Coordination polymer complexes of 4,4′-dipyridyldisulfide and AgX (X = PF6−, ClO4−, OTs−, NO3−, BF4−) with twisted rhomboid networks, 2-D sheets, and 1-D chain structures
Ryo
Horikoshi
a,
Tomoyuki
Mochida
*ab,
Noriko
Maki
a,
Sachiko
Yamada
c and
Hiroshi
Moriyama
a
aDepartment of Chemistry, Faculty of Science, Toho University, Funabashi, Chiba 274-8510, Japan. E-mail: mochida@chem.sci.toho-u.ac.jp
bPRESTO, Japan Science and Technology Corporation, Japan
cDepartment of Biomolecular Science, Faculty of Science, Toho University, Funabashi, Chiba 274-8510, Japan
First published on 28th November 2001
Abstract
Several AgI coordination polymers linked by the –S–S– bridged ligand 4-PDS (= 4,4′-dipyridyldisulfide) were prepared and their crystal structures characterized. The isomorphous complexes [Ag(4-PDS)2·PF6] 1 and [Ag(4-PDS)2·ClO4] 2 have twisted rhomboidal frameworks that consist of four AgI ions and four 4-PDS N-donor ligands, and show inclusion of the counter anions in their chiral cavities. The AgI ion has a tetrahedral coordination, and the rhomboidal units are assembled in the form of a 2-D sheet structure. The complex [Ag(4-PDS)·OTs] 3 (where OTs− = p-toluenesulfonate) shows a 2-D sheet structure, constructed from 1-D zigzag chains bridged by interchain Ag–Ag interactions. The AgI ion has a T-shaped coordination environment, and the OTs− anions are located between the sheets. The complex [Ag(4-PDS)·NO3·(CH3CN)0.5] 4 is a 1-D zigzag coordination polymer with a chiral chain structure, incorporating acetonitrile as a guest molecule between the chains.
Introduction
The synthesis and characterization of supramolecular coordination compounds has been investigated extensively in the last decade.1 These coordination compounds afford a variety of assembled structures and cavities, and also provide a reaction environment for guest molecules.2 Although various metal ions are used for constructing coordination polymers, the AgI ion in particular is known to produce a variety of coordination environments such as linear, T-shaped, tetrahedral, and octahedral.1d When combined with bidentate ligands, this leads to various types of polymeric structures such as 1-D (linear chain,3 zigzag chain,4 helix,5 stair-type,6 ladder7 and square mesh8), 2-D (honeycomb,9 brick,10 entanglement11 and pillar12), and 3-D network13 structures.In order to realize coordination polymers with unique extended networks, we have combined the AgI ion with the bidentate ligand of 4,4′-dipyridyldisulfide (Scheme 1), abbreviated as 4-PDS, with various counter anions. The advantages of the 4-PDS ligand are that: i) the twisted structure, due to the –S–S– bridge, may lead to topologically interesting molecular assemblies; ii) the presence of the chalcogen moiety may lead to unique network structures9 because of its bonding ability to soft metal ions; and in particular, iii) the twisted structure accompanies the axial chirality, generating the P- and the M-forms of optical antipodes, as shown in Scheme 1. Thus, the introduction of 4-PDS into coordination polymers is of special interest in terms of chiral crystal engineering, or for realizing optical properties14 such as fluorescence or non-linear phenomena. We have recently reported on the complexation of the 4-PDS ligand with M(hfac)2 (M = Cu, Mn, hfac = 1,1,1,5,5,5-hexafluoroacetylacetone), which afforded 1-D coordination polymers with helical or zigzag chain structures.15 Kitagawa and co-workers have also reported on a coordination polymer with Cd(NO3)2.16 We further combined the 4-PDS ligand with various AgI salts and obtained the following coordination polymers: [Ag(4-PDS)2·PF6] 1; [Ag(4-PDS)2·ClO4] 2; [Ag(4-PDS)·OTs] 3; and [Ag(4-PDS)·NO3·(CH3CN)0.5] 4, that show various kinds of interesting network structures.
