Lactiplantibacillus plantarum DMDL 9010 alleviates dextran sodium sulfate (DSS)-induced colitis and behavioral disorders by facilitating microbiota-gut-brain axis balance
Yan-yan
Huang
a,
Ya-ping
Wu
a,
Xiang-ze
Jia
ab,
Jin
Lin
a,
Lan-fang
Xiao
a,
Dong-mei
Liu
 *a and
Ming-hua
Liang
*a
*a and
Ming-hua
Liang
*a
aSchool of Food Science and Engineering, South China University of Technology, Guangzhou 510640, Guangdong, China. E-mail: liudm@scut.edu.cn; liangmh@scut.edu.cn; Fax: +86 20-87113848; Tel: +86 20-222368198 Tel: +86-13609795325 Tel: +86-15017539128
bDepartment of Health Sciences and Technology, ETH Zürich, Schmelzbergstrasse 9, Zürich, Switzerland
First published on 23rd November 2021
Abstract
Previous studies have found that probiotic supplements can ameliorate mental behavioral disorders. This study investigated the effects of Lactiplantibacillus plantarum DMDL 9010 (LP9010) intake on the depression-like behavior induced by dextran sodium sulfate (DSS) and its possible mechanism. Male C57BL/6N mice were fed with DSS to establish the model of ulcerative colitis. LP9010 intake reduced the DSS-induced inflammatory response, and repaired intestinal barrier damage, as well as lightened depression-like behavior. LP9010 supplementation also inhibited neuroinflammation by up-regulating the levels of neurotransmitters, especially 5-HT, NE, DA, and 5-HIAA. Moreover, the intake of LP9010 reorganized the gut microbiome by increasing the relative abundance of Bacteroidetes and Firmicutes, and decreasing the relative abundance of Proteobacteria and Verrucomicrobia. Furthermore, treatment with LP9010 increased the levels of short-chain fatty acids, such as butyric acid and propionic acid. In conclusion, LP9010 intake was a promising probiotic intervention strategy for the prevention of colitis-induced behavioral disorders through the microbiota-gut-brain axis.
1. Introduction
Depression, an epidemic disease of the 21st century, is faced with various problems such as high recurrence rate and strong side effects1 due to its high morbidity and mortality. Inflammatory bowel disease (IBD) is a chronic form of intestinal inflammation, including Crohn's disease (CD) and ulcerative colitis (UC).2,3 IBD has been recognized as a global disease, and will predictably become a global epidemic in the next few years.4 Various aspects can cause IBD, but the specific pathogenesis is still unclear. Intestinal inflammation can induce neuroinflammation,5 which potentially becomes one of the prominent pathogeneses of depression. Increasing numbers of evidence suggest that gut microbes are involved in a variety of stressful conditions, including depression, anxiety and irritable bowel syndrome.1 For now, large numbers of clinical studies are devoted to finding effective drugs for patients with inflammatory colitis accompanied by depression.6 Therefore, it is of great significance to discover drugs that achieve multi-therapeutic treatments for UC and depression.Recently, treatment for depression with probiotics has become a research hotspot.7,8 Chemosynthetic drugs have traditionally been used in the clinical treatment of depression. However, they would cause the intestinal environment imbalance of patients with inflammatory enteritis.9 Extensive animal experiments have shown that Lactobacillus, short-chain fatty acids (SCFAs)-producing probiotics,10 could relieve depression-like behavior.7,11 Previous studies have also shown that germ-free mice fed with Lactiplantibacillus plantarum PS128 for a long period of time significantly increased the levels of 5-hydroxytryptamine (5-HT) and dopamine (DA) in the striatum, as well as significantly improved depressive behavior.12 Additionally, there is growing evidence that the intake of probiotics (such as L. Casei Strain Shirota, Bifidobacterium Pseudocatenulatum CECT7765, and L. Helveticus R0052) could improve depressive behavior or mood in depressed patients.13 Therefore, probiotics and prebiotics can be used as an effective means to improve the gut microbial environment and relieve inflammatory enteritis and depression.
The connection and communication between the brain and gut flora would interact through the microbiota-gut-brain (MGB) axis.14,15 Previous studies have shown that the brain-derived neurotrophic factor (BDNF) is not only involved in the regulation of neuroplasticity and the repair of stress nerve injury to prevent neuronal degeneration,16 but also can increase the level of 5-HT in brain tissue by affecting signal transduction.17 High levels of pro-inflammatory cytokines such as interleukin-1β (IL-1β) could inhibit the utilization of tryptophan and 5-HT, further regulating the levels of neurotransmitters.1 In addition, intestinal microbial metabolites including SCFAs such as butyrate, acetate, and propionate might play a role in treating inflammatory (intestinal) diseases and alleviating nervous system dysfunction.4,16 Therefore, it is of great significance to evaluate the pathway of neurotransmitters, BDNF and inflammatory factors in MGB.
