Synthesis, characterisation and some reactions of alkynyl derivatives [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) CX]
CX]
John F.
Gallagher
*a,
Peter
Butler
b,
Richard D. A.
Hudson
b and
Anthony R.
Manning
*b
aSchool of Chemical Sciences, Dublin City University, Dublin 9, Ireland. E-mail: John.Gallagher@dcu.ie
bDepartment of Chemistry, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: Anthony.Manning@ucd.ie
First published on 10th December 2001
Abstract
Oxalic acid catalyses the hydrolysis of the Ni(II) acetylide [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2] 1, to the alkynylaldehyde [Ni(η5-C5H5)(PPh3)C
CCH(OEt)2] 1, to the alkynylaldehyde [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2, in high yield. Condensation reactions of 2 with phenylhydrazine and dinitrophenylhydrazine in the presence of acetic acid, and with malononitrile and 3-phenyl-5-isoxazolone (C9H7NO2) in the presence of triethylamine yield [Ni(η5-C5H5)(PPh3)C
CCHO] 2, in high yield. Condensation reactions of 2 with phenylhydrazine and dinitrophenylhydrazine in the presence of acetic acid, and with malononitrile and 3-phenyl-5-isoxazolone (C9H7NO2) in the presence of triethylamine yield [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] derivatives where X = CH
CX] derivatives where X = CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H53, CH
NNHC6H53, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2-2,4 4,
CH
NNHC6H3(NO2)2-2,4 4,
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)25, and CH
C(CN)25, and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO26. The reactivity of [Ni(η5-C5H5)(PPh3)C
C9H5NO26. The reactivity of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] complexes towards [Co2(CO)8] is a function of X. Thus 1 and 2, where X = CH(OEt)2 or CHO, react readily to give the bridging alkyne derivatives [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
CX] complexes towards [Co2(CO)8] is a function of X. Thus 1 and 2, where X = CH(OEt)2 or CHO, react readily to give the bridging alkyne derivatives [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2}{Co2(CO)6}] 7, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
CCH(OEt)2}{Co2(CO)6}] 7, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8, but 5, where X is the strongly electron-withdrawing CH
CCHO}{Co2(CO)6}] 8, but 5, where X is the strongly electron-withdrawing CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2 group, does not react even after 24 h at room temperature. Furthermore, coordination of the alkyne to a Co2(CO)6 fragment appears to inhibit the normal reactions of the group X in 7 and 8. Thus the acetal grouping in 7 does not undergo oxalic acid-catalysed hydrolysis to an aldehyde in 8, and the aldehyde function in 8 does not undergo a Knoevenagel condensation with CH2(CN)2. The IR spectra of 1, and 3–6 show a single ν(C
C(CN)2 group, does not react even after 24 h at room temperature. Furthermore, coordination of the alkyne to a Co2(CO)6 fragment appears to inhibit the normal reactions of the group X in 7 and 8. Thus the acetal grouping in 7 does not undergo oxalic acid-catalysed hydrolysis to an aldehyde in 8, and the aldehyde function in 8 does not undergo a Knoevenagel condensation with CH2(CN)2. The IR spectra of 1, and 3–6 show a single ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) band the frequency of which
decreases along the series X = CH(OEt)2 > CH
C) band the frequency of which
decreases along the series X = CH(OEt)2 > CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H5 > CH
NNHC6H5 > CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2-2,4 > CH
NNHC6H3(NO2)2-2,4 > CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2
≈ CH
C(CN)2
≈ CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2; that of 2 is anomalous in that it can show two ν(C
C9H5NO2; that of 2 is anomalous in that it can show two ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) bands. The UV-visible spectra of 1–6 show a strong charge transfer absorption band which increases in wavelength 1 < 3 < 2 < 4 < 5 < 6. These spectroscopic data and the 13C chemical shifts suggest that the (η5-C5H5)(Ph3P)Ni
moiety is a donor and, when X is an acceptor, charge separated cumulenic mesomers such as Ni+
C) bands. The UV-visible spectra of 1–6 show a strong charge transfer absorption band which increases in wavelength 1 < 3 < 2 < 4 < 5 < 6. These spectroscopic data and the 13C chemical shifts suggest that the (η5-C5H5)(Ph3P)Ni
moiety is a donor and, when X is an acceptor, charge separated cumulenic mesomers such as Ni+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X− contribute to the description of the bonding in 1–6. This is not reflected in the molecular dimensions of 1, 2 and 5 as determined by X-ray diffraction. However, the crystal structure of [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
X− contribute to the description of the bonding in 1–6. This is not reflected in the molecular dimensions of 1, 2 and 5 as determined by X-ray diffraction. However, the crystal structure of [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}], 8, shows that the C2Co2 cluster core is severely distorted because of the strong donor (Ni) and acceptor (CHO) substituents on the acetylenic carbon atoms.
CCHO}{Co2(CO)6}], 8, shows that the C2Co2 cluster core is severely distorted because of the strong donor (Ni) and acceptor (CHO) substituents on the acetylenic carbon atoms.
Introduction
Transition-metal σ-acetylide complexes have attracted considerable interest as precursors in the expanding field of organometallic derivatives that contain π-conjugated systems. These have potential applications in nonlinear optics,1 liquid crystal technology, dendrimer science and nanostructured materials for molecular devices.2,3 Attention has focused on the acetylide group because of its accessibility and the ease with which it can be incorporated into organometallic complexes and multimetallic assemblies, and because C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C can provide a facile pathway for electron delocalization between interacting metal centres.3–5 In this context, nickel cyclopentadienyl alkynyl derivatives having the general formulae [Ni(η5-C5H5)(PR3)C
C can provide a facile pathway for electron delocalization between interacting metal centres.3–5 In this context, nickel cyclopentadienyl alkynyl derivatives having the general formulae [Ni(η5-C5H5)(PR3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR]6,7 are of current
interest in donor–π-acceptor systems.5,8–10
CR]6,7 are of current
interest in donor–π-acceptor systems.5,8–10
Here, we report studies into the synthesis, structures, and spectra of a series of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] complexes which were obtained from the alkynylaldehyde [Ni(η5-C5H5)(PPh3)C
CX] complexes which were obtained from the alkynylaldehyde [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] by condensation reactions with hydrazines and some active methylene compounds (Scheme 1). We have found that some, but not all of these alkynes, form [(μ-alkyne){Co2(CO)6}] complexes, and that the presence of the Co2(CO)6 moiety appears to affect some of the characteristic reactions of the group X. The structures of [Ni(η5-C5H5)(PPh3)C
CCHO] by condensation reactions with hydrazines and some active methylene compounds (Scheme 1). We have found that some, but not all of these alkynes, form [(μ-alkyne){Co2(CO)6}] complexes, and that the presence of the Co2(CO)6 moiety appears to affect some of the characteristic reactions of the group X. The structures of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2, [Ni(η5-C5H5)(PPh3)C
CCHO] 2, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2] 5,
and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
C(CN)2] 5,
and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8, have been determined by single crystal X-ray diffraction and compared with those of related compounds.8–12
CCHO}{Co2(CO)6}] 8, have been determined by single crystal X-ray diffraction and compared with those of related compounds.8–12
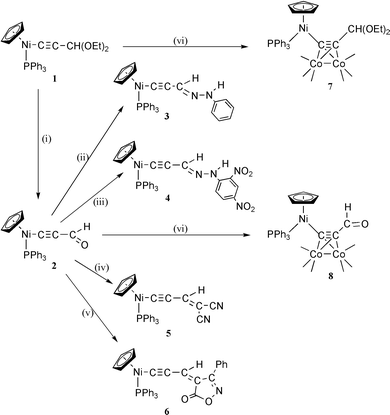 | ||
| Scheme 1 Synthetic route to the complexes 1–8 (CO ligands have been omitted from the Co2(CO)6 moiety for the sake of clarity). (i) H2O/oxalic acid; (ii) PhNHNH2/H+; (iii) C6H3(NO2)2NHNH2/H+; (iv) CH2(CN)2/Et3N; (v) C9H7NO2/Et3N; (vi) Co2(CO)8. | ||
Experimental
[Ni(η5-C5H5)(PPh3)Br] was prepared according to literature methods.13 Other chemicals were purchased from the usual sources.All reactions were carried out under N2 in dried and deoxygenated solvents unless stated otherwise. They were monitored by IR or NMR spectroscopy where appropriate.
IR spectra were recorded on a Perkin-Elmer 1710 FT spectrometer (peak positions are in cm−1 with relative peak heights in parentheses) and UV-visible spectra were recorded on a UNICAM UV2 spectrometer (band positions are in nm with intensities ε in dm3 mol−1 cm−1). NMR spectra were obtained in CDCl3 solution on a Jeol JNM-GX270 FT-NMR spectrometer. 1H (270 MHz) and 13C (67.8 MHz) chemical shifts are reported downfield from tetramethylsilane as internal standard; 31P (109.3 MHz) spectra are referenced to 85% phosphoric acid with downfield shifts reported as positive. Analyses were carried out in the Microanalytical Laboratory, University College Dublin.
Preparation of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCH(OEt)2], 1
CCH(OEt)2], 1
A solution of CuI (5 mg, 0.026 mmol), [Ni(η5-C5H5)(PPh3)Br] (0.5 g, 1.1 mmol) and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2 (0.14 g, 1.1 mmol) in Et3N (50 cm3) was stirred overnight. The solvent was removed under reduced pressure and the residue dissolved in Et2O. This solution was filtered, chromatographed (basic alumina/Et2O), and recrystallized from Et2O–hexane to give green crystals of [Ni(η5-C5H5)(PPh3)C
CCH(OEt)2 (0.14 g, 1.1 mmol) in Et3N (50 cm3) was stirred overnight. The solvent was removed under reduced pressure and the residue dissolved in Et2O. This solution was filtered, chromatographed (basic alumina/Et2O), and recrystallized from Et2O–hexane to give green crystals of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH(OEt)2] 1 (0.43 g, 80%), mp 76–77 °C (Found: C, 70.6; H, 6.0%. C30H31NiO2P requires C, 70.2; H, 6.1%). νmax/cm−1 (C
CH(OEt)2] 1 (0.43 g, 80%), mp 76–77 °C (Found: C, 70.6; H, 6.0%. C30H31NiO2P requires C, 70.2; H, 6.1%). νmax/cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2112 (CH2Cl2); (C
C) 2112 (CH2Cl2); (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2109 (KBr); λmax/nm (CH2Cl2)
305 (ε/dm3 mol−1 cm−1 15 200 ); λmax/nm (CH3CN) 303 (ε/dm3 mol−1 cm−1 14 900); δH(CDCl3) 7.26–7.72 [15 H, m, Ph], 5.16 [5 H, s, C5H5], 4.92 [1 H, s, C
C) 2109 (KBr); λmax/nm (CH2Cl2)
305 (ε/dm3 mol−1 cm−1 15 200 ); λmax/nm (CH3CN) 303 (ε/dm3 mol−1 cm−1 14 900); δH(CDCl3) 7.26–7.72 [15 H, m, Ph], 5.16 [5 H, s, C5H5], 4.92 [1 H, s, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH], 3.10 [4 H, q, J(HH) 6.8 Hz, OCH2] and 0.89 [6 H, t, J(HH) 6.8 Hz, CH3]; δC(CDCl3) 134.0 [d, J(CP) 11 Hz, o-Ph], 133.9 [d, J(CP) 48 Hz, i-Ph], 130.2 [d, J(CP) 2 Hz, p-Ph], 128.2 [d, J(CP) 10 Hz, m-Ph], 114.2 [s, NiC
CCH], 3.10 [4 H, q, J(HH) 6.8 Hz, OCH2] and 0.89 [6 H, t, J(HH) 6.8 Hz, CH3]; δC(CDCl3) 134.0 [d, J(CP) 11 Hz, o-Ph], 133.9 [d, J(CP) 48 Hz, i-Ph], 130.2 [d, J(CP) 2 Hz, p-Ph], 128.2 [d, J(CP) 10 Hz, m-Ph], 114.2 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 92.7 [s, η5-C5H5], 82.0 (d, J(CP) 40 Hz, NiC
C], 92.7 [s, η5-C5H5], 82.0 (d, J(CP) 40 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 71.7 [s, C
C], 71.7 [s, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH], 59.3 s, OCH2] and 15.1 [s, CH3]; δP(CDCl3)
41.24.