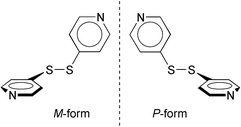 | ||
| Scheme 1 Optical antipodes of 4,4′-dipyridyldisulfide (4-PDS). | ||
Experimental
General methods
All reagents were commercially available. Infrared spectra were recorded on a JASCO FT-IR 230 spectrometer as KBr pellets for 1, 3, and 4, and on a JASCO FT-IR 5300 spectrometer as a Nujol mull for 2 in the 4000–400 cm−1 range. Elemental analyses were performed by using a Yanagimoto C–H–N recorder (MT-3). Solid state UV-vis spectra were recorded on a JASCO V-570 UV/VIS/NIR spectrophotometer. Emission spectra were obtained on a SPEX FluoroMax spectrophotometer.Preparations
Crystal structure analyses
All the X-ray data were collected using Mo-Kα radiation on a Rigaku AFC-5S four-circle diffractometer. Crystal data, data collection parameters and analysis statistics for 1–4 are listed in Table 1. Selected interatomic distances and angles are given in Table 2. All calculations were performed using the teXsan crystallographic software package.17 These structures were solved by direct methods (SIR9218 or SHELX-9719) and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically, and absorption correction was applied (ψ-scans). The acetonitrile molecule in 4 was found from the Fourier map, and the position was fixed during refinements. Though some of the hydrogen atoms could be located by the Fourier syntheses, all hydrogen atoms were inserted at the calculated positions using a rigid model.CCDC reference numbers 172107–172110.
See http://www.rsc.org/suppdata/dt/b1/b106032p/ for crystallographic data in CIF or other electronic format.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
a
R
1
=
Σ![[double-rule fence; left big]](https://www.rsc.org/images/entities/char_e0ec.gif) Fo|
−
|Fc Fo|
−
|Fc![[double-rule fence; right big]](https://www.rsc.org/images/entities/char_e0ed.gif) /Σ|Fo|; Rw
= [Σw(F2o
−
F2c)2/Σw(F2o)2]1/2. /Σ|Fo|; Rw
= [Σw(F2o
−
F2c)2/Σw(F2o)2]1/2.
|
||||
| Formula | C20F6H16N4PS4Ag | C20ClH16N4O4S4Ag | C17H15N2O3S3Ag | C11H9.5N3.5O3S2Ag |
| Formula weight | 693.45 | 647.93 | 499.37 | 410.71 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C 2/c | C 2/c | P21/n | P21/c |
| a/Å | 11.068(6) | 10.739(3) | 10.611(6) | 4.957(2) |
| b/Å | 19.811(5) | 19.527(1) | 9.640(3) | 19.291(3) |
| c/Å | 11.485(6) | 11.208(1) | 18.684(3) | 15.335(2) |
| β/° | 95.30(4) | 92.29(2) | 94.06(2) | 91.76(2) |
| V/Å3 | 2507(1) | 2348.4(7) | 1906(1) | 1465.6(7) |
| Z | 4 | 4 | 4 | 4 |
| μ(Mo-Kα)/cm−1 | 12.62 | 13.62 | 14.02 | 16.67 |
| T/K | 296 | 100 | 296 | 296 |
| Measured reflections | 3121 | 2929 | 4888 | 3872 |
| Observed reflections | 2999 | 2816 | 4380 | 3373 |
| R 1, Rwa | 0.039 0.120 | 0.030, 0.106 | 0.112, 0.305 | 0.096, 0.261 |
| Symmetry codes used to generate equivalent atoms. For 1: (i) −x + 2, y, −z + 3/2. For 2: (i) x − 1/2, y − 1/2, z; (ii) −x + 1/2, y − 1/2, −z + 1/2; (iii) −x, y, −z + 1/2. For 3: −x + 1, −y, −z; (ii) −x + 1/2, y + 1/2, −z + 1/2; (iii) −x + 1, −y + 1, −z. | |||