Previous studies showed that Lactiplantibacillus plantarum DMDL 9010 (LP9010) possessed efficient nitrite-degrading capacity, cholesterol-reducing ability, good acid and bile salt tolerance,18–21 and had the potential to be used as a starter culture in fermented goods.22,23 To explore the therapeutic functionality of LP9010, the ulcerative colitis model was established by instillation of dextran sodium sulfate (DSS) in male C57BL/6N mice. The effects of LP9010 ingestion on the inflammatory response, intestinal barrier, neurotransmitter, intestinal microflora and short-chain fatty acids were investigated. Furthermore, the influence of LP9010 on DSS induced depression-like behavior and the underlying mechanism of entero-brain axis were further demonstrated. This study provides a new approach for LP9010 in probiotic therapy of depression.
2. Materials and methods
2.1 Materials
Lactiplantibacillus plantarum DMDL 9010 (LP9010) (CGMCC no. 5172) was previously isolated from naturally fermented mustard and preserved in the China General Microbiological Culture Collection Center (CGMCCC). Dextran sodium sulfate (DSS) with a molecular weight of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was purchased from Aladdin Biochemical Technology (Shanghai, China).
000 was purchased from Aladdin Biochemical Technology (Shanghai, China).
2.2 Animals and DSS-induced colitis model
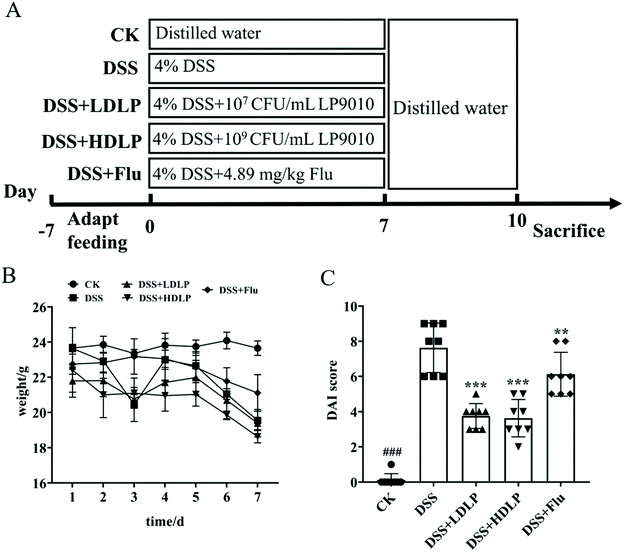
All animal experiments should be conducted in keeping with the procedures and permits approved by the Animal Ethics Review Committee of South China Agricultural University (SYXK 2019-0136), and in accordance with the Regulations of the People's Republic of China on the Management of Laboratory Animals. Forty C57BL/6N male mice (aged 4–5 weeks) were purchased from Zhejiang Vitong Lihua Experimental Animal Technology Co., Ltd (Zhejiang, China). All mice were familiarized (23–25 °C and a 12 h light/dark cycle) with the normal diet for 7-day adaptation.2 After being weighed, the mice were randomly divided into 5 groups (n = 8 for each group): CK, mice administered orally with saline and receiving distilled water; DSS, mice administered orally with saline and receiving 4% DSS solution; DSS + LDLP and DSS + HDLP, mice administered orally with 0.2 mL/10 g weight per day LP9010 (107 CFU mL−1 for DSS + LDLP, and 109 CFU mL−1 for DSS + HDLP) and receiving 4% DSS solution; DSS + Flu, mice administered orally with 4.89 mg kg−1 fluoxetine hydrochloride solution and receiving 4% DSS solution. The model of acute colitis was established by drinking 4% DSS solution freely for 7 days. The gavage strategy of mice in the five groups was strictly implemented according to Fig. 1A. All mice were weighed and observed every day. At days 8–10, all mice were treated with distilled water. The open field test (OFT) was conducted on the morning of day 8, and a light–dark test (LDT) was conducted on the morning of days 9–10. At day 10, all mice were euthanized.2.3 Assessment of disease activity index (DAI)
The obtained stool samples of each mouse were measured according to the fecal occult blood test kit from Nanjing Jiancheng, Inc. (Nanjing, China). The hematochezia, consistency of stool, and weight loss of mice were added together to calculate the DAI according to the previous study.2 The hematochezia and consistency of stool were assessed by the scoring system: 0, no observable blood (negative); 1, trace blood (light blue); 2, slight blood (blue); 3, obvious blood (dark blue); 4, gross blood (black) and 0, normal; 1, loose stool; 2, mild diarrhea; 3, diarrhea; 4, gross diarrhea. The percentage of weight change from day 0 until the end of the trial was calculated to evaluate the loss of weight (0, no weight loss; 1, 1–5%; 2,5–10%; 3, 10–20%; 4, >20%).2.4 Morphological studies
The colonic tissue of mice in each group was weighed and pictures were taken with a steel ruler as a reference. Morphological studies were mentioned in Kuang's methods.2 Briefly, fresh colon tissue (1 cm) near the anal side was soaked with 4% paraformaldehyde fix solution, embedded with paraffin, and sectioned at 4 μm intervals. Then, the sections were stained with hematoxylin and eosin (H&E) to illustrate histological changes. The microscope (Eclipse Ci-L, Nikon, Japan) was employed to take pictures, and Image-Pro Plus 6.0 (Media Cybemetics, USA) software was used to measure the intestinal wall thickness.2.5 Behavioral assessments