CCH], 59.3 s, OCH2] and 15.1 [s, CH3]; δP(CDCl3)
41.24.
Preparation of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO], 2
CCHO], 2
A solution of oxalic acid (0.03 g, 0.21 mmol) in H2O (10 cm3) was added to one of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH(OEt)2] 1 (0.1 g, 0.2 mmol) in tetrahydrofuran (10 cm3). The reaction mixture was stirred for 1 h at room temperature and then shaken with CHCl3 (100 cm3) and water (50 cm3). The CHCl3 layer was washed with water, dried with magnesium sulfate, and chromatographed (alumina/CH2Cl2–Et2O). The green band was evaporated to dryness and the residue crystallised from dichloromethane–hexane mixtures to give green crystals of [Ni(η5-C5H5)(PPh3)C
CH(OEt)2] 1 (0.1 g, 0.2 mmol) in tetrahydrofuran (10 cm3). The reaction mixture was stirred for 1 h at room temperature and then shaken with CHCl3 (100 cm3) and water (50 cm3). The CHCl3 layer was washed with water, dried with magnesium sulfate, and chromatographed (alumina/CH2Cl2–Et2O). The green band was evaporated to dryness and the residue crystallised from dichloromethane–hexane mixtures to give green crystals of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2 (0.07 g, 80%), mp 118–119 °C (Found: C, 71.6; H, 5.0%. C26H21NiOP
requires C, 71.1; H, 4.8%); νmax/cm−1 (C
CCHO] 2 (0.07 g, 80%), mp 118–119 °C (Found: C, 71.6; H, 5.0%. C26H21NiOP
requires C, 71.1; H, 4.8%); νmax/cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2079(7) and 2034(3), (CO) 1625(10), 1622(10) (CH2Cl2); (C
C) 2079(7) and 2034(3), (CO) 1625(10), 1622(10) (CH2Cl2); (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2081(7) and 2034(3), (CO) 1627(10) (KBr); λmax/nm (CH2Cl2) 364 (ε/dm3 mol−1 cm−1 16 100 ); λmax/nm (CH3CN) 359 (ε/dm3 mol−1 cm−1 16 000); δH(CDCl3) 8.41 [1 H, s, CHO], 7.37–7.70 [15 H, m, Ph] and 5.22 [5 H, s, C5H5]; δC(CDCl3) 174.4 [s, CHO], 133.7 [d, J(CP) 11 Hz, o-Ph], 132.9 (d, J(CP) 49 Hz, i-Ph], 130.5 (d, J(CP) 2 Hz, p-Ph), 128.3 (d, J(CP) 11 Hz, m-Ph], 124.6 [s, NiC
C) 2081(7) and 2034(3), (CO) 1627(10) (KBr); λmax/nm (CH2Cl2) 364 (ε/dm3 mol−1 cm−1 16 100 ); λmax/nm (CH3CN) 359 (ε/dm3 mol−1 cm−1 16 000); δH(CDCl3) 8.41 [1 H, s, CHO], 7.37–7.70 [15 H, m, Ph] and 5.22 [5 H, s, C5H5]; δC(CDCl3) 174.4 [s, CHO], 133.7 [d, J(CP) 11 Hz, o-Ph], 132.9 (d, J(CP) 49 Hz, i-Ph], 130.5 (d, J(CP) 2 Hz, p-Ph), 128.3 (d, J(CP) 11 Hz, m-Ph], 124.6 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C],
122.8 (d, J(CP) 43 Hz, NiC
C],
122.8 (d, J(CP) 43 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C] and 93.27 [s, C5H5]; δP(CDCl3) 42.64.
C] and 93.27 [s, C5H5]; δP(CDCl3) 42.64.
The reaction of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO] with hydrazines
CCHO] with hydrazines
[Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2 (0.5 g, 1.1 mmol) and phenylhydrazine, H2NNHC6H5 (0.11 g, 1.1 mmol), were dissolved in dichloromethane (50 cm3). A few drops of glacial acetic acid were added to the mixture which was then stirred for 2 h at 35 °C. The solution colour changed from brown to red. The solvent was removed in vacuo and the residue dissolved in CH2Cl2, layered with n-hexane and stored at −20 °C overnight. Filtration gave [Ni(η-C5H5)(PPh3)C
CCHO] 2 (0.5 g, 1.1 mmol) and phenylhydrazine, H2NNHC6H5 (0.11 g, 1.1 mmol), were dissolved in dichloromethane (50 cm3). A few drops of glacial acetic acid were added to the mixture which was then stirred for 2 h at 35 °C. The solution colour changed from brown to red. The solvent was removed in vacuo and the residue dissolved in CH2Cl2, layered with n-hexane and stored at −20 °C overnight. Filtration gave [Ni(η-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H5] 3 as a brick-red powder (0.55 g, 90%), decomp. > 150 °C (Found: C, 72.1; H, 5.1; N, 5.4%. C32H27NiN2P requires C, 72.6; H, 5.1; N, 5.3%); νmax/cm−1
(C
NNHC6H5] 3 as a brick-red powder (0.55 g, 90%), decomp. > 150 °C (Found: C, 72.1; H, 5.1; N, 5.4%. C32H27NiN2P requires C, 72.6; H, 5.1; N, 5.3%); νmax/cm−1
(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2090(4.5), (C
C) 2090(4.5), (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) 1593(10) (CH2Cl2); (C
N) 1593(10) (CH2Cl2); (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2091(4.3), (C
C) 2091(4.3), (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) 1595(10) (KBr); λmax/nm (CH2Cl2) 350 (ε/dm3 mol−1 cm−1 12 200); λmax/nm (CH3CN) 354 (ε/dm3 mol−1 cm−1 10 100); δH(CDCl3) 6.51–7.76 [20 H, m, Ph and Ph3P), 5.25 [5 H, s, C5H5], 5.09 [1 H, s, NNH] and 4.22 [1 H, s, CH]; δC(CDCl3) 120.1–143.0 (Ph, PPh3), 113.0 [s, CH
N) 1595(10) (KBr); λmax/nm (CH2Cl2) 350 (ε/dm3 mol−1 cm−1 12 200); λmax/nm (CH3CN) 354 (ε/dm3 mol−1 cm−1 10 100); δH(CDCl3) 6.51–7.76 [20 H, m, Ph and Ph3P), 5.25 [5 H, s, C5H5], 5.09 [1 H, s, NNH] and 4.22 [1 H, s, CH]; δC(CDCl3) 120.1–143.0 (Ph, PPh3), 113.0 [s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N], 93.1 [s, NiC
N], 93.1 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 92.4 [s, η5-C5H5] and 91.4 [d, J(CP) 48 Hz, NiC
C], 92.4 [s, η5-C5H5] and 91.4 [d, J(CP) 48 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C].
C].
A similar procedure but with NH2NHC6H5 replaced by NH2NHC6H3(NO2)2-2,4 gave [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2-2,4] 4 (0.67 g, 95%) decomp. > 150 °C (Found: C, 61.2; H, 4.0; N, 8.7%. C32H25NiN4O4P requires C, 62.1; H, 4.1; N, 9.0%); νmax/cm−1 (C
NNHC6H3(NO2)2-2,4] 4 (0.67 g, 95%) decomp. > 150 °C (Found: C, 61.2; H, 4.0; N, 8.7%. C32H25NiN4O4P requires C, 62.1; H, 4.1; N, 9.0%); νmax/cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2082 and (NO2) 1617, 1600 (CH2Cl2); (C
C) 2082 and (NO2) 1617, 1600 (CH2Cl2); (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2082 and (NO2) 1615, 1601, (KBr); λmax/nm (CH2Cl2) 394 (ε/dm3 mol−1 cm−1 19 600 ); λmax/nm (CH3CN) 394 (ε/dm3
mol−1 cm−1 18 500); δH(CDCl3) 9.17 [1 H, s, CH
C) 2082 and (NO2) 1615, 1601, (KBr); λmax/nm (CH2Cl2) 394 (ε/dm3 mol−1 cm−1 19 600 ); λmax/nm (CH3CN) 394 (ε/dm3
mol−1 cm−1 18 500); δH(CDCl3) 9.17 [1 H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N], 8.26 [3 H, m, C6H3], 6.50–7.81 [15 H, m Ph3P], 6.23 [1 H, m, NNH) and 5.20 [5 H, s, C5H5]; δC 118–144.4 [m, C6H3(NO2)2], 133.8 [d, J(CP) 41 Hz, o-Ph), 133.1 [d, J(CP) 50 Hz, i-Ph], 130.5 [d, J(CP) 2 Hz, p-Ph], 128.2 [d, J(CP) 11 Hz, m-Ph], 117.0 [s, CH
N], 8.26 [3 H, m, C6H3], 6.50–7.81 [15 H, m Ph3P], 6.23 [1 H, m, NNH) and 5.20 [5 H, s, C5H5]; δC 118–144.4 [m, C6H3(NO2)2], 133.8 [d, J(CP) 41 Hz, o-Ph), 133.1 [d, J(CP) 50 Hz, i-Ph], 130.5 [d, J(CP) 2 Hz, p-Ph], 128.2 [d, J(CP) 11 Hz, m-Ph], 117.0 [s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N], 110.9 [s, NiC
N], 110.9 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 108.3 [d, J(CP) 48 Hz, NiC
C], 108.3 [d, J(CP) 48 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C] and 93.5 [s, η5-C5H5].