|---|---|---|---|
| Complex 1 | |||
| Ag–N(1) | 2.333(3) | Ag–N(2) | 2.297(3) |
| S(1)–S(2) | 2.028(2) | N(1)–Ag–N(1i) | 106.1(1) |
| N(1)–Ag–N(2) | 117.4(1) | N(2)–Ag–N(2i) | 109.7(1) |
| N(2)–Ag–N(1i) | 103.4(1) | ||
| Complex 2 | |||
| Ag–N(1) | 2.318(2) | Ag–N(2i) | 2.282(2) |
| S(1)–S(2) | 2.033(1) | N(1i)–Ag–N(1ii) | 107.8(1) |
| N(1i)–Ag–N(2) | 117.47(8) | N(2)–Ag–N(2iii) | 115.6(1) |
| N(2)–Ag–N(1i) | 99.56(8) | ||
| Complex 3 | |||
| Ag–N(1i) | 2.175(8) | Ag–N(2ii) | 2.167(8) |
| Ag–Ag(iii) | 3.044(2) | S(1)–S(2) | 1.984(6) |
| N(1)–Ag–N(2) | 162.6(3) | N(1)–Ag–Ag(i) | 102.1(2) |
| N(2)–Ag–Ag(i) | 88.5(2) | ||
| Complex 4 | |||
| Ag–N(1) | 2.139(9) | Ag–N(2) | 2.145(8) |
| S(1)–S(2) | 2.022(4) | N(1)–Ag–N(2) | 166.2(4) |
Results and discussion
A. Two-dimensional coordination polymers with a rhomboidal framework: [Ag(4-PDS)2·PF6] 1 and [Ag(4-PDS)2·ClO4] 2
The molecular structure and coordination environment around the AgI ion in 1 are shown in Fig. 1(a), together with the atomic numbering scheme. Selected bond lengths and angles are listed in Table 2. Each 4-PDS unit acts as a bidentate ligand and exhibits a typical twisted conformation (vide infra). The AgI ion in 1 shows tetrahedral coordination, in which the N(1)–Ag–N(2*) and N(1)–Ag–N(1*) angles are 117.4(1) and 106.1(1)°, and the Ag–N(1) and Ag–N(2*) bond lengths are 2.333(3) and 2.297(3) Å, respectively. A local coordination environment scheme similar to that found in 1 is found in [{Ag(pyz)3}(BF4)] (pyz = pyrazine).20 Complex 2, with a slightly smaller ClO4− counter anion, is isomorphous with 1. The coordination geometries are almost comparable for both complexes, as shown in Table 2. | ||
| Fig. 1 (Top, a) An ORTEP29 drawing of 1 with the atom numbering scheme, showing the silver coordination environment. Displacement ellipsoids are shown at the 50% probability level. (Middle, b) The twisted rhomboidal framework of 1. Only one PF6− counter anion is shown for clarity. Also shown is a schematic illustration of the framework. (Bottom, c) Packing diagram of 1 viewed along the c-axis. Only one PF6− counter anion is shown for clarity. | ||
Fig. 1(b) shows the unique twisted rhomboidal framework of 1 formed by the four 4-PDS ligands and the four AgI ions. Interestingly, the shape of the unit is somewhat analogous to a Möbius strip, as schematically illustrated in the figure. The rhomboidal cavity accommodates two PF6− counter anions. The units are further interconnected and extend as a 2-D sheet, as shown in Fig. 1(c). It is noteworthy that the cavity is a chiral one, consisting only of either the M- or P-form of the ligand. Thus the assembled 2-D sheet is chiral, as schematically shown in Scheme 2(a), although the structures of 1 and 2 have a centre of symmetry (space group C2/c) and show no chirality. The centrosymmetry of the crystals originates from alternate stacking of sheets with opposite chirality.