OPT and LDT were generally applied to evaluate depression and anxiety-like behaviors.1 OPT was usually used to determine the exploration desire of mice. A locomotor monitoring box (50 cm × 50 cm × 50 cm) was equally divided into 16 parts. Each mouse's spontaneous movement was monitored (5 min) by a computerized video-tracking system (SuperMaze, China). Six mice in each group were randomly selected for OPT determination. In LDT, each mouse was placed in the center of the device (40 cm × 30 cm × 35 cm) for 10 min, containing two chambers of equal scope, one bright and another dark. Each mouse's behavior was observed by a computerized video-tracking system (SuperMaze, China). Four mice in each group were randomly selected for LDT determination.2.6 Assessment of neurotransmitters
The brain tissue samples (20 mg) of mice were washed with sterile saline. The deionized water (200 μL) and the mixed acetonitrile–methanol solution (800 μL) were added to the brain tissue samples. The mixed samples were ultrasonicated at 4 °C, followed by vortexing and quickly freezing by liquid nitrogen. Then, the mixtures were restored at 25 °C, and the above steps were repeated three times to obtain brain tissue homogenate. Brain homogenate was centrifuged at 4 °C (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 15 min), and 600 μL of supernatant was collected. Then, the lyophilized supernatant was mixed with acetonitrile aqueous solution (400 mL, 50%). The mixed samples were filtered with 0.22 μm filter and detected by LC-MS (AB Sciex Triple TOF 5600+), equipped with a Kinetex C18 100 A column (2.6 μm, 2.1 × 50 mm, Waters, USA). The mobile phase consisted of solution A (0.05% HCOOH-Waters) and solution B (0.05% HCOOH–CH3CN). The procedure of the gradient elution was as follows: 0.1 → 2.0 → 4.5 → 8.0 → 9.0 → 9.1 → 11 min, corresponding to solvent B: 2 → 2 → 10 → 95 → 95 → 2 → 2% at a flow speed of 0.4 mL min−1. The reference external standards of 5-hydroxytryptamine (5-HT), gamma-aminobutyric acid (GABA), dopamine (DA), norepinephrine (NE) and 5-hydroxyindole-3-acetic acid (5-HIAA) were bought from Sigma-Aldrich company (USA).
000g, 15 min), and 600 μL of supernatant was collected. Then, the lyophilized supernatant was mixed with acetonitrile aqueous solution (400 mL, 50%). The mixed samples were filtered with 0.22 μm filter and detected by LC-MS (AB Sciex Triple TOF 5600+), equipped with a Kinetex C18 100 A column (2.6 μm, 2.1 × 50 mm, Waters, USA). The mobile phase consisted of solution A (0.05% HCOOH-Waters) and solution B (0.05% HCOOH–CH3CN). The procedure of the gradient elution was as follows: 0.1 → 2.0 → 4.5 → 8.0 → 9.0 → 9.1 → 11 min, corresponding to solvent B: 2 → 2 → 10 → 95 → 95 → 2 → 2% at a flow speed of 0.4 mL min−1. The reference external standards of 5-hydroxytryptamine (5-HT), gamma-aminobutyric acid (GABA), dopamine (DA), norepinephrine (NE) and 5-hydroxyindole-3-acetic acid (5-HIAA) were bought from Sigma-Aldrich company (USA).
2.7 16S rDNA sequencing analysis
The total fecal DNA of each sample was obtained from the colonic substance of mice by using the HiPure Stool DNA Kit (Magen, Guangzhou, China). The V3–V4 region of the 16S ribosomal RNA gene was amplified by PCR procedures according to the pervious study24 using primers (341F: 5′-CCTACGGGNGGCWGCAG-3′; 806R: 5′-GGACTACHVGGGTATCTAAT-3′). Obtained amplicons were then pooled in equimolar and double-end sequenced (2 × 250) on an Illumina platform according to the reported procedures.24 All sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity level using UPARSE (VERSION 9.2.64) and bioinformatics analysis was conducted. The sequencing data was performed in R project Venn Diagram package (version 1.6.16), R project Vegan package (version 2.5.3), and Linear discriminant analysis Effect Size (LEfSe) software (version 1.0).2.8 Enzyme-linked immunosorbent assay (ELISA)
Blood samples of each group were centrifuged at 4000g for 10 min to obtain serum and stored at −80 °C for further analysis. The brain tissues were lysed by lysate (Enzyme-linked Biotechnology Co., Ltd, Shanghai, China) through a tissue crushing apparatus (SCIENTZ-IID, Ningbo Xinzhi Biological Technology Co., Ltd, China). After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min, the supernatant of each sample was taken for detection. The levels of cytokines (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and transforming growth factor-β (TGF-β)) in the serums and BDNF in the brain tissues were each quantified using mouse ELISA kit from Enzyme-linked Biotechnology Co., Ltd (Shanghai, China).
000g for 15 min, the supernatant of each sample was taken for detection. The levels of cytokines (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and transforming growth factor-β (TGF-β)) in the serums and BDNF in the brain tissues were each quantified using mouse ELISA kit from Enzyme-linked Biotechnology Co., Ltd (Shanghai, China).
