ref. 8(a).
- For recent glycosidations using 2-iodo-2-deoxyglycosyl acetate and trichloroacetimidate donors, see: (a) W. R. Roush, K. Briner and D. P. Sebesta, Synlett, 1993, 264 CrossRef CAS; (b) W. R. Roush, R. A. Hartz and D. J. Gustin, J. Am. Chem. Soc., 1999, 121, 1990 CrossRef CAS; (c) W. R. Roush, B. W. Gung and C. E. Bennett, Org. Lett., 1999, 1, 891 CrossRef CAS; (d) W. R. Roush and S. Narayan, Org. Lett., 1999, 1, 899 CrossRef CAS; (e) W. R. Roush and C. E. Bennett, J. Am. Chem. Soc., 1999, 121, 3541 CrossRef CAS; (f) W. R. Roush and C. E. Bennett, J. Am. Chem. Soc., 2000, 122, 6124 CrossRef CAS; (g) D. Lafont, P. Boullanger and M. Rosenzweig, J. Carbohydr. Chem., 1998, 17, 1377 Search PubMed; (h) A. Kirschning, Eur. J. Org. Chem., 1998, 2267 CrossRef CAS.
- Montmorillonite K-10 was purchased from Aldrich Chemical Company, Inc. and dried at 200 °C/1 mmHg for 12 h before use.
- Nafion-H® was purchased from Wako Pure Chemical Industries, Ltd. as Nafion® NR-50 and dried at 25 °C/1 mmHg for 2 h before use.
- SO4/ZrO2 was purchased from Wako Pure Chemical Industries, Ltd. and dried at 200 °C/1 mmHg for 12 h before use.
- For activation of an anomeric C–F bond with a protic acid, see: (a) T. Mukaiyama, H. Jona and K. Takeuchi, Chem. Lett., 2000, 696 CrossRef CAS; (b) H. Jona, K. Takeuchi and T. Mukaiyama, Chem. Lett., 2000, 1278 CAS; (c) H. Jona, H. Mandai and T. Mukaiyama, Chem. Lett., 2001, 426 CrossRef CAS ; see also ref. 2(f).
- We and the group of Mukaiyama found that MS 5A is superior to other MS such as MS 3A and MS 4A in glycosidations using a protic acid, see: refs. 2(f) and 12(b).
Footnote |
| † Taking into account of the surface areas of the used montmorillonite K-10 and Nafion-H®, the activity of montmorillonite K-10 as an acid is comparable to that of Nafion-H®. |
| This journal is © The Royal Society of Chemistry 2002 |