C] and 93.5 [s, η5-C5H5].
The reaction of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO] with malononitrile and 3-phenyl-5-isoxazolone
CCHO] with malononitrile and 3-phenyl-5-isoxazolone
A solution of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2 (0.1 g, 0.22 mmol) and CH2(CN)2 (0.015 g, 0.22 mmol) in CH2Cl2 (10 cm3) was stirred for 10 min. Et3N (3 drops) was added. A rapid colour change from green to deep red took place. The mixture was stirred for 2 h, and then the solvent removed at reduced pressure. The residue was crystallised from chloroform–hexane mixtures at −20 °C overnight and then filtered to give a red powder, [Ni(η5-C5H5)(PPh3)C
CCHO] 2 (0.1 g, 0.22 mmol) and CH2(CN)2 (0.015 g, 0.22 mmol) in CH2Cl2 (10 cm3) was stirred for 10 min. Et3N (3 drops) was added. A rapid colour change from green to deep red took place. The mixture was stirred for 2 h, and then the solvent removed at reduced pressure. The residue was crystallised from chloroform–hexane mixtures at −20 °C overnight and then filtered to give a red powder, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2] 5 (0.098 g, 90%), decomp. > 150 °C (Found: C, 71.4; H, 4.4; N, 5.8%. C29H21N2NiP requires C, 71.4; H, 4.3; N, 5.7%); νmax/cm−1 (C
C(CN)2] 5 (0.098 g, 90%), decomp. > 150 °C (Found: C, 71.4; H, 4.4; N, 5.8%. C29H21N2NiP requires C, 71.4; H, 4.3; N, 5.7%); νmax/cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)
2050(10), (CN) 2218(1), 2226(1), (C
C)
2050(10), (CN) 2218(1), 2226(1), (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 1534(2.1) (CH2Cl2); (C
C) 1534(2.1) (CH2Cl2); (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2049(10), (CN) 2216(1), 2225(1), (C
C) 2049(10), (CN) 2216(1), 2225(1), (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 1532(2.4) (KBr); λmax/nm (CH2Cl2) 469 (ε/dm3 mol−1 cm−1 13 000); λmax/nm (CH3CN) 463 (ε/dm3 mol−1 cm−1 11 200); δH(CDCl3) 7.33–7.62 [15 H, m, Ph3P], 6.43 [1 H, s, CH], 5.25 [5H, s, C5H5]; δC(CDCl3) 152.8 [d, J(CP) 48 Hz, NiC
C) 1532(2.4) (KBr); λmax/nm (CH2Cl2) 469 (ε/dm3 mol−1 cm−1 13 000); λmax/nm (CH3CN) 463 (ε/dm3 mol−1 cm−1 11 200); δH(CDCl3) 7.33–7.62 [15 H, m, Ph3P], 6.43 [1 H, s, CH], 5.25 [5H, s, C5H5]; δC(CDCl3) 152.8 [d, J(CP) 48 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 143.7 [s, HC
C], 143.7 [s, HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C], 134.1 [d, J(CP) 11 Hz, o-Ph], 133.2 (d, J(CP) 50 Hz, i-Ph], 131.4 (d, J(CP) 3 Hz, p-Ph], 129.1 [d, J(CP) 11 Hz, m-Ph], 121.5 [s, NiC
C], 134.1 [d, J(CP) 11 Hz, o-Ph], 133.2 (d, J(CP) 50 Hz, i-Ph], 131.4 (d, J(CP) 3 Hz, p-Ph], 129.1 [d, J(CP) 11 Hz, m-Ph], 121.5 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C],
114.5 [s, C
C],
114.5 [s, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N], 114.9 [s, C
N], 114.9 [s, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N], 94.2 [s, η5-C5H5] and 86.3 [d, J(CP) 2.1 Hz, C(CN)2].
N], 94.2 [s, η5-C5H5] and 86.3 [d, J(CP) 2.1 Hz, C(CN)2].
Using the same procedure 3-phenyl-5-isoxazolone, C9H7NO2 and [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2 gave purple [Ni(η5-C5H5)(PPh3)C
CCHO] 2 gave purple [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2] 6 (0.11g, 85%), decomp. > 50 °C (Found: C, 72.1; H, 4.5; N, 2.3%. C32H25NiN4O4P requires C, 72.2; H, 4.5; N, 2.4%); νmax/cm−1 (C
C9H5NO2] 6 (0.11g, 85%), decomp. > 50 °C (Found: C, 72.1; H, 4.5; N, 2.3%. C32H25NiN4O4P requires C, 72.2; H, 4.5; N, 2.4%); νmax/cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2050(6), (CO) 1690(10) (CH2Cl2); (C
C) 2050(6), (CO) 1690(10) (CH2Cl2); (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) 2051(6), (CO) 1692(10), (KBr); λmax/nm (CH2Cl2) 511 (ε/dm3 mol−1 cm−1 27 800 ); λmax/nm (CH3CN)
507 (ε/dm3 mol−1 cm−1 26 600); δH(CDCl3) 6.95–7.59 [21 H, m, Ph3P, Ph, CH
C) 2051(6), (CO) 1692(10), (KBr); λmax/nm (CH2Cl2) 511 (ε/dm3 mol−1 cm−1 27 800 ); λmax/nm (CH3CN)
507 (ε/dm3 mol−1 cm−1 26 600); δH(CDCl3) 6.95–7.59 [21 H, m, Ph3P, Ph, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ] and 5.22 [5H, s, C5H5]; δC(CDCl3) 140.3 [d, J(CP) 48 Hz, NiC
] and 5.22 [5H, s, C5H5]; δC(CDCl3) 140.3 [d, J(CP) 48 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 133.9 [d, J(CP) 12 Hz, o-Ph], 133.0 (d, J(CP) 50 Hz, i-Ph], 128.7–131.4 [m, Ph, isoxazolone], 130.7 [d, J(CP) 2 Hz, p-Ph], 128.6 [d, J(CP) 11 Hz, m-Ph], 120.7 [s, NiC
C], 133.9 [d, J(CP) 12 Hz, o-Ph], 133.0 (d, J(CP) 50 Hz, i-Ph], 128.7–131.4 [m, Ph, isoxazolone], 130.7 [d, J(CP) 2 Hz, p-Ph], 128.6 [d, J(CP) 11 Hz, m-Ph], 120.7 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C] and 96.7 [s, η5-C5H5].
C] and 96.7 [s, η5-C5H5].
The reaction of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CX] {X = CH(OEt)2, CHO and CHC(CN)2} with [Co2(CO)8]
CX] {X = CH(OEt)2, CHO and CHC(CN)2} with [Co2(CO)8]
[Co2(CO)8] (0.07 g, 0.22 mmol) was added to a solution of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] {0.22 mmol; X = CH(OEt)2 and CHO} in tetrahydrofuran (20 cm3). After 2 h the solution was filtered, the solvent removed at reduced pressure and the residue chromatographed (alumina/petroleum ether, bp 40–60 °C). The product was recrystallised from ether–hexane mixtures to give red crystals.
CX] {0.22 mmol; X = CH(OEt)2 and CHO} in tetrahydrofuran (20 cm3). After 2 h the solution was filtered, the solvent removed at reduced pressure and the residue chromatographed (alumina/petroleum ether, bp 40–60 °C). The product was recrystallised from ether–hexane mixtures to give red crystals.
There was no reaction between [Co2(CO)8] and 5 where X = CHC(CN)2.
[{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2}{Co2(CO)6}] 7 (0.14 g, 80%), decomp. > 200 °C (Found: C, 63.1; H, 4.5%. C36H31O8PCo2Ni requires C, 63.5, H, 4.6%); νmax/cm−1 terminal (CO) 2058(8), 2031(6) and 1997(10) (CH2Cl2); terminal (CO) 2056(8), 2031(6) and 1998(10) (KBr); λmax/nm (CH2Cl2) 400 (ε/dm3 mol−1 cm−1 12 900); λmax/nm (CH3CN) 401 (ε/dm3 mol−1 cm−1 13 300); δH(CDCl3) 7.24–7.75 [15 H, m,
Ph3P], 5.17 [5 H, s, C5H5], 4.90 [1 H, s, CH(OEt)2], 3.05 [4 H, q, J(HH) 7.3 Hz, CH2] and 0.9 [6 H, t, J(HH), CH3]; δC(CDCl3) 206.4 [s, terminal-CO], 134.2 [d, J(CP) 12 Hz, o-Ph], 133.6 [d, J(CP) 50 Hz, i-Ph], 130.5 [d, J(CP) 2 Hz, p-Ph], 128.0 [d, J(CP) 11 Hz, m-Ph], 106.5 [s, NiC
CCH(OEt)2}{Co2(CO)6}] 7 (0.14 g, 80%), decomp. > 200 °C (Found: C, 63.1; H, 4.5%. C36H31O8PCo2Ni requires C, 63.5, H, 4.6%); νmax/cm−1 terminal (CO) 2058(8), 2031(6) and 1997(10) (CH2Cl2); terminal (CO) 2056(8), 2031(6) and 1998(10) (KBr); λmax/nm (CH2Cl2) 400 (ε/dm3 mol−1 cm−1 12 900); λmax/nm (CH3CN) 401 (ε/dm3 mol−1 cm−1 13 300); δH(CDCl3) 7.24–7.75 [15 H, m,
Ph3P], 5.17 [5 H, s, C5H5], 4.90 [1 H, s, CH(OEt)2], 3.05 [4 H, q, J(HH) 7.3 Hz, CH2] and 0.9 [6 H, t, J(HH), CH3]; δC(CDCl3) 206.4 [s, terminal-CO], 134.2 [d, J(CP) 12 Hz, o-Ph], 133.6 [d, J(CP) 50 Hz, i-Ph], 130.5 [d, J(CP) 2 Hz, p-Ph], 128.0 [d, J(CP) 11 Hz, m-Ph], 106.5 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 96.0 [s, C5H5], 75.2 [d, J(CP) 48 Hz, NiC
C], 96.0 [s, C5H5], 75.2 [d, J(CP) 48 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 70.3 [s, CH(OEt)2], 60.1 [s, CH2CH3] and 15.1 [s, CH2CH3].
C], 70.3 [s, CH(OEt)2], 60.1 [s, CH2CH3] and 15.1 [s, CH2CH3].