2.9 Determination of SCFAs in colon content
HPLC was used to evaluate the contents of SCFAs in feces, including acetic acid, propionic acid, isobutyric acid, n-butyric acid and isovaleric acid. Briefly, 100 mg fecal samples of each mouse were respectively soaked in a saturated solution of sodium chloride and crushed. The H2SO4 solution (50%, 0.15 mL) was added to the sample at 4 °C for 30 min, and then centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min) to obtain the supernatant. Subsequently, the supernatant was filtered through a 0.22 μm filter. The mobile phase was 0.005 mol L−1 H2SO4 at a flow rate of 0.6 mL min−1. A Rezex ROA organic acid H+ column (300 × 7.8 mm, Phenomenex, USA) and UV detector (210 nm) were used for HPLC.
000g, 10 min) to obtain the supernatant. Subsequently, the supernatant was filtered through a 0.22 μm filter. The mobile phase was 0.005 mol L−1 H2SO4 at a flow rate of 0.6 mL min−1. A Rezex ROA organic acid H+ column (300 × 7.8 mm, Phenomenex, USA) and UV detector (210 nm) were used for HPLC.
2.10 Data and statistics analysis
The one-way ANOVA analysis was performed to compare the variables between the two groups. All parameters were analyzed using GraphPad 8.0.1 and SPSS 19.0. The R 3.3.2 software was used to carry out the heatmap analysis. Data were reported as the mean ± SD, and a difference at p < 0.05 was considered as a statistical significance.3. Results
3.1 Changes in the body weight and DAI score of mice
On the seventh day of DSS exposure (Fig. 1A), the body weights of mice in the DSS (20.22 ± 0.90 g), DSS + LDLP (20.62 ± 1.08 g), DSS + HDLP (19.85 ± 0.53 g), and DSS + Flu (20.69 ± 1.49 g) groups decreased significantly compared with the CK group (23.09 ± 1.37 g) (p < 0.05) (Fig. 1B). The body weight of mice with DSS-induced colitis (DSS, DSS + LDLP, DSS + HDLP and DSS + Flu group) was significantly decreased because of the disease condition. The phenotype might be worse with the further decrease of body weight. The changes in DAI were recorded as shown in Fig. 1C. Compared with the DSS group, intragastric administration of LP9010 and fluoxetine hydrochloride (Flu) significantly alleviated weight loss, liquefied feces and hematochezia, and downregulated the DAI score in mice (p < 0.05) (Fig. 1C). Among them, different concentrations of LP9010 had a similar effect on alleviating DSS-induced colitis. All in all, the modeling was successful according to the changing trend of the body weight of mice and DAI score (Fig. 1C).3.2 Effects of LP9010 on DSS-induced histopathological evaluations in the colon
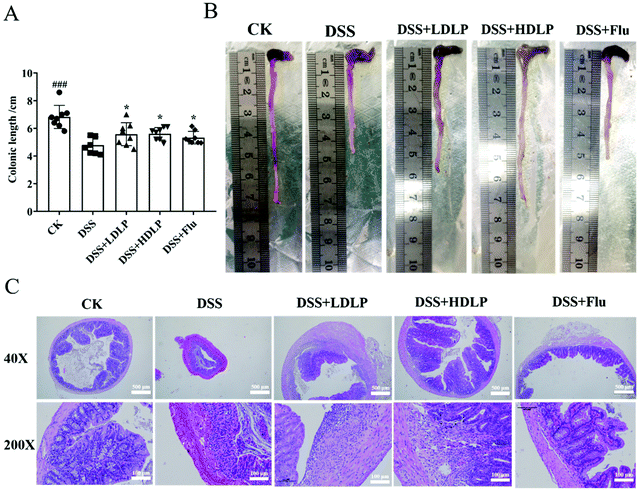
As mentioned earlier, behavioral disorders might be reported to be highly associated with intestinal barrier dysfunction and inflammation.25 To analyze the effect of LP9010 on DSS-induced colonic tissue damage, the colonic length and colonic status of each mouse were observed. DSS significantly damaged colon tissues, shortening them by 28.57% (p < 0.001) (Fig. 2A and B). Compared with the DSS group, intragastric administration of LDLP, HDLP and Flu significantly increased the colonic length (p < 0.05) (Fig. 2A and B). Moreover, the effect of LP9010 on improving the colon tissue showed a dose-dependent trend (Fig. 2A and B). Histological evaluation of the colon could directly evaluate the degree of pathological injury of mouse colitis.2 In the colon tissue of the DSS group, H&E staining showed that multiple ulcers and large areas of inflammatory infiltrate, whereas the CK group had a complete structure without an ulcer or inflammatory infiltration (200×, Fig. 2C). Inflammatory lesions were also found in colon tissues of the DSS + LDLP, DSS + HDLP and DSS + Flu groups (Fig. 2C), and the severity of inflammation was lower than that of the DSS group, which was consistent with Ding's results.26 Additionally, the DSS + HDLP group was significantly better than the DSS + LDLP group in the histological evaluation of the colon (Fig. 2C). As shown in Fig. 2C under a 40× microscope, the DSS group had extensive inflammatory infiltration in mucosal layer, and the crypt disappeared compared to the CK group. More importantly, histological evaluation of the colon was significantly improved in both DSS + LDLP and DSS + HDLP groups compared to the DSS group. Furthermore, compared with the treatment group (DSS + LDLP and DSS + HDLP), the DSS + Flu group had a significantly thinner mucosal layer, probably due to the side effects of Flu (Fig. 2C). These results suggested that ingestion of LP9010 had a protective effect on DSS-induced intestinal damage.3.3 Effects of LP9010 on behavior disorder in mice with DSS-induced colitis
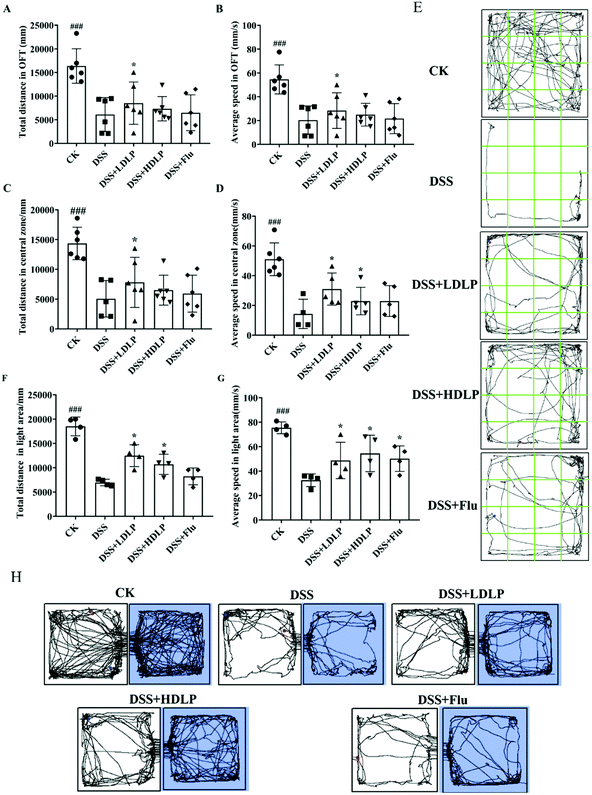
Depression was generally accompanied by symptoms such as decreased interest and motivation, with a decrease in voluntary activity and desire to explore.13 Generally, OFT and LDT were often used in the evaluation of depression, anxiety and other behavioral disorders.1 A more disorganized total movement path of the mice in the OFT and faster average speed indicated that the spontaneous activities were in a more active state.1 As shown in Fig. 3, the total distance and average speed of mice in the entire and central areas of OFT in the DSS group were significantly lower than those in the CK group (Fig. 3A–D), demonstrating that DSS could significantly reduce the autonomous activities and exploration desire of mice. The DSS + Flu group showed an increase in the total distance and average speed of movement across the entire and central area compared to the DSS group (Fig. 3A–D). The effects of LP9010 on depression-like behavior disorders by gavage (Fig. 3A–D) were consistent with the previous study,27 enhancing the depression-like behavior via probiotics supplementation. The gavage of LP9010 and Flu had similar effects on improving the depression-like behavior, and recovering the autonomic activity and exploration desire of mice (Fig. 3E). There was no significant difference among the DSS + LDLP, DSS + HDLP and DSS + Flu groups.In order to further explore the autonomous activities and exploration desire of mice, the LDT experiment was investigated in Fig. 3F and G. In the DSS group, the movement distance and average speed in the bright box were significantly decreased (p < 0.001), which proved that DSS could decrease the autonomous activity and exploration desire of the mice.26 Both LP9010 and Flu were effective in restoring the autonomous activity and exploration desire in mice. Mice could freely move between the light box and the dark box, and the movement track is shown in Fig. 3H. The complexity of the autonomous tracks represented the active degree of the mouse.1 Mice in the DSS group had significantly reduced movement trajectories in both light and dark boxes. The recovery of mice in the DSS + Flu group was limited and slightly better than that in the DSS group. The mice movement trajectories of the administration of LP9010 were significantly more complex than those of the DSS group (Fig. 3H). Additionally, the trajectory complexity of the DSS + LDLP group was similar to that of the DSS + Flu group, and the trajectory of the DSS + HDLP group was clearly more complex than that of the DSS + Flu group (Fig. 3H). Therefore, LP9010 showed a tendency to improve exploration and movement in depressed mice.
