New Schiff base zinc(II) complexes exhibiting second harmonic generation
Cara
Evans†
and
Dominique
Luneau
*
Laboratoire de Chimie Inorganique et Biologique (UMR 5046), DRFMC, CEA-Grenoble, 17 rue des Martyrs, 38054 Grenoble cedex 09, France. E-mail: luneau@drfmc.ceng.cea.fr
First published on 6th December 2001
Abstract
Four bis(salicylaldiminato)zinc(II) complexes have been synthesized from four Schiff bases obtained by the condensation of chiral (R)-(+)-1-phenylethylamine with salicylaldehyde (1), 5-nitrosalicylaldehyde (2), 3,5-dichlorosalicylaldehyde (3) and 5-methoxysalicylaldehyde (4). The complexes crystallize in the noncentrosymmetric space groups P212121 (1), P21 (2), and C2 (3, 4). The geometry around the zinc(II) metal center is pseudo-tetrahedral with two oxygen and two nitrogen atoms from the ligands and has the Λ absolute configuration. Powders of the four compounds exhibit second harmonic generation (SHG) of intensity between that of 3-methyl-4-nitropyridine-1-oxide (POM) and N-(4-nitrophenyl)-(S)-prolinol (NPP).
Introduction
In the last decade a growing number of reviews1–3 and publications have highlighted the utility of organometallic complexes, in which organic chromophores are bound to metal centers, for second harmonic generation (SHG).4–20 Molecular polarizabilites (β) are frequently larger for the metallic complex than for the free chromophore (ligand) because of metal-to-ligand or ligand-to-metal charge transfer and because of the involvement of the orbitals on the metal.7,8,10,12,18 Another appealing aspect for the design of nonlinear optical (NLO) properties is that metal centers may serve as anchors in the engineering of three-dimensional geometries (tetrahedral, octahedral . . .), giving rise to octupolar molecules.10,21 Moreover, the combination of organic and inorganic elements affords materials of relatively high mechanical and thermal stability, as is also observed for organic chromophores in inorganic host matrixes.22However, despite the large molecular hyperpolarizabilities of many organometallic compounds, few organometallic crystals exhibit SHG (nonzero second-order susceptibility χ2). This is because the molecules are arranged centrosymmetrically in the solid state. One possible way to control the crystallization of organometallic complexes in noncentrosymmetric space groups is to incorporate chiral chromophores.19 Following this approach we report herein four zinc complexes (1–4) that exhibit SHG. The complexes were obtained from chiral Schiff bases as schematized in Fig. 1. The synthesis and crystal structure but not the NLO properties of compound 1 were published several years ago.23 Our aim was to characterize the SHG properties of 1 and to investigate the effect of varying its substituents [R1 = R2 = H (1); R1 = NO2, R2 = H (2); R1 = R2 = Cl (3); R1 = OMe, R2 = H (4)] (Fig. 1). The choice of zinc(II) as the metal center was guided by transparency considerations. An important requirement for SHG applications is transparency in a large spectral range in order to avoid absorption of the second harmonic.24 In this regard Zn(II), Cd(II) and Hg(II) are interesting because of the lack of d–d transitions.13–15,18 Furthermore, other work has shown that, from a series of complexes with different metal centers, those with Zn(II) generally exhibit the largest hyperpolarizability.8,18