[{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8 (0.14 g, 85%), decomp. 150 °C (Found: C, 53.0; H, 3.0%. C32H21O7PCo2Ni requires C, 53.0, H, 2.9%); νmax/cm−1 terminal (CO) 2072(6), 2034(6) and 2007(10), (CHO) 1654(8) (CH2Cl2); terminal (CO) 2071(6), 2032(6) and 2006(10), (CHO) 1654(1) (KBr); λmax/nm (CH2Cl2) 406 (ε/dm3 mol−1 cm−1 12 000); λmax/nm (CH3CN) 402 (ε/dm3 mol−1 cm−1 14 300); δH(CDCl3) 8.43 [1 H, s, CHO],
7.42–7.70 [15 H, m, Ph3P] and 5.18 [5 H, s, C5H5]; δC(CDCl3) 201.8 [s, terminal CO], 185.7 [s, CHO], 133.8 [d, J(CP) 11 Hz, o-Ph], 132.5 [d, J(CP) 50 Hz, i-Ph], 131.2 [d, J(CP) 2 Hz, p-Ph], 128.8 [d, J(CP) 10 Hz, m-Ph], 118.0 [s, NiC
CCHO}{Co2(CO)6}] 8 (0.14 g, 85%), decomp. 150 °C (Found: C, 53.0; H, 3.0%. C32H21O7PCo2Ni requires C, 53.0, H, 2.9%); νmax/cm−1 terminal (CO) 2072(6), 2034(6) and 2007(10), (CHO) 1654(8) (CH2Cl2); terminal (CO) 2071(6), 2032(6) and 2006(10), (CHO) 1654(1) (KBr); λmax/nm (CH2Cl2) 406 (ε/dm3 mol−1 cm−1 12 000); λmax/nm (CH3CN) 402 (ε/dm3 mol−1 cm−1 14 300); δH(CDCl3) 8.43 [1 H, s, CHO],
7.42–7.70 [15 H, m, Ph3P] and 5.18 [5 H, s, C5H5]; δC(CDCl3) 201.8 [s, terminal CO], 185.7 [s, CHO], 133.8 [d, J(CP) 11 Hz, o-Ph], 132.5 [d, J(CP) 50 Hz, i-Ph], 131.2 [d, J(CP) 2 Hz, p-Ph], 128.8 [d, J(CP) 10 Hz, m-Ph], 118.0 [s, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C], 107.1 [d, J(CP) 48 Hz, NiC
C], 107.1 [d, J(CP) 48 Hz, NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C] and 96.6 [s, C5H5].
C] and 96.6 [s, C5H5].
Structures of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO] 2, [Ni(η5-C5H5)(PPh3)C
CCHO] 2, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) C(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
C(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO}{Co2(CO)6}] 8
CCHO}{Co2(CO)6}] 8
The crystal data for 2, 5 and 8 are summarised in Table 1. The structures were solved using the Patterson function of SHELXS97,14 and refined by full-matrix least squares using SHELXL97.14 The diagrams were obtained using the PLATON15 and ORTEX16 programs. The molecular structures and atom labelling for 2, 5 and 8 are illustrated in Figs. 1, 2 and 3, respectively. Selected bond lengths and angles are listed in Table 2 together with those previously
reported for 1.10
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2, [Ni(η5-C5H5)(PPh3)C
CCHO] 2, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHC(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
CCHC(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8
CCHO}{Co2(CO)6}] 8
| 2 | 5 | 8 | |
|---|---|---|---|
| Empirical formula | C26H21NiOP | C29H21N2NiP | C32H21Co2NiO7P |
| Formula weight | 439.11 | 487.16 | 725.03 |
| Temperature/K | 297(2) | 290(1) | 294(2) |
| Wavelength/Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system, space group | Orthorhombic, Pbca | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Monoclinic, I2/a |
| a/Å | 10.2026(14) | 10.6684(9) | 18.698(3) |
| b/Å | 16.4365(18) | 11.0455(10) | 16.8087(11) |
| c/Å | 26.152(2) | 11.7757(8) | 21.090(3) |
| α/° | 90 | 95.774(7) | 90 |
| β/° | 90 | 113.486(7) | 109.150(14) |
| γ/° | 90 | 106.520(8) | 90 |
| Volume/Å3 | 4385.5(8) | 1183.46(17) | 6261.3(14) |
| Z, Calculated density/Mg m−3 | 8, 1.330 | 2, 1.367 | 8, 1.538 |
| Absorption coefficient/mm−1 | 0.971 | 0.907 | 1.739 |
| Reflections collected/unique | 6439/5249 | 4396/3658 | 7152/7152 |
| Final R indices {I > 2σ(I)] | R 1 = 0.062, wR2 = 0.099 | R 1 = 0.037, wR2 = 0.046 | R 1 = 0.047, wR2 = 0.078 |
| R indices (all data) | R 1 = 0.168, wR2 = 0.129 | R 1 = 0.093, wR2 = 0.096 | R 1 = 0.143, wR2 = 0.092 |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2] 1, [Ni(η5-C5H5)(PPh3)C
CCH(OEt)2] 1, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2, [Ni(η5-C5H5)(PPh3)C
CCHO] 2, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
C(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8
CCHO}{Co2(CO)6}] 8
| 1 | 2 | 5 | 8 b | |
|---|---|---|---|---|
a
Cg1 is the centroid of the η5–C5H5 ligand.
b
The range of bond lengths for Co–CO is 1.765(6) to 1.815(6) Å and for C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O is 1.126(6) to 1.144(5) Å and the Co–C O is 1.126(6) to 1.144(5) Å and the Co–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O bond angles are in the range 176.2(6)–178.7(6)° (cf. refs. 20, 24–26). Co1–C1–C2 64.6(2), Co2–C1–C2 67.2(3), Co1–C2–C1 76.8(2), Co2–C2–C1 74.1(3)°. O bond angles are in the range 176.2(6)–178.7(6)° (cf. refs. 20, 24–26). Co1–C1–C2 64.6(2), Co2–C1–C2 67.2(3), Co1–C2–C1 76.8(2), Co2–C2–C1 74.1(3)°.
|
||||
| Ni1–P1 | 2.1376(10) | 2.1418(11) | 2.1451(6) | 2.1430(11) |
| Ni1–C1 | 1.840(5) | 1.830(5) | 1.833(2) | 1.867(4) |
| C1–C2 | 1.193(5) | 1.205(6) | 1.214(3) | 1.333(5) |
| C2–C3 | 1.474(5) | 1.432(8) | 1.402(3) | 1.460(5) |
| C11–C12 | 1.391(5) | 1.364(7) | 1.346(5) | 1.386(6) |
| C12–C13 | 1.389(5) | 1.418(7) | 1.385(5) | 1.376(6) |
| C13–C14 | 1.406(6) | 1.370(7) | 1.391(5) | 1.400(6) |
| C14–C15 | 1.369(6) | 1.398(7) | 1.356(5) | 1.373(6) |
| C11–C15 | 1.396(6) | 1.426(7) | 1.394(5) | 1.405(6) |
| Ni1–C11 | 2.059(4) | 2.127(5) | 2.111(3) | 2.099(4) |
| Ni1–C12 | 2.128(4) | 2.099(6) | 2.123(3) | 2.152(4) |
| Ni1–C13 | 2.094(4) | 2.097(5) | 2.062(30) | 2.084(4) |
| Ni1–C14 | 2.133(4) | 2.121(5) | 2.134(30) | 2.119(4) |
| Ni1–C15 | 2.128(4) | 2.066(4) | 2.101(3) | 2.144(4) |
| Ni1–Cg1a | 1.7458(4) | 1.7354(6) | 1.7523(4) | 1.7612(6) |
| C3–O1/C3–C4* | 1.147(6) | 1.345(4)* | 1.179(5) | |
| C3–H3 | 0.93(3) | 0.96(3) | 0.93 | |
| Others | C4–C5 1.434(4) | Co1–Co2 2.4854(11) | ||
| C4–C7 1.419(5) | Co1–C1 2.084(3) | |||
| C7–N8 1.134(5) | Co2–C1 2.053(4) | |||
| Co1–C2 1.934(4) | ||||
| Co2–C2 1.969(5) | ||||
| P1–Ni1–C1 | 90.90(10) | 95.65(14) | 89.93(7) | 93.34(11) |
| Ni1–C1–C2 | 178.7(3) | 176.4(4) | 176.6(2) | 154.2(3) |
| Cg1–Ni1–P1 | 136.6 | 134.24(4) | 137.27(3) | 131.19(4) |
| Cg1–Ni1–C1 | 132.6 | 130.10(13) | 131.92(9) | 135.46(10) |
| C1–C2–C3 | 173.9(4) | 174.6(6) | 177.8(3) | 145.0(4) |
| C2–C3–O1 (C4)* | 130.8(6) | 125.0(3)* | 126.3(5) | |
| Ni1–C1–Co1 | 128.5(2) | |||
| Ni1–C1–Co2 | 134.3(2) | |||
![Molecular structure and atom labeling for [Ni(η5-C5H5)(PPh3)CCCHO] 2. Displacement ellipsoids are drawn at the 30% probability level.](/image/article/2002/DT/b104442g/b104442g-f1.gif) | ||
Fig. 1 Molecular structure and atom labeling for [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2. Displacement ellipsoids are drawn at the 30% probability level. CCHO] 2. Displacement ellipsoids are drawn at the 30% probability level. | ||
![Molecular structure and atom labeling for [Ni(η5-C5H5)(PPh3)CCCHC(CN)2] 5. Displacement ellipsoids are drawn at the 30% probability level.](/image/article/2002/DT/b104442g/b104442g-f2.gif) | ||
Fig. 2 Molecular structure and atom labeling for [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHC(CN)2] 5. Displacement ellipsoids are drawn at the 30% probability level. CCHC(CN)2] 5. Displacement ellipsoids are drawn at the 30% probability level. | ||
![Molecular structure and atom labeling for [{μ-η1:η1-Ni(η5-C5H5)(PPh3)CCCHO}{Co2(CO)6}] 8. Displacement ellipsoids are drawn at the 10% probability level.](/image/article/2002/DT/b104442g/b104442g-f3.gif) | ||
Fig. 3 Molecular structure and atom labeling for [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8. Displacement ellipsoids are drawn at the 10% probability level. CCHO}{Co2(CO)6}] 8. Displacement ellipsoids are drawn at the 10% probability level. | ||
CCDC reference numbers 165064–165066.
See http://www.rsc.org/suppdata/dt/b1/b104442g/ for crystallographic data in CIF or other electronic format.