3.4 Effects of LP9010 on neurotransmitter changes in mice with DSS-induced colitis
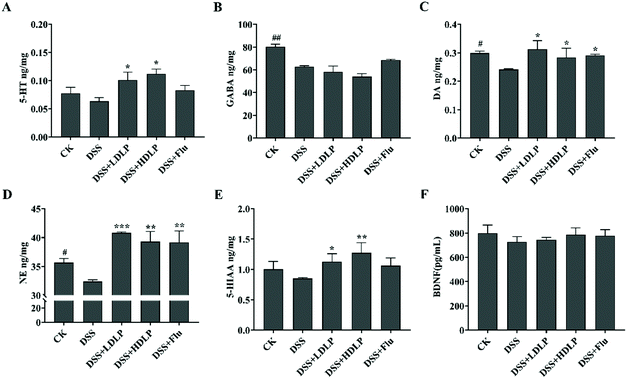
The relative levels of neurotransmitters, such as GABA, NE, DA, 5-HT and 5-HIAA, were closely related to psychoneurotic dysfunction and depression.28 Among them, the reduced availability of 5-HT within the brain was a dominant feature in the pathogenesis of depression.29 As shown in Fig. 4, compared with the CK group, the levels of various neurotransmitters in the mice brain in the DSS group were significantly decreased, especially the levels of GABA (Fig. 4B), DA (Fig. 4C), and NE (Fig. 4D) (p < 0.05). Compared with the DSS group, the levels of neurotransmitters 5-HT (Fig. 4A), DA (Fig. 4C), NE (Fig. 4D), and 5-HIAA (Fig. 4E) in all experimental groups were significantly increased (p < 0.05). In addition, intragastric administration of LP9010 inhibited neuroinflammation by increasing the levels of 5-HT and its metabolite 5-HIAA in depression-like mice, as well as the levels of NE and DA, and there was no significant difference between the different concentrations of LP9010 (Fig. 4, p > 0.05). The effect of Flu on the enhancement of various neurotransmitters was different due to the inhibition of the reuptake of 5-HT.28 Different doses of LP9010 were more effective than Flu in increasing the levels of 5-HT and 5-HIAA (Fig. 4). Interestingly, low concentrations of LP9010 were more effective than Flu in increasing NE and DA levels (Fig. 4). BDNF was an important brain-derived neurotrophic factor.28 However, there was no significant difference in BDNF among all groups (Fig. 4F, p > 0.05). The central BDNF and 5-HT systems are synergistic, and 5-HT up-regulates hippocampal BDNF-TrkB signaling to increase BDNF expression and synthesis.30,31 Therefore, further research is needed on the specific ways of the treatment of depression by Lactobacillus.3.5 Effects of LP9010 on the intestinal microbial structure in mice with DSS-induced colitis
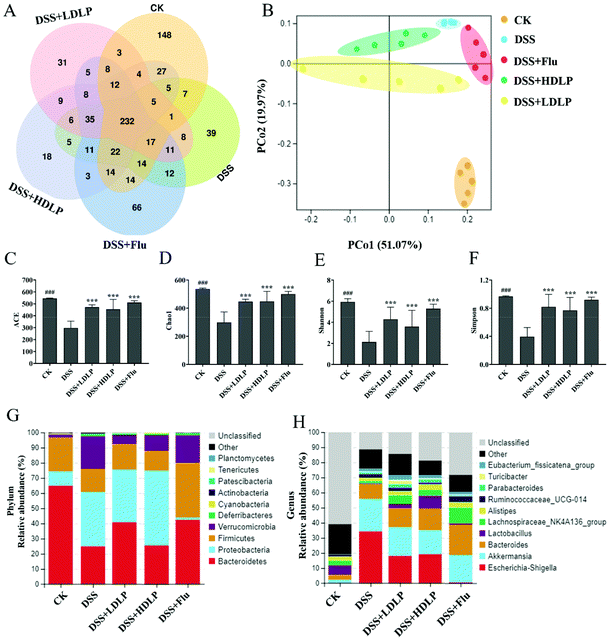
The V3–V4 region of the 16S rDNA gene in intestinal contents of mice was sequenced. OTU cluster analysis was performed on the CK, DSS, DSS + Flu, DSS + LDLP and DSS + HDLP groups. The number of unique OTU values in each group were all greater than 18, and the number of common OTU in all groups was 232, as shown in Fig. 5A. β-Diversity analysis reflected significant microbial community differences between multiple samples.32 Principal Coordinate Analysis (PCoA) was usually used to compare the evolutionary distance between morphological or functional community-specific OTUs to more fully reflect community similarity.2,32Fig. 5B plotted the clustering composed by each group of microbiotas. Each point represents a sample point. Different colors represent different groups, and the distance among them reflects the degree of difference. There were significant differences in the biological community among groups (Fig. 5B). The intestinal microflora structure of mice was significantly different from that of the DSS group after intragastric administration of LP9010 and Flu (Fig. 5B). The α diversity analysis, including ACE, Chao1, Shannon and Simpson indices, focused on reflecting the species diversity in the community.2 The results of α diversity analysis are shown in Fig. 5C, D, E, and F, which proved that intragastric administration of LP9010 could change the species diversity and abundance of DSS-induced colitis and depression mice. The Chao 1, Shannon and Simpson indices in the DSS group were significantly lower than those in the CK group (p < 0.05), indicating that DSS significantly reduced the diversity and richness of intestinal microflora in mice. Intragastric administration of LP9010 could significantly improve the situation and increase the richness of intestinal microflora in mice (Fig. 5, p < 0.05).The average relative abundance levels of the top ten bacterial groups in the mice gut were further analyzed at the phylum and genus levels (Fig. 5G and H). The dominant bacterial phyla in the control (CK) group were Bacteroidetes (64.86%), Firmicutes (22.24%), Proteobacteria (9.43%) and Verrucomicrobia (2.07%) (Fig. 5G). DSS significantly changed the relative abundances of Proteobacteria and Verrucomicrobia with an increase to 35.78% and 21.46%, respectively, and Bacteroidetes and Firmicutes with a decrease to 24.95% and 15.27%, respectively (Fig. 5G). Compared to the DSS group, the DSS + Flu group could increase the relative abundance of Bacteroidetes and Firmicutes to 42.39% and 36.07%, respectively (Fig. 5G). Accordingly, the relative abundances of Proteobacteria and Verrucomicrobia decreased to 1.47% and 18.19%, respectively. High concentrations of LP9010 were effective in reducing the relative abundance of Verrucomicrobia (10.40%), and low concentrations of LP9010 was effective in reducing the relative abundance of Verrucomicrobia (6.19%) and increasing Bacteroidetes (35.53%) (Fig. 5G). At the genus level, DSS could cause a significant decline in the relative levels of the Lactobacillus and Lachnospiraceae_NK4A136_group. Meanwhile, the relative levels of Escherichia-Shigella, Akkermansia, Parabacteroides and Eubacterium_fissicatena_group increased (Fig. 5H). Gavage of LP9010 reduced the relative level of Akkermansia and increased Bacteroides, Lactobacillus, Lachnospiraceae_NK4A136_group, Alistipes, Parabacteroides and Eubacterium_fissicatena_group. Lactobacillus, a typical probiotic, had been found to improve the preference for sugar water and increase the levels of 5-HT in the hippocampus and prefrontal lobes of mice.28 Specifically, the diversity of intestinal microflora was evaluated at the phylum and genus levels, and significant differences were found between the DSS group and the LP9010 groups (DSS + LDLP and DSS + HDLP).