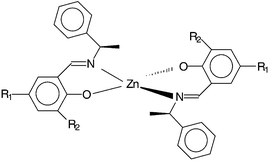 | ||
| Fig. 1 Chemical structure of the complexes ZnII(Ln)2 (n = 1–4). R1 = R2 = H (1); R1 = NO2, R2 = H (2); R1 = R2 = Cl (3); R1 = OMe, R2 = H (4). | ||
Results
Second-order NLO properties
All compounds 1–4 give strong SHG signals when incident light is from a 1.064 μm laser. The second harmonic intensities of all four compounds are between those of POM and NPP and increase as 2 < 1 < 3 < 4.Description of the structures
Views of the molecular structures of compounds 2–4 are shown in Fig. 2–4, respectively. Selected bond lengths (Å) and angles (deg.) for compounds 1–4 are listed in Table 1. In the four complexes 1–4 the Zn(II) metal center is in a pseudo-tetrahedral environment with two bidentate Schiff base ligands (L1–4) acting as chelates through their phenolate oxygen and azomethine nitrogen atoms (Fig. 1–4). All compounds crystallize in noncentrosymmetric space groups and exhibit the same configuration around the metal center. When the geometry is pseudo-tetrahedral, two different optical isomers, Λ and Δ are normally possible. However, for compounds 2–4 we found only the Λ configuration. This configuration was also found for 1, and circular dichroism spectra of this material in chloroform have demonstrated that the Λ configuration is preponderant in solution.23 The preference for the Λ(R,R) configuration in compounds 1–4 is thought to be driven by the minimization of steric hindrance between the two ligands in the coordination sphere.23| 1A | 1B | 2A | 2B | 3 | 4 | |
|---|---|---|---|---|---|---|
| Zn–O1 | 1.907(4) | 1.916(4) | 1.924(2) | 1.927(2) | 1.918(7) | 1.914(2) |
| Zn–O2 | 1.929(4) | 1.902(3) | 1.937(2) | 1.925(2) | ||
| Zn–N1 | 1.992(4) | 1.996(4) | 2.017(2) | 1.999(3) | 2.01(1) | 2.017(2) |
| Zn–N2 | 1.992(5) | 1.986(3) | 2.010(2) | 1.997(2) | ||
| O1–Zn–O2 | 117.7(2) | 111.6(2) | 114.84(9) | 104.8(1) | ||
| O1–Zn–O1# | 130.1(4) | 117.3(1) | ||||
| O1–Zn–N1 | 97.6(2) | 113.1(2) | 95.49(8) | 96.60(8) | 93.1(3) | 96.39(8) |
| O1–Zn–N1# | 110.5(3) | 113.00(8) | ||||
| O1–Zn–N2 | 112.0(2) | 95.5(2) | 111.3(1) | 118.28(9) | ||
| O2–Zn–N1 | 110.8(2) | 97.1(2) | 109.39(9) | 117.2(1) | ||
| O2–Zn–N2 | 94.6(2) | 113.5(2) | 94.17(9) | 97.22(9) | ||
| N1–Zn–N2 | 125.8(2) | 126.6(2) | 132.70(9) | 122.6(1) | ||
| N1–Zn–N1# | 122.5(5) | 122.3(1) | ||||
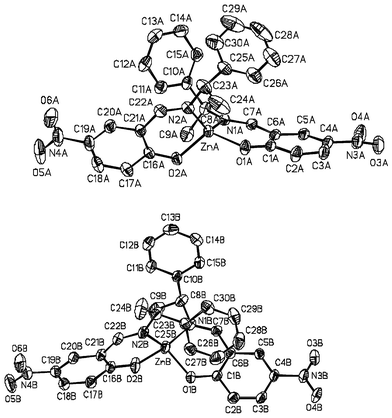 | ||
| Fig. 2 View of the molecular structures A and B for complex ZnII(L2)2 (2). Ellipsoids are drawn at the 30% probability level. | ||
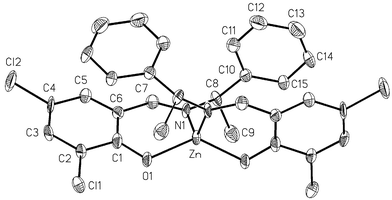 | ||
| Fig. 3 View of the molecular structure for complex ZnII(L3)2 (3). Ellipsoids are drawn at the 30% probability level. | ||
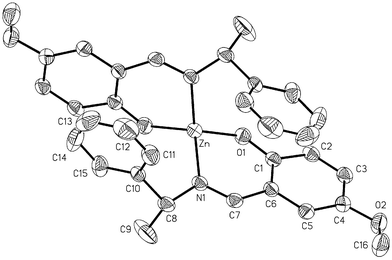 | ||
| Fig. 4 View of the molecular structure for complex ZnII(L4)2 (4). Ellipsoids are drawn at the 30% probability level. | ||
1 crystallizes in the P212121 orthorhombic space group.23 The asymmetric unit comprises two independent molecules, A and B, which show slight differences in the Zn(II) coordination sphere (Table 1). For both molecules there is a pseudo-C2 axis running through the Zn(II) ion and bisecting the N–Zn–N and O–Zn–O angles. In molecules A and B the dihedral angles between the two planes O1ZnN1 and O2ZnN2 are 81.7° and 84.9°, respectively, so that the geometry around Zn is nearly tetrahedral with a slight distortion toward a cis-planar geometry.