 | ||
| Scheme 2 Schematic illustration of the chirality and structures of coordination polymers from 4-PDS and AgI ions: (a) for 1 and 2, (b) for 3, and (c) for 4. | ||
The coordination environment around the AgI ion in these complexes bears some resemblance to that in [{Ag2(bpethy)5}(BF4)2] (bpethy = 1,2-bis(4-pyridyl)ethyne),11 although the bpethy complex has a square cavity structure because of the linear shape of the ligand. Thus, the formation of the unique twisted cavity in the present case can be understood in terms of the characteristic torsion of the 4-PDS ligand. Indeed, coordination polymers of [Cd(py–X–py)2·(NO3)2] (py = 4-pyridyl, X = CH2 or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)21 and [Cd(4-PDS)2(H2O)2]·2NO3·2EtOH·2H2O,16 formed by more flexible ligands, show flat cavities.
CH2)21 and [Cd(4-PDS)2(H2O)2]·2NO3·2EtOH·2H2O,16 formed by more flexible ligands, show flat cavities.
B. A two-dimensional coordination polymer formed by the interchain Ag–Ag interactions: [Ag(4-PDS)·OTs] 3
The molecular structure and the coordination environment around the AgI ion in 3 are shown in Fig. 2(a), together with the atomic numbering scheme. Selected bond lengths and angles are listed in Table 2. Fig. 2(b) shows the 2-D sheet structure of 3, constructed from 1-D zigzag chains bridged by interchain Ag–Ag interactions. The AgI ion has a T-shaped coordination, with N(1)–Ag–N(2) and N(1)–Ag–Ag* bond angles of 162.6(3) and 102.1(2)°, and Ag–N(1), Ag–N(2), and Ag⋯Ag* distances of 2.175(8), 2.167(8), and 3.044(2) Å, respectively. Fig. 2(c) shows the packing structure of 3 including the OTs− counter anions. These anions are located between the sheets, with the molecular planes oriented parallel to the 2-D sheet of the coordination polymers. From the viewpoint of chirality, the 1-D unit consists of alternate linkages of the M- and P-forms of the ligands with the AgI ions. The chain is thus represented as –M–(Ag)–P–(Ag)–M–(Ag)–P–, as schematically shown in Scheme 2(b), resulting in an achiral structure with centrosymmetric space group P21/n. | ||
| Fig. 2 (Top, a) An ORTEP drawing of 3 with the atom numbering scheme, showing the silver coordination environment. Displacement ellipsoids are shown at the 50% probability level. (Middle, b) The 2-D sheet structure of 3. (Bottom, c) Packing diagram of 3 viewed along the b-axis. The OTs− anions are omitted for clarity. | ||
A local coordination environment of AgI similar to that exhibited in 3 is found in [Ag(bpp)·OTf·EtOH] (where bpp = 1,3-bis(4-pyridyl)propane, OTf = trifluoromethanesulfonate). The AgI⋯AgI distances of several coordination polymers retrieved from the Cambridge Structure Database are compared in Table 3, in order of increasing bond distance. Although the nature of the interactions is not fully understood, Ag⋯Ag distances of 2.50–3.00 Å are considered to originate from weak d10–d10 aurophilic interactions.22 Even in compounds with longer Ag⋯Ag distances, e.g., [Ag(NO3)3(py2S)2·2H2O], an Ag⋯Ag distance shorter than for other interactions, such as S⋯S or π–π, is claimed to be indirect evidence of Ag⋯Ag interactions.23 Solid state UV-Vis absorption measurement showed that 3 has a small absorption band at 425 nm, and a fluorescence is observed at 600 nm when this band is excited. As reported by Che and co-workers, the absorption and the fluorescence properties may originate from the d10–d10 interaction.24 This interpretation is further supported by the fact that no fluorescence was observed for complexes 1, 2, and 4, which have no Ag⋯Ag interactions.