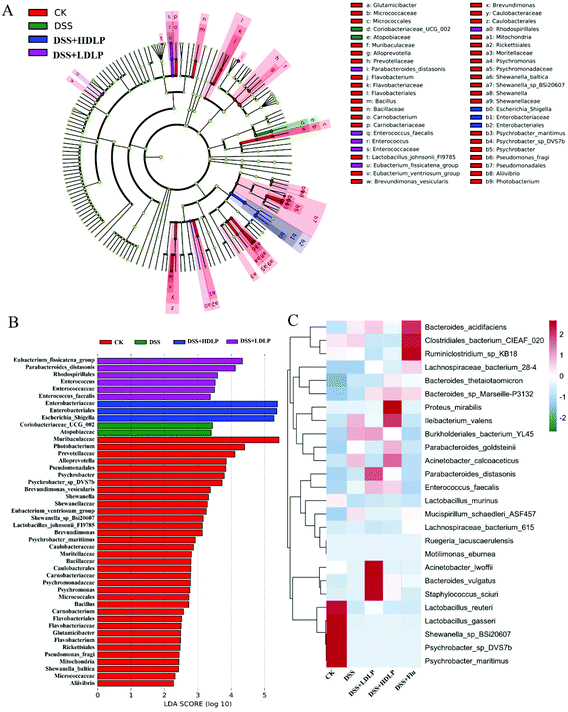
LefSe (Fig. 6A) and Linear Discriminant Analysis (LDA) (Fig. 6B) were conducted to find biological markers for further study of the indicator bacteria in each group. It could be known that LP9010 could affect the enrichment of some bacteria by gavage. Further heat map analysis (Fig. 6C) showed the representative species and clustering differences. Compared with the CK group, the relative levels of Lactobacillus_reuteri, Lactobacillus_gasseri, Shewanella_sp_BSi20607, Psychrobacter_sp_DVS7b, and Psychrobacter_maritimus in the DSS group decreased. Intragastric administration of LP9010 could promote the relative levels of Bacteroides_sp_marseille-P3132 and Enterococcus_faecalis. Notably, intragastric administration of LP9010 could promote the production of GABA and improve depressive symptoms by increasing the relative level of Parabacteroides, and this effect did not depend on the dose of LP9010.
3.6 Effects of LP9010 on inflammatory factors in mice with DSS-induced colitis
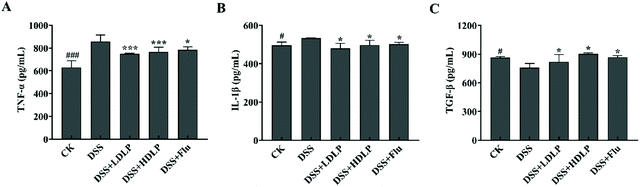
Inflammatory factors can be used as biomarkers for the diagnosis of depression. Generally, the central nervous system of depressed patients receives signals from activated inflammatory factors, which interfere with the growth and differentiation of neurons and affect depression.33 The serum cytokine levels of mice were determined, as shown in Fig. 7. DSS significantly increased the levels of pro-inflammatory cytokines TNF-α and IL-1β, especially TNF-α (p < 0.001), which had a role in generating inflammation. Studies have shown that increases in inflammatory biomarkers such as IL-6, TNF-α, and C-reactive protein were associated with depression.28 Meanwhile, the level of anti-inflammatory cytokine TGF-β in the serum of mice in the DSS group was significantly lower than that in the CK group (p < 0.05). TGF-β plays a role in the inhibition of inflammation in the gut. Both LP9010 and Flu could decrease the level of pro-inflammatory cytokines and increase the level of anti-inflammatory cytokines (Fig. 7). Low concentrations of LP9010 effectively reduced the levels of two pro-inflammatory cytokines (TNF-α and IL-1β) (p < 0.001), but had minor stimulative effect on anti-inflammatory cytokines (TGF-β) (p < 0.05), and high doses of LP9010 had similar trends with low doses of LP9010 (Fig. 7). Flu significantly decreased the level of TNF-α and increased the level of TGF-β.3.7 Effects of LP9010 on SCFAs in mice with DSS-induced colitis
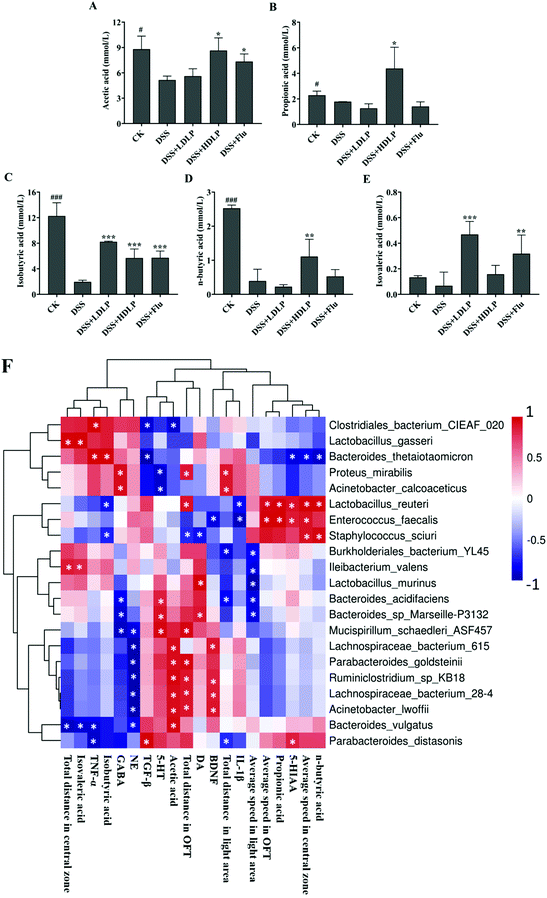
Substantial evidence shows that SCFAs can cross the blood–brain barrier and directly act as a signal factor of the endocrine system.4,16 The results of SCFAs determination in mouse feces are shown in Fig. 8A–E. DSS reduced the levels of various SCFAs in the feces of mice, indicating that the intestinal environment in mice was damaged by DSS. Intragastric administration of LP9010 and Flu could increase the contents of SCFAs (Fig. 8A–E). Among them, no significant difference was observed between different concentrations of LP9010 in improving the effects of various SCFAs (p > 0.05). It was worth noting that a high concentration of LP9010 showed significant effects on the contents of butyric acid and propionic acid, which were importantly related to the regulation of depression.283.8 Pearson's correlation