2 crystallizes in the P21 monoclinic space group. As for 1, the asymmetric unit comprises two different molecules, A and B. The dihedral angles between the two planes O1ZnN1 and O2ZnN2 are 80.47° and 94.61°, respectively, in molecule A and molecule B. The conformation around the zinc is nearly tetrahedral with distortion toward a cis-planar geometry for molecule A and a trans-planar geometry for molecule B.
3 crystallizes in the C2 monoclinic space group. The asymmetric unit is comprised of a half molecule with the zinc(II) metal ion located on the two-fold axis bisecting the N–Zn–N and O–Zn–O angles. The dihedral angle between the two planes O1ZnN1 is 77.07°. The conformation around the zinc has a strong distortion toward a cis-planar geometry.
4 crystallizes in the C2 monoclinic space group. As in the case of 3, the asymmetric unit comprises half a molecular complex. The Zn metal ion is in a special position on the two fold axis bisecting the N–Zn–N and O–Zn–O angles. The dihedral angle between the two planes O1ZnN1 is 84.67°, corresponding to a slight distortion of the geometry from tetrahedral toward cis-planar.
Spectroscopic properties
The UV-visible spectra of the solids (KBr pellets) and solutions (CH2Cl2, acetone) of complexes 1–4 are very similar. The absorption maxima for the lowest optical transitions are below 400 nm (Table 2). Compound 2 is blue-shifted compared to 1 because of the acceptor effect of the nitro substituent. The maxima of 3 and 4 are red-shifted relative to 1 because the chloro or methoxy substituents act as donors in addition to the phenylato oxygen.| Solid | Acetone | CH2Cl2 | ||
|---|---|---|---|---|
| Complex | λ max | λ max (εmax) | λ max (εmax) | |
| 1 | 379 | 372 (8400) | 374 (8300) | |
| 2 | 362 | 361 (33000) | 361 (37000) | |
| 3 | 394 | 386 (13300) | 389 (13000) | |
| 4 | 398 | 394 (3100) | 395 (3000) |
Discussion
All compounds 1–4 give strong SHG signals when illuminated with a Nd3+ ∶ YAG laser (λ = 1.064 μm). Second harmonic signal intensities are between those of POM and NPP and increase as 2 < 1 < 3 < 4. This trend is surprising; we had expected compound 2 to give the strongest SHG signal. The intensity of the second harmonic depends on the structure of both the molecules and the crystals they form. In the ligand of 1 there is no substituent, and charge transfer (CT) is along the O(phenolato) → CN(imino) direction. The substituents NO2, Cl, and OMe in compounds 2–4 tune the CT of 1. In the ligand of 2 there are two CT directions: O → NO2 and O → CN(imino). The large value of β calculated for the 4-nitrophenolate anion25 would predict a significant contribution to the hyperpolarizability of 2 from O → NO2 CT. However, its effect is weakened by the O → CN CT. In the chromophores of 3 and 4, on the other hand, the chloro and methoxy substituents have cooperative effects for the charge transfer directions, which are Cl → CN and O → CN (3) and MeO → CN and O → CN (4).In considering the effect of crystallization of the chromophores, we assume that the Zn(II) ion has an identical impact on the molecular hyperpolarizabilities of compounds 1–4 and that its main role is structural anchorage of the chromophore. Then only the polarizabilities of the chromophores and their orientations in point groups 2 and 222 should be taken into account. Compounds 2–4 crystallize in point group 2, for which four coefficients dijk of the χ2 tensor are nonzero. 1, on the other hand, crystallizes in point group 222, for which only one coefficient of χ2 is nonzero.26 In the crystal structure of 2, the two chromophores of molecule B are nearly in opposition; the angle between the two O → NO2 charge transfer directions is 7°. Such a situation may contribute to cancellation of the dijk coefficients of the χ2 tensor. This factor, in addition to molecular charge transfer considerations, may explain why 2 exhibits the lowest SHG signal.