| Compounda | Ag⋯Ag distance/Å | Ref. |
|---|---|---|
| a bpy = bipyridine, OTs = p-toluenesulfonate, tren(mim)3 = tris{2-[2-(1-methyl)imidazolyl]methyliminoethyl}amine, bpp = 1,3-bis(4-pyridyl)propane, OTf = trifluoromethanesulfonate, pydz = pyridazine, 3,3′-dcpa = 3,3′-dicyanodiphenylacetylene, py2S = 4,4′-bispyridylsulfide. | ||
| [Ag(4,4′-bpy)·NO3] | 2.78(1) | 22a |
| [Ag(4,4′-bpy)·NO3] | 2.970(2) | 13b |
| [Ag(4-PDS)·OTs] | 3.044(2) | This work |
| [Ag2(tren(mim)3)]n(NO3)2n·nH2O | 3.053(1) | 3a |
| [Ag(bpp)·OTf·EtOH] | 3.089(1) | 5d |
| [Ag(2,4′-bpy)·ClO4] | 3.1526(6) | 5b |
| [Ag(pydz)2·BF4] | 3.282(1) | 5a |
| [Ag(3,3′-dcpa)·OTf·C6H6] | 3.377(1) | 22b |
| [Ag3(NO3)3(Py2S)2·2H2O] | 3.436(2) | 23 |
C. A one-dimensional coordination polymer with a simple zigzag chain structure: [Ag(4-PDS)·NO3·(CH3CN)0.5] 4
The molecular structure and the coordination environment around the AgI ion in 4 are shown in Fig. 3(a), together with the atomic numbering scheme. Selected bond lengths and angles are listed in Table 2. This is a simple 1-D zigzag coordination polymer, as shown in Fig. 3(b). Each AgI ion is coordinated by two N atoms from the 4-PDS ligand in a linear geometry in which the Ag–N(1), Ag–N(2) bond distances and the N(1)–Ag–N(2) bond angle are 2.139(9) and 2.145(8) Å and 166.2(4)°, respectively. | ||
| Fig. 3 (Top, a) An ORTEP drawing of 4 with the atom numbering scheme, showing the silver coordination environment. Displacement ellipsoids are shown at the 50% probability level. (Middle, b) The 1-D zigzag chain of 4. (Bottom, c) Packing diagram of 4 viewed along the a-axis. | ||
Fig. 3(c) shows the packing structure of 4, and includes the counter anion and CH3CN molecules, which are interposed between the chains. Although the NO3− anion is located beside the AgI ion, there seems to be no coordination bond between them since the Ag⋯O(3) distance of 2.86(2) Å is a little longer than a typical Ag⋯O interaction distance.4,5a,9,22a,25 Each 1-D chain unit is chiral, and is represented by either –M–(Ag)–M–(Ag)–M–(M-chain) or –P–(Ag)–P–(Ag)–P–(P-chain), as schematically shown in Scheme 2(b). The complex consists of a 1 ∶ 1 ratio of P- and M-chains, and is achiral with a centrosymmetric space group (P21/c).