Results and discussion
Synthesis and reactivity studies
Transition metal acetylides of the general type MC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX provide an attractive starting point for the synthesis of organometallic donor–π-acceptor systems. They may often be easily prepared from a CuI–catalysed reaction of metal halides with terminal alkynes HC
CX provide an attractive starting point for the synthesis of organometallic donor–π-acceptor systems. They may often be easily prepared from a CuI–catalysed reaction of metal halides with terminal alkynes HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX,7 and when X contains a suitable functional group, it may be modified by conventional organic reactions. In the present work, the reaction of [Ni(η5-C5H5)(PPh3)Br] with the alkyne acetal HC
CX,7 and when X contains a suitable functional group, it may be modified by conventional organic reactions. In the present work, the reaction of [Ni(η5-C5H5)(PPh3)Br] with the alkyne acetal HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2 gives [Ni(η5-C5H5)(PPh3)C
CCH(OEt)2 gives [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2] 1, which is hydrolysed in the presence of oxalic acid to the aldehyde [Ni(η5-C5H5)(PPh3)C
CCH(OEt)2] 1, which is hydrolysed in the presence of oxalic acid to the aldehyde [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO] 2. This undergoes condensation reactions with hydrazines
in the presence of acetic acid to give the hydrazones [Ni(η5-C5H5)(PPh3)C
CCHO] 2. This undergoes condensation reactions with hydrazines
in the presence of acetic acid to give the hydrazones [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHAr] {Ar = C6H53, or C6H3(NO2)2-2,4 4}, and with active methylene compounds such as malononitrile or phenylisoxazolone in the presence of triethylamine to give [Ni(η5-C5H5)(PPh3)C
NNHAr] {Ar = C6H53, or C6H3(NO2)2-2,4 4}, and with active methylene compounds such as malononitrile or phenylisoxazolone in the presence of triethylamine to give [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2] 5, and [Ni(η5-C5H5)(PPh3)C
C(CN)2] 5, and [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2] 6, respectively (Scheme 1).
C9H5NO2] 6, respectively (Scheme 1).
Compounds 1–6 are alkynes and would be expected to react with [Co2(CO)8] to give the well-known alkyne complexes [(μ2-alkyne)Co2(CO)6]. Thus 1 and 2, respectively, gave the derivatives [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2}{Co2(CO)6}] 7, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
CCH(OEt)2}{Co2(CO)6}] 7, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}] 8, within two hours. However 5, [Ni(η5-C5H5)(PPh3)C
CCHO}{Co2(CO)6}] 8, within two hours. However 5, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHC(CN)2],
does not undergo this reaction even after 24 h at room temperature, so clearly the ability of the alkynes [Ni(η5-C5H5)(PPh3)C
CCHC(CN)2],
does not undergo this reaction even after 24 h at room temperature, so clearly the ability of the alkynes [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] to react with [Co2(CO)8] depends on X and is inhibited by the strongly electron-withdrawing C(CN)2 group. Furthermore, some characteristic reactions of X appear to be affected by complexation of C
CX] to react with [Co2(CO)8] depends on X and is inhibited by the strongly electron-withdrawing C(CN)2 group. Furthermore, some characteristic reactions of X appear to be affected by complexation of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C to the {Co2(CO)6} moiety so that the acetal group in 7 is not hydrolysed to the aldehyde in the presence of oxalic acid, and the aldehyde group in 8 does not undergo a Knoevenagel condensation with CH2(CN)2 or react with the Wittig reagent prepared from [Ph3PCH2Br]Br and nBuLi. These reactions are facile for the free [Ni(η5-C5H5)(PPh3)C
C to the {Co2(CO)6} moiety so that the acetal group in 7 is not hydrolysed to the aldehyde in the presence of oxalic acid, and the aldehyde group in 8 does not undergo a Knoevenagel condensation with CH2(CN)2 or react with the Wittig reagent prepared from [Ph3PCH2Br]Br and nBuLi. These reactions are facile for the free [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX].
CX].
A rationalization of these observations will be discussed below.
Spectra and bonding
The IR spectra of complexes 1–8 show many absorption bands, but the ones which yield useful structural information are those due to the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), aldehyde ν(CO) and ν(CN) modes of the C
C), aldehyde ν(CO) and ν(CN) modes of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX moieties and the ν(CO) vibrations of the Co2(CO)6 groups.
CX moieties and the ν(CO) vibrations of the Co2(CO)6 groups.
The bands due to the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) vibrations of the [Ni(η5-C5H5)(PPh3)C
C) vibrations of the [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] complexes are readily identified. Their frequencies (Table 3) decrease along the series X = CH(OEt)2 > CH
CX] complexes are readily identified. Their frequencies (Table 3) decrease along the series X = CH(OEt)2 > CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H5 > CH
NNHC6H5 > CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2 > CH
NNHC6H3(NO2)2 > CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2
≈ CH
C(CN)2
≈ CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2. This may be correlated with the increasing electron-withdrawing power of X and rationalized by assuming that the ground state electronic structure of these complexes may be described as a resonance hybrid of three mesomers: the acetylenic form A and charge-separated forms B, C and D (Fig. 4).
C9H5NO2. This may be correlated with the increasing electron-withdrawing power of X and rationalized by assuming that the ground state electronic structure of these complexes may be described as a resonance hybrid of three mesomers: the acetylenic form A and charge-separated forms B, C and D (Fig. 4).
| IR spectraa | 13C spectrab | UV/visible spectrac | |||||
|---|---|---|---|---|---|---|---|
| X |
ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)d C)d |
ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)e C)e |
C1 | C2 | C5H5 | λ max (ε)e | λ max (ε)f |
| a Peak positions in cm−1 with relative peak heights in parentheses for 2. b Chemical shifts in ppm downfield from Me4Si as an internal standard. In parentheses are multiplicity (d = doublet) and coupling constants JPC in Hz. Other resonances are singlets. Spectra recorded in CDCl3 solution. c Absorption band maxima in nm with band intensities in parentheses in 103 dm3 mol−1 cm−1. d Spectra measured as KBr discs. e Spectra measured in CH2Cl2 solution. f Spectra measured in CH3CN solution. | |||||||
| CH(OEt)2, 1 | 2109 | 2112 | 82.0 (d, 40) | 114.2 | 92.7 | 305 (15.2) | 303 (14.9) |
| CHO, 2 | 2081 (2), 2034 (3) | 2079 (2), 2034 (3) | 122.8 (d, 43) | 124.6 | 93.3 | 364 (16.1) | 359 (16.0) |
| CHNNHC6H5, 3 | 2091 | 2090 | 91.4 (d, 48) | 93.1 | 92.4 | 350 (12.0) | 354 (10.1) |
| CHNNHC6H3(NO2)2-2,4, 4 | 2082 | 2082 | 108.3 (d, 48) | 110.9 | 93.5 | 394 (19.6) | 394 (18.5) |
| CHC(CN)2, 5 | 2049 | 2050 | 152.8 (d, 48) | 121.5 | 94.2 | 469 (13.0) | 463 (11.2) |
| CHC9H5NO2, 6 | 2051 | 2050 | 140.3 (d, 48) | 120.7 | 96.7 | 511 (27.8) | 507 (26.6) |
![Resonance structures of the [Ni(η5-C5H5)(PPh3)CCX] complexes.](/image/article/2002/DT/b104442g/b104442g-f4.gif) | ||
Fig. 4 Resonance structures of the [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] complexes. CX] complexes. | ||
A is always the most important, but there will be an increasing contribution from B, C and D as the electron-withdrawing ability of X increases. The decreasing C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond order results in a lower ν(C
C bond order results in a lower ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) frequency as has been observed by Gladysz and co-workers in the metalloalkynes Re(η5-C5Me5)(NO)(PPh3)C
C) frequency as has been observed by Gladysz and co-workers in the metalloalkynes Re(η5-C5Me5)(NO)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(OMe)(fluorenyl)17 which show ν(C
CC(OMe)(fluorenyl)17 which show ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) bands at 2018 and 2172 cm−1 whereas in [Re(η5-C5Me5)(NO)(PPh3)C
C) bands at 2018 and 2172 cm−1 whereas in [Re(η5-C5Me5)(NO)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAr2]+ they are found at 1993 and 1902 cm−1 (H2CAr2
= fluorene).17
CAr2]+ they are found at 1993 and 1902 cm−1 (H2CAr2
= fluorene).17
The IR spectrum of 2 (X = CHO) (Table 4) is different from those of 1, and 3–6. It displays two ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) absorption bands in the solid state at 2034 and 2081 cm−1 (intensity ratio 1 ∶ 3). In solution, the relative intensities vary with the ca. 2080 cm−1 band increasing in importance along the series MeCN ≈ CH2Cl2 (1 ∶ 3) < toluene ≈ tetrahydrofuran (1 ∶ 5) < hexane (0 ∶ 1) so that for the latter solvent the ca. 2030 cm−1 band is absent. These changes are reversible. The aldehydic ν(CO) band of 2 has two equal components in acetonitrile or dichloromethane solutions ca. 1621 and 1626 cm−1,
but not in toluene, tetrahydrofuran, hexane, or in the solid state. Its frequencies are very low even when compared with that of benzaldehyde (1714 and 1702 cm−1 in hexane and CH2Cl2, respectively) and PhC
C) absorption bands in the solid state at 2034 and 2081 cm−1 (intensity ratio 1 ∶ 3). In solution, the relative intensities vary with the ca. 2080 cm−1 band increasing in importance along the series MeCN ≈ CH2Cl2 (1 ∶ 3) < toluene ≈ tetrahydrofuran (1 ∶ 5) < hexane (0 ∶ 1) so that for the latter solvent the ca. 2030 cm−1 band is absent. These changes are reversible. The aldehydic ν(CO) band of 2 has two equal components in acetonitrile or dichloromethane solutions ca. 1621 and 1626 cm−1,
but not in toluene, tetrahydrofuran, hexane, or in the solid state. Its frequencies are very low even when compared with that of benzaldehyde (1714 and 1702 cm−1 in hexane and CH2Cl2, respectively) and PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO (1661 and 1672 cm−1). This frequency variation may be attributed to (a) coupling of the ν(C
CCHO (1661 and 1672 cm−1). This frequency variation may be attributed to (a) coupling of the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) and ν(C
C) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibrations which would raise the frequency of the former and lower that of the latter but which is likely to be limited, and (b) the contribution made to the ground state electronic structure of 2 by the cumulenic mesomers B and C (Fig. 4) with their CO− moieties. The latter also accounts for the marked solvent-dependence of this absorption band. Hydrogen-bonding of the negatively charged oxygen atom to solvents such as dichloromethane and acetonitrile (but not
hexane) would be expected to lower the frequency of the ν(CO) vibration still further. However, we are unable to account for the presence of two ν(C
O) vibrations which would raise the frequency of the former and lower that of the latter but which is likely to be limited, and (b) the contribution made to the ground state electronic structure of 2 by the cumulenic mesomers B and C (Fig. 4) with their CO− moieties. The latter also accounts for the marked solvent-dependence of this absorption band. Hydrogen-bonding of the negatively charged oxygen atom to solvents such as dichloromethane and acetonitrile (but not
hexane) would be expected to lower the frequency of the ν(CO) vibration still further. However, we are unable to account for the presence of two ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) bands in the spectrum of 2. This feature is commonly observed for MC
C) bands in the spectrum of 2. This feature is commonly observed for MC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX complexes and is usually attributed to Fermi resonance [e.g.ref. 18]. It may be due to the presence of two species, but there is no evidence for this in the case of 2 either in the solid state (X-ray crystallography) or in solution (NMR spectra).