Conclusion
This paper reports the synthesis and characterization of four new Schiff base Zn(II) complexes that exhibit intense second harmonic generation. As expected from the chirality of the ligands, these materials crystallize in noncentrosymmetric space groups. Only one optical isomer, Λ, is observed. The compounds are light-colored and show a large range of transparency above 400 nm. The various substituents tune the SHG properties and also the thermal stability of the compounds, as reflected by their melting points.Experimental
Synthesis of the complexes
All reagents and solvents were reagent grade and were used without further purification. The complexes were synthesized using procedures reported for 1.23Crystallography
Data were collected at room temperature (298 K) with a Bruker SMART CCD diffractometer equipped with a normal focus X-ray tube having a molybdenum target. The data were processed through the SAINT reduction software.27 Empirical absorption corrections were carried out by SADABS.28 The structures were solved and refined on F2 using the SHELXTL software.29 All non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms were included in the final refinement model in calculated positions with isotropic thermal parameters. Crystal structure and refinement data for compounds 1–4 are summarized in Table 3.| 1 a | 2 | 3 | 4 | |
|---|---|---|---|---|
| a Crystal data obtained from ref. 23. b I > 2σ(I), R(F) = Σ‖Fo| − |Fc‖/Σ|Fo|. c All data, Rw(F2) = Σ[w(Fo2 − Fc2)2/ΣwFo4]1/2. | ||||
| Formula | C30H28N2O2Zn | C30H26N4O6Zn | C30H24N2O2Cl4Zn | C32H32N2O4Zn |
| M | 513.91 | 603.92 | 651.68 | 573.97 |
| T/K | 293(2) | 298(2) | 298(2) | 298(2) |
| Cryst. syst. | Orthorhombic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P212121 | P21 | C2 | C2 |
| a/Å | 17.738(4) | 15.4512(8) | 13.928(2) | 17.569(2) |
| b/Å | 29.632(9) | 10.1073(5) | 10.949(2) | 8.758(8) |
| c/Å | 9.968(2) | 18.0725(9) | 10.208(2) | 11.981(1) |
| β/deg. | 90 | 90.541(1) | 118.246(3) | 130.283(1) |
| V/Å3 | 5239(2) | 2822.3(2) | 1371.4(4) | 1406.4 |
| Z | 8 | 4 | 2 | 2 |
| μ/mm−1 | 0.98 | 0.920 | 1.318 | 0.921 |
| d/g cm−3 | 1.303 | 1.421 | 1.578 | 1.421 |
| λ/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| R(F)b | 0.0725 | 0.0311 | 0.0859 | 0.0312 |
| R w (F2)c | 0.0880 | 0.2006 | 0.0835 | |
CCDC reference numbers 165067–165069.
See http://www.rsc.org/suppdata/dt/b1/b104360a/ for crystallographic data in CIF or other electronic format.
Optical measurements
The efficiency of the crystals for frequency doubling was determined by the Kurtz and Perry powder test30 using a pulsed Nd3+ ∶ YAG laser (λ = 1.06 μm). Prior to measurement the crystalline compounds were ground into powders, sieved to an average particle size between 250–300 μm, and packed into pellets of 2 mm path length. The references used were urea, POM [3-methyl-4-nitropyridine-1-oxide] and NPP [N-(4-nitrophenyl)-(S)-prolinol]. Absorption spectra of KBr pellets and of solutions in acetone and dichloromethane were obtained on a Varian Cary5E spectrometer.Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Commissariat à l'Energie Atomique (CEA), and the Université Joseph-Fourier. The authors thank Dr. René Masse for encouragement and fruitful discussions. C. E. is grateful for a Chateaubriand Fellowship from the French government.References
- S. R. Marder, Metal containing Materials for Nonlinear Optics, in Inorganic Materials, ed. D. W. Bruce and D. O'Hare, John Wiley and Sons, Sussex, England, 1992 Search PubMed.
- N. J. Long, Angew. Chem., Int. Ed. Engl., 1995, 34, 21 CrossRef CAS.
- T. Verbiest, S. Houbrechts, M. Kauranen, K. Clays and A. Persoons, J. Mater. Chem., 1997, 7, 2175 RSC.
- J. C. Calabrese and W. Tam, Chem. Phys. Lett., 1987, 133, 244 CrossRef CAS.
- J. C. Calabrese, L.-T. Cheng, J. C. Green, S. R. Marder and W. Tam, J. Am. Chem. Soc., 1991, 113, 7227 CrossRef CAS.
- H. S. Nalwa and S. Kobayashi, J. Porphyins Phthalocyanines, 1998, 2, 21 Search PubMed.