A local coordination environment similar to that exhibited in complex 4, is found in [Ag(4-bpbd)NO3·CH3CN] (4-bpbd = 1,4-bis(4-pyridyl)butadiyne).26 Complex 4 shows no significant interaction between the chains; the interchain distances of Ag⋯Ag, Ag⋯O(2*) and S(1)⋯S(1*) are ca. 4.9, 3.37(1), and 3.73 Å, respectively, which are longer than the usual contact distances encountered. This contrasts with the py–S–py bridged coordination polymer [Ag3(NO3)3(py2S)2·2H2O], which shows several interchain interactions, such as Ag⋯Ag and π–π type.23
D. Assembled structures of 4-PDS coordination polymers
The AgI complexes with 4-PDS are found to display interesting assembled structures and dimensionalities as shown above, in contrast to the relatively simple 1-D coordination polymers of [4-PDS·M(hfac)2], on which we have reported previously.15 We have assumed that the coordinational variation of AgI as well as the presence of counter anions has led to the structural variety in the present complexes. It is known that the crystal structures of coordination polymers are highly influenced by the shape of the counter anion, which may act as the template of the coordination polymer. For example, the anion dependence of the coordination polymer assembly for the complex from AgI and bpsb (= 1,2-bis[(2-pyrimidinyl)sulfanylmethyl]benzene) has been demonstrated by Hong and co-workers.9 We speculate that the counter anions may serve as a template in the formation of complexes 1 and 2, producing the Möbius-strip-like cavity accommodating the anions.We next focus on the conformation of the 4-PDS ligand. The bpe (= 1,2-bis(4-pyridyl)ethane) ligand, bearing the –CH2–CH2– bridging group, is more flexible than 4-PDS, thus the ligand conformation of bpe is highly affected by intermolecular interactions.27 In contrast, the 4-PDS ligand maintains its characteristic conformation as described above, despite the difference in the coordination schemes. The S–S bond length and the C–S–S–C torsion angles in 4-PDS existing in the present complexes are listed in Table 4, together with those for [4-PDS·M(hfac)2] 2 (M = Cu, Mn). These values are very close to the typical values found in aromatic disulfides,28 regardless of the metal species, coordination environment, and counter anion. Thus, the disulfide moiety is shown to be rather rigid, maintaining its characteristic shape even when coordinated to metal ions. This characteristic has produced the structural uniqueness of the present compounds.
The most interesting feature of the present system is the chirality of the ligand and its consequence for the assembled structures. The chirality of the present complexes is schematically shown in Scheme 2. It is interesting to note that, as described above, chiral structural units are found as a 2-D sheet in 1 and 2, and as a 1-D chain in 4. In M(hfac)2 complexes, [4-PDS·Cu(hfac)2] shows 1-D chiral chains, similar to complex 4, whereas [4-PDS·Mn(hfac)2] shows 1-D achiral chains, similar to 3. The complex [Cd(4-PDS)2(H2O)2]·2NO3·2EtOH·2H2O16 has an achiral 1-D chain structure. Thus, all the complexes so far obtained are achiral crystals, with centrosymmetric space groups. From these comparisons, a general structural correlation is found for the system: when the 1-D chain unit is achiral, represented as –P–(M)–M–(M)–P–, it forms a zigzag chain structure as exhibited by 3 and [4-PDS·Mn(hfac)2], while complexes with chiral chains form a helical chain structures such as exhibited by 4 and [4-PDS·Cu(hfac)2]. The twisted structure of the chiral cavities in 1 and 2 is also ascribable to be the consequence of the axial chirality of the bridging ligand.
In conclusion, we have prepared coordination polymers from AgI and 4-PDS, demonstrating the dimensionality from 1-D to 2-D structures. This study also showed that the twisted ligand is interesting from the viewpoint of asymmetrical crystal engineering. As we could obtain 1-D and 2-D chiral structural units in this study, the construction of a chiral crystal seems to be feasible, the structure and physical properties of which would be highly intriguing.
Acknowledgements
We thank Ms. Y. Sato (School of Pharmaceutical Sciences, Toho University) for elemental analyses. We also thank Prof. Dr. Y. Yamamoto and Mr. J. Seta (Toho University) for their help in measuring infrared spectra.References
- For example:
(a) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS
; (b) S. Kitagawa and M. Kondo, Bull. Chem. Soc. Jpn., 1998, 71, 1739 CAS
; (c) M. J. Zaworotko, Chem. Commun., 2001, 1 RSC
; (d) M. Munakata, L.-P. Wu and T. Kuroda-Sowa, Adv. Inorg. Chem., 1999, 46, 173 Search PubMed
and references cited therein.