CX complexes and is usually attributed to Fermi resonance [e.g.ref. 18]. It may be due to the presence of two species, but there is no evidence for this in the case of 2 either in the solid state (X-ray crystallography) or in solution (NMR spectra).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO]
CCHO]
| Solvent |
ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) absorption bandsa C) absorption bandsa |
ν(CO) absorption bandsa |
|---|---|---|
a
Frequency (cm−1) with relative peak heights in parentheses.
b
Values for PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO. CCHO.
|
||
| CH3CN | 2078, 2031 (3 ∶ 1) | 1626, 1621 (1 ∶ 1) |
| CH2Cl2 | 2079, 2034 (3 ∶ 1) | 1625, 1622 (1 ∶ 1) |
| C6H5CH3 | 2082, 2026 (5 ∶ 1) | 1635 |
| THF | 2082, 2026 (5 ∶ 1) | 1635 |
| Hexane | 2086 | 1645 |
| KBr | 2081, 2034 (3 ∶ 1) | 1627 |
| CH2Cl2b | 2192 | 1661 |
| CH3C6H5b | 2190 | 1664 |
| Hexaneb | 2193 | 1672 |
The IR spectra of 7 and 8 show absorption bands due to the ν(CO) ligands of the Co2(CO)6 moiety (Table 5). Their frequencies are a function of X and are ca. 10 cm−1 higher when X is the electron-withdrawing CHO group as compared with CH(OEt)2. This is attributed to the contribution that mesomers such as G (Fig. 5) make to the electronic structure of these complexes. The same frequency relationship is observed for the ν(CO) bands of [{μ-PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX}{Co2(CO)6}] (X = CH(OEt)29, and CHO 10),19 but whereas the frequencies for 9
and 10 are similar to those of other [(μ-alkyne)Co2(CO)6] derivatives, they are ca. 30 cm−1 higher on average than are those of 7 and 8. This is attributed to the electron-donating ability of the (η5-C5H5)(Ph3P)Ni moiety and the contribution made by mesomers such as H to an overall description of the bonding in 7 and 8 (Fig. 5). Similar mesomers clearly do not contribute to the bonding of 9 and 10. Similar effects have been observed for [{μ-η1:η1-(η5-C5H5)(OC)2FeC
CX}{Co2(CO)6}] (X = CH(OEt)29, and CHO 10),19 but whereas the frequencies for 9
and 10 are similar to those of other [(μ-alkyne)Co2(CO)6] derivatives, they are ca. 30 cm−1 higher on average than are those of 7 and 8. This is attributed to the electron-donating ability of the (η5-C5H5)(Ph3P)Ni moiety and the contribution made by mesomers such as H to an overall description of the bonding in 7 and 8 (Fig. 5). Similar mesomers clearly do not contribute to the bonding of 9 and 10. Similar effects have been observed for [{μ-η1:η1-(η5-C5H5)(OC)2FeC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH}{Co2(CO)6}] {ν(CO) =
2060, 2011, 1986, 1961 cm−1}.20 The spectra of 8 and 10 also show an additional weaker band at 1654 and 1663 cm−1 respectively due to their aldehydic ν(CO) vibration. This is much higher than that of the uncomplexed aldehyde 2 but very little changed from PhC
CH}{Co2(CO)6}] {ν(CO) =
2060, 2011, 1986, 1961 cm−1}.20 The spectra of 8 and 10 also show an additional weaker band at 1654 and 1663 cm−1 respectively due to their aldehydic ν(CO) vibration. This is much higher than that of the uncomplexed aldehyde 2 but very little changed from PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO. The relative frequencies reflect the greater electron-richness of the Co2C2 cluster in 8 as compared with 10. It points to some Ni⋯CHO electronic interaction in 8 that is greatly reduced as compared with that in the parent alkyne 2.
CCHO. The relative frequencies reflect the greater electron-richness of the Co2C2 cluster in 8 as compared with 10. It points to some Ni⋯CHO electronic interaction in 8 that is greatly reduced as compared with that in the parent alkyne 2.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX}{Co2(CO)6}] {X = CH(OEt)27 and CHO 8} and [{μ-η1:η1-PhC
CX}{Co2(CO)6}] {X = CH(OEt)27 and CHO 8} and [{μ-η1:η1-PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX}{Co2(CO)6}] {X = CH(OEt)29 and CHO 10}
CX}{Co2(CO)6}] {X = CH(OEt)29 and CHO 10}
| Compound | Absorption bands | ||||
|---|---|---|---|---|---|
| a Peak positions (cm−1) with relative peak heights in parentheses. The bands at ca. 1660 cm−1 are due to the aldehyde group. | |||||
| 7 {X = CH(OEt)2} | 2058 (8) | 2031 (6) | 1997 (10, br) | ||
| 8 {X = CHO} | 2072 (6) | 2034 (6) | 2007 (10, br) | 1654 (1) | |
| 9 {X = CH(OEt)2} | 2093 (3) | 2056 (10) | 2030 (8) | 2024 (10) | |
| 10 {X = CHO} | 2102 (4) | 2067 (10) | 2041 (10) | 2035 (sh) | 1663 (1) |
![Resonance structures of [{μ-η1-η1-Ni(η5-C5H5)(PPh3)CCCHO}{Co2(CO)6}].](/image/article/2002/DT/b104442g/b104442g-f5.gif) | ||
Fig. 5 Resonance structures of [{μ-η1-η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}]. CCHO}{Co2(CO)6}]. | ||
The 1H NMR spectra of 1–8 show the resonances characteristic of the η5-C5H5 and PPh3 ligands, and the group X. They require no further discussion.
The 13C NMR spectra show the anticipated resonances. The most interesting are two due to the alkynyl C atoms (Table 3). One is a doublet. It is assigned to the Ni–C1 atom coupled to the coordinated P atom. The other is a singlet and is assigned to the remote Ni–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C2 atom as there is no coupling to 31P. The chemical shifts of both are a function of X so that when X is electron-donating such as CH(OEt)2, the C1 resonance is found upfield (δ 82.0) of that due to C2 (δ 114.2). Both carbon atoms are deshielded when X is electron-withdrawing, but C1 is affected more than C2 so that when X is, for example, CH
C2 atom as there is no coupling to 31P. The chemical shifts of both are a function of X so that when X is electron-donating such as CH(OEt)2, the C1 resonance is found upfield (δ 82.0) of that due to C2 (δ 114.2). Both carbon atoms are deshielded when X is electron-withdrawing, but C1 is affected more than C2 so that when X is, for example, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2, the C1 resonance is found downfield (δ 152.8) of that due to C2 (δ 121.5). C1 is increasingly
deshielded along the series X = CH(OEt)2, CH
C(CN)2, the C1 resonance is found downfield (δ 152.8) of that due to C2 (δ 121.5). C1 is increasingly
deshielded along the series X = CH(OEt)2, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H5, CH
NNHC6H5, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2-2,4, CHO, CH
NNHC6H3(NO2)2-2,4, CHO, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2, CH
C9H5NO2, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2. These effects are consistent with the suggestion made above that the charge separated mesomers B, C and D (Fig. 4) make an increasing contribution to the ground state structure of these complexes as the electron-withdrawing ability of X increases. The series for C1 arises, in part, from its increasing carbene character/decreasing alkyne character as a consequence of rehybridisation of the NiCCX chain, and, in part, from its increasing positive charge as a consequence of charge separation. The series is very similar to that observed for the effect of X on the ν(C
C(CN)2. These effects are consistent with the suggestion made above that the charge separated mesomers B, C and D (Fig. 4) make an increasing contribution to the ground state structure of these complexes as the electron-withdrawing ability of X increases. The series for C1 arises, in part, from its increasing carbene character/decreasing alkyne character as a consequence of rehybridisation of the NiCCX chain, and, in part, from its increasing positive charge as a consequence of charge separation. The series is very similar to that observed for the effect of X on the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) frequency except for the anomalous IR spectrum of 2.
C2 becomes increasingly deshielded for X = CH
C) frequency except for the anomalous IR spectrum of 2.
C2 becomes increasingly deshielded for X = CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H5, CH
NNHC6H5, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2-2,4, CH(OEt)2, CH
NNHC6H3(NO2)2-2,4, CH(OEt)2, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2, CH
C9H5NO2, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2, CHO. This is a somewhat different series from that for C1, but similarly it has the electron-withdrawing X giving rise to the most deshielded carbon atoms. The differences between the two series are probably a reflection of the differing contributions made by mesomers A–D.
C(CN)2, CHO. This is a somewhat different series from that for C1, but similarly it has the electron-withdrawing X giving rise to the most deshielded carbon atoms. The differences between the two series are probably a reflection of the differing contributions made by mesomers A–D.
The UV-visible spectrum of 1 (Table 3) shows a relatively intense absorption band at 305 nm. It is present in the spectra of 2–6 but its wavelength increases with the increasing electron-withdrawing ability of X to 469 nm when X = CHC(CN)2 and 511 nm when X = CHC9H5NO2. It is assigned to an electronic transition from the ground state of these complexes to an excited state. As discussed above, the electronic structure of the ground state may be described as a resonance hybrid in which the alkynyl mesomer A predominates over the charge separated cumulenic mesomers. In the excited state, the same mesomers probably contribute to an overall description of the bonding but B, C and D are now the more important. Consequently, the electron-withdrawing groups X preferentially stabilise the excited state, reduce the energy of the electronic transition and increase the wavelength of the observed absorption band. The increasing conjugation present in 5 and 6 may also contribute to lowering the energy of this electronic transition as may the presence of the NHC6H3(NO2)2-2,4 moiety in 4.
The NMR and UV-visible spectra of 7 and 8 are similar to those of other [μ-(alkyne)Co2(CO)6] species and will not be discussed further.