- T. Thami, P. Bassoul, M. A. Petit, J. Simon, A. Gort, M. Barzoukas and A. Villaeys, J. Am. Chem. Soc., 1992, 114, 915 CrossRef CAS.
- M. Bourgault, C. Mountassir, H. Le Bozec, I. Ledoux, G. Pucetti and J. Zyss, J. Chem. Soc., Chem. Commun., 1993, 1623 RSC.
- M. Bourgault, K. Baum, H. Le Bozec, G. Pucetti, I. Ledoux and J. Zyss, New J. Chem., 1998, 22, 517 RSC.
- C. Dhenaut, I. Ledoux, I. Samuel, D. W., J. Zyss, M. Bourgault and H. Le Bozec, Nature, 1995, 374, 339 CrossRef.
- T. Renouard, H. LeBozec, S. Brasselet, I. Ledoux and J. Zyss, Chem. Commun., 1999, 871 RSC.
- S. Di Bella, I. Fragala, I. Ledoux and T. J. Marks, J. Am. Chem. Soc., 1995, 117, 9481 CrossRef CAS.
- Y.-p. Tian, C.-y. Duan, C.-y. Zhao, X.-z. You, T. C. W. Mak and Z.-y. Zhang, Inorg. Chem., 1997, 36, 1247 CrossRef CAS.
- C. C. Evans, R. Masse, J. F. Nicoud and M. Bagieu-Beucher, J. Mater. Chem., 2000, 10, 1419 RSC.
- H. Zhang, D. E. Zelmon, G. E. Price and B. K. Teo, Inorg. Chem., 2000, 39, 1868 CrossRef CAS.
- A. Houlton, N. Jasim, R. M. G. Roberts, J. Silver, D. Cunningham, P. McArdle and T. Higgins, J. Chem. Soc., Dalton Trans., 1992, 2235 RSC.
- F. Averseng, P. G. Lacroix, I. Malfant, G. Lenoble, P. Cassoux, K. Nakatani, I. MalteyFanton, J. A. Delaire and A. Aukauloo, Chem. Mater., 1999, 11, 995 CrossRef CAS.
- P. G. Lacroix, S. Di Bella and I. Ledoux, Chem. Mater., 1996, 8, 541 CrossRef CAS.
- G. Lenoble, P. G. Lacroix, J. C. Daran, S. Di Bella and K. Nakatani, Inorg. Chem., 1998, 37, 2158 CrossRef CAS.
- F. Averseng, C. Lepetit, P. G. Lacroix and J.-P. Tuchagues, Chem. Mater., 2000, 12, 2225 CrossRef CAS.
- J. Zyss and I. Ledoux, Chem. Rev., 1994, 94, 77 CrossRef CAS.
- R. Masse, M. Bagieu-Beucher, J. Pécaut, J. P. Lévy and J. Zyss, Nonlinear Opt., 1993, 5, 413 Search PubMed.
- H. Sakiyama, H. Okawa, N. Matsumoto and S. Kida, J. Chem. Soc., Dalton Trans., 1990, 2935 RSC.
- J. Zyss, in Conjugated Polymeric Matenals: Opportunities in Electronics, Optoelectronics and Molecular Electronics, ed. J.-L. Brédas and R. R. Chance, Kluwer Academic Publishers, Dordrecht, 1990 Search PubMed.
- C. C. Evans, M. Bagieu-Beucher, R. Masse and J.-F. Nicoud, Chem. Mater., 1998, 10, 847 CrossRef CAS.
- J. Zyss and J.-L. Oudar, Phys. Rev. A, 1982, 26, 2028 CrossRef CAS.
- SAINT, ver. 4.050, Bruker Analytical X-ray Instruments, Inc., Madison, WI, 1998 Search PubMed.
- G. M. Sheldrick, SADABS, Program for Siemens area detector absorption correction, Institut für Anorganische Chemie, Universität Göttingen, 1994 Search PubMed.
- SHELXTL, ver. 5.030, Bruker Analytical X-ray Instruments, Inc., Madison, WI, 1998 Search PubMed.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798 CrossRef CAS.
Footnote |
| † Present address: Department of Chemical Engineering, Rm. 3357, Engineering II, University of California Santa Barbara, Santa Barbara, CA 93106, USA. |
| This journal is © The Royal Society of Chemistry 2002 |