- For example:
(a) S. Leininger, B. Olenyuk and J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS
; (b) M. Fujita, Chem. Soc. Rev., 1998, 27, 417 RSC
and references cited therein.
-
(a) S.-P. Yang, H.-L. Zhu, X.-H. Yin, X.-M. Chen and L.-N. Ji, Polyhedron, 2000, 19, 2237 CrossRef CAS
; (b) W. Su, R. Cao, M. Hong, W.-T. Wong and J. Lu, Inorg. Chem. Commun., 1999, 2, 241 CrossRef CAS
.
- C. Richardson and P. J. Steel, Inorg. Chem. Commun., 1998, 1, 260 CrossRef CAS
.
-
(a) L. Carlucci, G. Ciani, D. M. Proserpio and A. Sironi, Inorg. Chem., 1998, 37, 5941 CrossRef CAS
; (b) M.-L. Tong, X.-M. Chen, B.-H. Ye and S. W. Ng, Inorg. Chem., 1998, 37, 5278 CrossRef CAS
; (c) U. Schroder, L. Beyer and J. Sieler, Inorg. Chem. Commun., 2000, 3, 630 CrossRef CAS
; (d) L. Carlucci, G. Ciani, D. W. v. Gudenberg and D. M. Proserpio, Inorg. Chem., 1997, 36, 3812 CrossRef CAS
.
- C. Kaes, M. W. Hosseini, C. E. F. Rickard, B. W. Skelton and A. H. White, Angew. Chem., Int. Ed., 1998, 37, 920 CrossRef CAS
.
- M.-L. Tong, X.-M. Chen and S. W. Ng, Inorg. Chem. Commun., 2000, 3, 436 CrossRef CAS
.
- Y. Suenaga, T. Kuroda-Sowa, M. Munakata and M. Maekawa, Polyhedron, 1998, 18, 191 CrossRef
.
- M. Hong, W. Su, R. Cao, M. Fujita and J. Lu, Chem. Eur. J., 2000, 6, 427 CrossRef CAS
.
- S. Liao, C.-Y. Su, C.-H. Yeung, A.-W. Xu, H.-X. Zhang and H.-Q. Liu, Inorg. Chem. Commun., 2000, 3, 405 CrossRef CAS
.
- L. Carlucci, G. Ciani and D. M. Preserpio, Chem. Commun., 1999, 449 RSC
.
- G. K. H. Shimizu, G. D. Enright, C. I. Ratciffe and J. A. Ripmeester, Chem. Commun., 1999, 461 RSC
.
-
(a) D. Venkataraman, S. Lee, J. S. Moore, P. Zhang, K. A. Hirsch, G. B. Gardner, A. C. Covey and C. L. Prentice, Chem. Mater., 1996, 8, 2030 CrossRef CAS
; (b) F. Robinson and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1995, 2413 RSC
; (c) Y. Suenaga, T. Kamiya, T. Kuroda-Sowa, M. Maekawa and M. Munakata, Inorg. Chim. Acta, 2000, 308, 17 CrossRef CAS
; (d) A. J. Blake, N. R. Champness, S. S. M. Chung, W.-S. Li and M. Schröder, Chem. Commun., 1997, 1675 RSC
; (e) W.-M. Bu, L. Ye and Y.-G. Fan, Inorg. Chem. Commun., 2000, 3, 194 CrossRef CAS
; (f) M.-L. Tong, S.-L. Zheng and X.-M. Chen, Chem. Commun., 1999, 561 RSC
; (g) I. Ino, J. C. Zhong, M. Munakata, T. Kuroda-Sowa, M. Maekawa, Y. Suenaga and Y. Kitamori, Inorg. Chem., 2000, 39, 4273 CrossRef CAS
; (h) X.-H. Bu, K. Biradha, T. Yamaguchi, M. Nishimura, T. Ito, K. Tanaka and M. Shionoya, Chem. Commun., 2000, 1953 RSC
; (i) L. Carlucci, G. Ciani, D. M. Preserpio and A. Sironi, Angew. Chem., Int. Ed. Engl., 1995, 34, 1895 CrossRef CAS
.