Crystal structures of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO] 2, [Ni(η5-C5H5)(PPh3)C
CCHO] 2, [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHC(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C
CCHC(CN)2] 5, and [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) CCHO}{Co2(CO)6}] 8
CCHO}{Co2(CO)6}] 8
The structures of 2, 5 and 8 are illustrated in Figs. 1, 2 and 3, respectively, and selected molecular dimensions are listed in Table 2 together with those previously reported for 1 (isomer B). All four compounds have the expected half-sandwich structures in which the C5 and P–Ni–C planes are virtually orthogonal. The P–Ni–C plane may be coincident with a σv plane of the C5 ring (α isomers in Fig. 6) or perpendicular to it (β isomer in Fig. 6). There are two possible α isomer (Fig. 6) and intermediate situations
are possible. Complex 2 adopts the αa structure, 5 the αb structure, and 1B and 8 the β structure but not completely so. The α isomers show a distortion of the C5 ring towards a diene with two short non-adjacent C–C bonds (C11–C12/C13–C14 in 2, and C11–C12/C14–C15 in 5) and one particularly short Ni–Cring bond (to C15 in 2 and C13 in 5). The β isomers show distortions of the ring towards an ene-allyl with a short ene C–C
bond (C14–C15 in both 1B and 8) and two longer bonds (C11–C15/C13–C14) and two Ni–Cring ring bonds (to C11 and C13) which are shorter than the other three; differences which are less marked for 1B than for 8. These distortions are a reflection of the loss of degeneracy of the cyclopentadienyl E1 and E2
π orbitals, their different energies and their differential occupancy in these complexes. This has been discussed in ref. 21 and many other papers cited therein.
![Distortions of the η5-C5H5 ligand in the [Ni(η5-C5H5)(PPh3CCX] complexes.](/image/article/2002/DT/b104442g/b104442g-f6.gif) | ||
Fig. 6 Distortions of the η5-C5H5 ligand in the [Ni(η5-C5H5)(PPh3C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] complexes. CX] complexes. | ||
The P–Ni–C angles lie in the range 89.9–95.7° and are comparable with values of 93.47(5)° in [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH],9 93.30(10)° in [Ni(1-Me-indenyl)(PPh3)C
CH],9 93.30(10)° in [Ni(1-Me-indenyl)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H5],11 and 95.4(7)° in the benzonitrile derivative [Ni(η5-C5H5)(PPh3)NCC6H4NH2][PF6].12 The Ni–P distances lie in the range 2.1376(10)–2.1451(6) Å. They are identical within experimental error and similar to those of related compounds.8–11
CC6H5],11 and 95.4(7)° in the benzonitrile derivative [Ni(η5-C5H5)(PPh3)NCC6H4NH2][PF6].12 The Ni–P distances lie in the range 2.1376(10)–2.1451(6) Å. They are identical within experimental error and similar to those of related compounds.8–11
The Ni–C–C–X systems are almost linear for 1, 2 and 5 with Ni–C1–C2 and C1–C2–C3 angles lying between 173.9(4)° and 178.7(3)°. Their Ni–C1 distances are, within experimental error, independent of X, and although their C1–C2 bond lengths increase along the series 1 < 2 < 5, the changes are small and scarcely outside of experimental error, but they do follow the trend expected if the mesomeric form B makes an increasing contribution to the overall bonding with increasing electron-withdrawing power of X. However, our observations are essentially in agreement with those of
Humphrey and co-workers who showed that Ni–Calkynyl and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond lengths do not vary significantly in a series of [Ni(η5-C5H5)(PPh3)(L)C
C bond lengths do not vary significantly in a series of [Ni(η5-C5H5)(PPh3)(L)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAr] complexes with NO2 substituents on the aromatic groups Ar.8
CAr] complexes with NO2 substituents on the aromatic groups Ar.8
In 5 the CHC(CN)2 moiety is planar and oriented almost orthogonal {81.53(10)°} to the P–Ni–C plane. The same is observed for the CHO group in 2, which contrasts with the situation in [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh] derivatives where the P–Ni–C plane and the C6 ring tend towards coplanarity.22 The C2–C3 distance in 2
{1.432(8) Å} is longer than that in 5
{1.402(3) Å}, which is consistent with the increasing contribution that mesomer B makes toward the bonding in 5 as compared with 2 (Fig. 4).
CPh] derivatives where the P–Ni–C plane and the C6 ring tend towards coplanarity.22 The C2–C3 distance in 2
{1.432(8) Å} is longer than that in 5
{1.402(3) Å}, which is consistent with the increasing contribution that mesomer B makes toward the bonding in 5 as compared with 2 (Fig. 4).
There are no intramolecular interactions of note in 5 and this is primarily due to the orientation of the rigid dicyanovinyl ligand with respect to the Ni(η5-C5H5)(PPh3) group; this contrasts with the acetal derivative [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2]10 where the flexible CH(OEt)2 group interacts with the PPh3 ligand. An intermolecular interaction C23–H23⋯π(C
CCH(OEt)2]10 where the flexible CH(OEt)2 group interacts with the PPh3 ligand. An intermolecular interaction C23–H23⋯π(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)i (i = 1 +
x, y, z) involving the alkynyl C1
C)i (i = 1 +
x, y, z) involving the alkynyl C1![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C2–C3, with C23–H23⋯C2 146°, H23⋯C2 2.63 Å and C23⋯C2 3.438(5) Å, is comparable to similar C–H⋯π(C
C2–C3, with C23–H23⋯C2 146°, H23⋯C2 2.63 Å and C23⋯C2 3.438(5) Å, is comparable to similar C–H⋯π(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)
interactions reported in Ni(η5-C5H5)(PPh3)C
C)
interactions reported in Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH,9 and by Desiraju and Steiner.23 There are two other intermolecular interactions of note C43–H43⋯{C31,⋯C36}ii (ii =
x, y, 1 +
z) and C45–H45⋯{C21,⋯C26}iii (iii = 1 −
x, 1 −
y, 1 −
z).
CH,9 and by Desiraju and Steiner.23 There are two other intermolecular interactions of note C43–H43⋯{C31,⋯C36}ii (ii =
x, y, 1 +
z) and C45–H45⋯{C21,⋯C26}iii (iii = 1 −
x, 1 −
y, 1 −
z).
The overall structure of [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO}{Co2(CO)6}], 8, (Fig. 3) is similar to that of other [(μ-η1:η1-alkyne)Co2(CO)6] complexes20,24–26 with a Co2C2 tetrahedral core. However, inspection of bond angles and bond lengths within this core reveals some interesting distortions. The Co–C distances to C1 (average 2.068 Å) are considerably longer than those to C2 (average 1.952 Å). A similar distortion is observed in [{μ-η1:η1-(η5-C5H5)(OC)2FeC
CCHO}{Co2(CO)6}], 8, (Fig. 3) is similar to that of other [(μ-η1:η1-alkyne)Co2(CO)6] complexes20,24–26 with a Co2C2 tetrahedral core. However, inspection of bond angles and bond lengths within this core reveals some interesting distortions. The Co–C distances to C1 (average 2.068 Å) are considerably longer than those to C2 (average 1.952 Å). A similar distortion is observed in [{μ-η1:η1-(η5-C5H5)(OC)2FeC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH}{Co2(CO)6}]20 whereas in [{μ-η1:η1-ButC
CH}{Co2(CO)6}]20 whereas in [{μ-η1:η1-ButC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CBut}{Co2(CO)6}] Co–C are comparable at 1.999 and 1.985 Å.24 It is a reflection of the electron-donating character of (η5-C5H5)(Ph3P)Ni and the electron-withdrawing character of CHO. It is consistent with the supposition that the alkyne–Co2 bonding consists of donation of the alkyne π-electrons to the metal atoms and back-donation of electrons from the metal atoms into the two vacant alkyne π* orbitals in which the latter is the more important and would be preferentially directed to the C atom bearing the most electronegative substituent. There is a second but minor distortion of the Co2C2 core as each C is bonded unequally to the two Co atoms with C1–Co1 >
C1–Co2 and C2–Co1 < C2–Co2 so that the Co–Co and C–C axes are not orthogonal. These solid-state differences involving the (μ-η1:η1-C2)/Co2(CO)6 system in 8 may support evidence for predisposed substitution sites in unsymmetrical alkynyl systems in Pauson–Khand reactions.27–30
CBut}{Co2(CO)6}] Co–C are comparable at 1.999 and 1.985 Å.24 It is a reflection of the electron-donating character of (η5-C5H5)(Ph3P)Ni and the electron-withdrawing character of CHO. It is consistent with the supposition that the alkyne–Co2 bonding consists of donation of the alkyne π-electrons to the metal atoms and back-donation of electrons from the metal atoms into the two vacant alkyne π* orbitals in which the latter is the more important and would be preferentially directed to the C atom bearing the most electronegative substituent. There is a second but minor distortion of the Co2C2 core as each C is bonded unequally to the two Co atoms with C1–Co1 >
C1–Co2 and C2–Co1 < C2–Co2 so that the Co–Co and C–C axes are not orthogonal. These solid-state differences involving the (μ-η1:η1-C2)/Co2(CO)6 system in 8 may support evidence for predisposed substitution sites in unsymmetrical alkynyl systems in Pauson–Khand reactions.27–30
As would be expected, the C1–C2 bond length in 8
{1.333(5) Å} is much longer than in 2
{1.205(6) Å} but well short of a normal C–C single bond of 1.53 Å.31 It is comparable to that found in most complexes of this class of compound24 but it is longer than that in [{μ-η1:η1-(η5-C5H5)(OC)2FeC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH}{Co2(CO)6}]{1.305(5) Å}.20 The Ni–C
CH}{Co2(CO)6}]{1.305(5) Å}.20 The Ni–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C angles of 154.2(3)° and 145.0(4)° in 8 differ greatly as is usually the case where the substituents on the coordinated C
C–C angles of 154.2(3)° and 145.0(4)° in 8 differ greatly as is usually the case where the substituents on the coordinated C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C differ greatly in their electron-withdrawing
capabilities, (e.g.ref. 25). They are different from the near linear 176.4(4) and 174.6(6)° angles in 2. The Ni–C and aldehyde C
C differ greatly in their electron-withdrawing
capabilities, (e.g.ref. 25). They are different from the near linear 176.4(4) and 174.6(6)° angles in 2. The Ni–C and aldehyde C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths of 1.867(4) Å and 1.179(5) Å, however, are longer in 8 than in 2 as a direct consequence of the μ-η1
∶
1-(C2) moiety bonding with the Co2 group. The orientation of the η5-C5H5 ring and the P–Ni–C fragment is of the β type with ene-allyl distortions within the cyclopentadienyl ring (see above). The Ni–Ccp bond lengths in 8
{2.084(4)–2.152(4) Å} are lengthened when compared with those in 2
{2.066(4)–2.127(5) Å}
and so is the nickel to cyclopentadienyl ring centroid distance, Ni1⋯Cg1 is 1.7612(6) Å in 8 and 1.7354(6) Å in 2.
O bond lengths of 1.867(4) Å and 1.179(5) Å, however, are longer in 8 than in 2 as a direct consequence of the μ-η1
∶
1-(C2) moiety bonding with the Co2 group. The orientation of the η5-C5H5 ring and the P–Ni–C fragment is of the β type with ene-allyl distortions within the cyclopentadienyl ring (see above). The Ni–Ccp bond lengths in 8
{2.084(4)–2.152(4) Å} are lengthened when compared with those in 2
{2.066(4)–2.127(5) Å}
and so is the nickel to cyclopentadienyl ring centroid distance, Ni1⋯Cg1 is 1.7612(6) Å in 8 and 1.7354(6) Å in 2.
Conclusions
In [Ni(η5-C5H5)(PPh3)C1![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C2X] complexes the (η5-C5H5)(Ph3P)Ni acts as an electron donor. When X is a suitable electron acceptor, the electronic interaction between the two results in a lowering of the ν(C
C2X] complexes the (η5-C5H5)(Ph3P)Ni acts as an electron donor. When X is a suitable electron acceptor, the electronic interaction between the two results in a lowering of the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) frequency, a deshielding of the C1 and C2 resonances (particularly the former), and an increase in the wavelength of the lowest energy charge transfer transition of the NiC
C) frequency, a deshielding of the C1 and C2 resonances (particularly the former), and an increase in the wavelength of the lowest energy charge transfer transition of the NiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX system. In the broadest terms, these effects increase in importance as the electron-withdrawing ability of X increases. Although the ordering of X is not the same for each spectroscopic parameter, it is clear that the more strongly electron-withdrawing X [CHO, CH
CX system. In the broadest terms, these effects increase in importance as the electron-withdrawing ability of X increases. Although the ordering of X is not the same for each spectroscopic parameter, it is clear that the more strongly electron-withdrawing X [CHO, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2 and CH
C(CN)2 and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2] have a greater effect than the less strongly electron-withdrawing [CH(OEt)2,
CH
C9H5NO2] have a greater effect than the less strongly electron-withdrawing [CH(OEt)2,
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHPh and CH
NNHPh and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3(NO2)2-2,4]. The two least ambiguous of the parameters are the ν(C
NNHC6H3(NO2)2-2,4]. The two least ambiguous of the parameters are the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) frequencies which provide a direct measure of the C
C) frequencies which provide a direct measure of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond order, and the chemical shift of C1 which depends on the charge separation in the molecule as well as hybridisation at C1. Both suggest that the electron withdrawing ability of X increases along the series CH(OEt)2 < CH
C bond order, and the chemical shift of C1 which depends on the charge separation in the molecule as well as hybridisation at C1. Both suggest that the electron withdrawing ability of X increases along the series CH(OEt)2 < CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHPh < CH
NNHPh < CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHC6H3-(NO2)2-2,4 < CHO < CH
NNHC6H3-(NO2)2-2,4 < CHO < CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9H5NO2
≈ CH
C9H5NO2
≈ CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2. The spectroscopic changes are attributed to the increasing contribution that charge-separated mesomers make to the overall electronic structure of these compounds, but the crystal structures of [Ni(η5-C5H5)(PPh3)C
C(CN)2. The spectroscopic changes are attributed to the increasing contribution that charge-separated mesomers make to the overall electronic structure of these compounds, but the crystal structures of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OEt)2], [Ni(η5-C5H5)(PPh3)C
CCH(OEt)2], [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHO]
and [Ni(η5-C5H5)(PPh3)C
CCHO]
and [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCHC(CN)2] show that this is not reflected in any significant structural changes in the Ni–C
CCHC(CN)2] show that this is not reflected in any significant structural changes in the Ni–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C system.
C–C system.
Although 1–8 are alkynes, not all of them form [{μ-η1:η1-Ni(η5-C5H5)(PPh3)C1![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C2X}{Co2(CO)6}] derivatives on reaction with [Co2(CO)8]. Such compounds may be isolated when X = CH(OEt)27, or CHO 8, but not when X is the strongly electron-withdrawing CH(CN)2. This is attributed to the relatively low C
C2X}{Co2(CO)6}] derivatives on reaction with [Co2(CO)8]. Such compounds may be isolated when X = CH(OEt)27, or CHO 8, but not when X is the strongly electron-withdrawing CH(CN)2. This is attributed to the relatively low C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond order in 6 as evidenced by its low ν(C
C bond order in 6 as evidenced by its low ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) frequency. The donor capability of the (η5-C5H5)(PPh3)Ni substituents greatly affects the ν(CO) frequencies of 7 and 8 which are lowered by ca. 35 cm−1 as compared with their [(μ-PhC
C) frequency. The donor capability of the (η5-C5H5)(PPh3)Ni substituents greatly affects the ν(CO) frequencies of 7 and 8 which are lowered by ca. 35 cm−1 as compared with their [(μ-PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX){Co2(CO)6}] counterparts, and in 8 where one cluster C atom is substituted by the electron donating (η5-C5H5)(PPh3)Ni and the other by the electron withdrawing CHO groups, the C2Co2 core is greatly distorted with significantly different C–Co distances. Furthermore, complexation of [Ni(η5-C5H5)(PPh3)C
CX){Co2(CO)6}] counterparts, and in 8 where one cluster C atom is substituted by the electron donating (η5-C5H5)(PPh3)Ni and the other by the electron withdrawing CHO groups, the C2Co2 core is greatly distorted with significantly different C–Co distances. Furthermore, complexation of [Ni(η5-C5H5)(PPh3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CX] to a Co2(CO)6 moiety appears to affect the reactivity of X, but the electronic communication between Ni and X is still apparent and has spectroscopic implications.
CX] to a Co2(CO)6 moiety appears to affect the reactivity of X, but the electronic communication between Ni and X is still apparent and has spectroscopic implications.
Acknowledgements
P.B. thanks Enterprise Ireland and Schering-Plough (Avondale) for financial support. J.F.G. thanks Enterprise Ireland (International collaboration grants) for funding several research visits to the University of Guelph, Canada and especially Professor George Ferguson for use of his Enraf–Nonius CAD4 diffractometer to collect data sets of 5 and 8. The purchase of a Bruker-AXS P4 diffractometer by Dublin City University in 1999 is genuinely appreciated by J.F.G.References
- N. J. Long, Angew. Chem., Int. Ed. Engl., 1995, 34, 21 CrossRef CAS.
- M. D. Ward, Chem. Soc. Rev., 1995, 121 RSC.
- H. Fink, N. J. Long, A. J. Martin, G. Opromolla, A. J. P. White, D. J. Williams and P. Zanello, Organometallics, 1997, 16, 2646 CrossRef CAS.
- K. R. J. Thomas, J. T. Lin and Y. S. Wen, J. Organomet. Chem., 1998, 575, 301 CrossRef.
- R. H. Naulty, M. P. Cifuentes, M. G. Humphrey, S. Houbrechts, C. Boutton, A. Persoons, G. A. Heath, D. C. R. Hockless, B. Luther-Davies and M. Samoc, J. Chem. Soc. Dalton Trans., 1997, 4167 RSC.
- H. Yamazaki, T. Nishido, Y. Matsumoto, S. Sumida and N. Hagihara, J. Organomet. Chem., 1966, 6, 86 CrossRef CAS.
- K. Sonogashira, T. Yakate, Y. Tohda, S. Takahashi and N. Hagihara, J. Chem. Soc., Chem. Commun., 1977, 291 RSC.
- I. R. Whittal, M. G. Humphrey and D. C. R. Hockless, Aust. J. Chem., 1998, 51, 219 CrossRef.
- J. F. Gallagher, P. Butler and A. R. Manning, Acta Crystallogr., Sect. C, 1998, 54, 342 CrossRef.
- P. Butler, J. F. Gallagher and A. R. Manning, Inorg. Chem. Commun., 1998, 1, 343 CrossRef CAS.
- R. Wang, F. Bélanger-Gariépy and D. Zargarian, Organometallics, 1999, 18, 5548 CrossRef CAS.
- A. R. Dias, M. H. Garcia, P. Mendes, M. F. M. Piedade, M. T. Duarte, M. J. Calhorda, C. Mealli, W. Wenseleers, A. W. Gerbrandij and E. Goovaerts, J. Organomet. Chem., 1998, 553, 115 CrossRef CAS.
- K. W Barnett, J. Chem. Educ., 1974, 51, 422 Search PubMed.
- G. M. Sheldrick, SHELXS97 and SHELXL97, University of Göttingen, Germany, 1997 Search PubMed.
- A. L. Spek, PLATON, University of Utrecht, The Netherlands, 1998 Search PubMed.
- P. McArdle, ORTEX, J. Appl. Crystallogr., 1995, 28, 65 Search PubMed.
- S. Szafert, P. Haquette, S. B. Falloon and J. A. Gladysz, J. Organomet. Chem., 2000, 604, 52 CrossRef CAS.
- R. Denis, L. Toupet, F. Paul and C. Lapinte, Organometallics, 2000, 19, 4240 CrossRef CAS.
- R. D. Hudson and A. R. Manning, unpublished work.
- M. Akita, M. Terada and Y. Moro-oka, Organometallics, 1992, 11, 1825 CrossRef CAS.
- P. J. Holland, M. E. Smith, R. A. Andersen and R. G. Bergman, J. Am. Chem. Soc., 1997, 119, 12815 CrossRef CAS.
- P. Butler, J. Gallagher and A. R. Manning, unpublished work.
- G. A. Desiraju and T. Steiner, Weak Hydrogen Bonds in Structural Chemistry and Biology, Oxford University Press, 1999 Search PubMed.
- D. Gregson and J. A. K. Howard, Acta Crystallgor., Sect. C, 1983, 39, 1024 CrossRef.
- S. Back, R. A. Gossage, M. Lutz, I. del Rio, A. L. Spek, H. Lang and G. van Koten, Organometallics, 2000, 19, 3296 CrossRef CAS.
- E. Louattani, J. Suades, A. Alvarez-Larena, J. F. Piniella and G. Germain, J. Organomet. Chem., 1996, 506, 121 CrossRef CAS.
- J. C. Anderson, B. F. Taylor, C. Viney and E. J. Wilson, J. Organomet. Chem., 1996, 519, 103 CrossRef CAS.
- C. M. Gordon, M. Kiszka, I. R. Dunkin, W. J. Kerr, J. S. Scott and J. Gebicki, J. Organomet. Chem., 1998, 554, 147 CrossRef CAS.
- R. J. Baxter, G. R. Knox, M. McLaughlin, P. L. Pauson and M. D. Spicer, J. Organomet. Chem., 1999, 579, 83 CrossRef CAS.
- R. J. Baxter, G. R. Knox, P. L. Pauson and M. D. Spicer, Organometallics, 1999, 18, 215 CrossRef CAS.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1 RSC.
| This journal is © The Royal Society of Chemistry 2002 |