-
(a) M. A. S. Goher and F. A. Mautner, Polyhedron, 2000, 19, 601 CrossRef CAS
; (b) M. A. S. Goher, Q.-C. Yang and T. C. W. Mak, Polyhedron, 2000, 19, 615 CrossRef CAS
; (c) G. Mago, M. Hinago, H. Miyasaka, N. Matsumoto and H. Okawa, Inorg. Chim. Acta, 1997, 254, 145 CrossRef CAS
.
- R. Horikoshi, T. Mochida and H. Moriyama, Inorg. Chem., 2001, 40, 2430 CrossRef CAS
.
- M. Kondo, M. Shimamura, S. Noro, Y. Kimura, K. Uemura and S. Kitagawa, J. Solid State Chem., 2000, 152, 113 CrossRef CAS
.
-
teXsan: Crystal Structure Analysis Package
, Molecular Structure Corporation, The Woodlands, TX, 1985 and 1999 Search PubMed
.
- A. Altomare, M. C. Burla, M. Camalli, M. Cascarano, C. Giacovazzo, A. Guagliardi and G. Polidori, J. Appl. Crystallogr., 1994, 27, 435 CrossRef
.
-
G. M. Sheldrick, SHELX-97: Program for the Solution of Crystal Structures, University of Göttingen, Germany, 1997 Search PubMed
.
- L. Carlucci, G. Ciani, D. M. Proserpio and A. Sironi, J. Am. Chem. Soc., 1995, 117, 4562 CrossRef CAS
.
- M. Fujita, M. Aoyagi and K. Ogura, Bull. Chem. Soc. Jpn., 1998, 71, 1799 CAS
.
-
(a) O. M. Yaghi and H. Li, J. Am. Chem. Soc., 1996, 118, 295 CrossRef CAS
; (b) K. A. Hirsch, S. R. Wilson and J. S. Moore, Inorg. Chem., 1997, 36, 2960 CrossRef CAS
.
- O.-S. Jung, S. H. Park, C. H. Park and K. Park, Chem. Lett., 1999, 923 CrossRef CAS
.
-
(a) R.-H. Uang, C.-K. Chan, S.-M. Peng and C.-M. Che, J. Chem. Soc., Chem. Commun., 1994, 2561 RSC
; (b) V. J. Catalano, H. M. Kar and B. L. Bennett, Inorg. Chem., 2000, 39, 121 CrossRef CAS
; (c) P. D. Harvey and H. B. Gray, J. Am. Chem. Soc., 1988, 110, 2145 CrossRef CAS
.
- R. L. LaDuca Jr., R. S. Rarig Jr., P. J. Zapf and J. Zubieta, Solid State Science, 2000, 2, 39 Search PubMed
.
- M. Maekawa, H. Konaka, Y. Suenaga, T. Kuroda-Sowa and M. Munakata, J. Chem. Soc., Dalton Trans., 2000, 4160 RSC
.
- Y.-B. Dong, M. D. Smith, R. C. Layland and H.-C. zur Loye, Inorg. Chem., 1999, 38, 5027 CrossRef CAS
.
-
(a) N. V. Raghavan and K. Seff, Acta Crystallogr., Sect. B, 1977, 33, 386 CrossRef
; (b) L. S. Higashi, M. Lundeen and K. Seff, J. Am. Chem. Soc., 1978, 100, 8101 CrossRef CAS
.
-
C. K. Johnson, ORTEP, Report ORNL-5138, Oak Ridge National Laboratory, Oak Ridge, TN, 1976 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2002 |