Pearson's correlation heatmaps of the relationships between the microbiota and the effects of LP9010 (OFT, LDT, neurotransmitter, inflammatory factors and SCFAs) are shown in Fig. 8F. The average speed in OFT is positively correlated with the abundance of Lactobacillus_reuteri and Enterococcus_faecalis. The average speed in the central zone is positively correlated with the abundance of Lactobacillus_reuteri, Enterococcus_faecalis and Staphylococcus_sciuri, and negatively correlated with the abundance of Bacteroides_thetaiotaomicron. Additionally, the total distance in the light area is positively correlated with the abundance of Proteus_mirabilis and Acinetobacter_calcoaceticus, and negatively correlated with the abundance of Burkholderiales_bacterium_YL45, Bacteroides_acidifaciens and Parabacteroides_distasonis. 5-HT is positively correlated with the abundance of Bacteroides_acidifaciens, Bacteroides_sp_Marseille-P3132 and Mucispirillum_schaedleri_ASF457, and negatively correlated with the abundance of Proteus_mirabilis and Acinetobacter_calcoaceticus. IL-1β is negatively correlated with the abundance of Lactobacillus_reuteri and Enterococcus_faecalis. Acetic acid is positively correlated with the abundance of Lachnospiraceae_bacterium_615, Parabacteroides_goldsteinii, Ruminiclostridium_sp_KB18, Lachnospiraceae_bacterium_28-4, Acinetobacter_lwoffii and Bacteroides_vulgatus.4. Discussion
Depression has attracted great attention all over the world1 due to its high morbidity and mortality. Inflammatory colitis accompanied by depression6 could be treated with SCFAs-producing probiotics.10 Therefore, LP9010 with efficient nitrite-degrading capacity, cholesterol-reducing ability, good acid and bile salt tolerance18–21 was proposed to treat with DSS-induced colitis and behavioral disorders in mice. In this study, acute colitis associated with depression was successfully modeled in mice by freely drinking 4% DSS aqueous solution. H&E section staining, LC-MS and behavioral experiments were used to detect the inflammatory infiltration in the colonic lamina propria of DSS model mice. The structure of the muscular and mucosal layers was abnormal with the absence of the close connection between cells, the formation of multiple ulcers, and the substantial reduction of goblet cells.MGB has been shown to be bidirectional, involving in complex behavioral, emotional, pain regulation and the development of the nervous system.28 Furthermore, intestinal flora can produce neuroactive substances such as GABA, 5-HT, BDNF, butyrate, and immunoreactive substances, such as anti-inflammatory cytokines (TGF-β).30,31 Through blood transport, these active substances could cross the blood–brain barrier to affect the central nervous system and modulate depression-like behavior.34 SCFAs produced by intestinal flora metabolism could play a role in promoting the colonization of beneficial bacteria and repairing intestinal barrier damage.1,13 Additionally, they were neuroactive and directly involved in the regulation of the central nervous system.28 In this study, it was found that intragastric administration of LP9010 could increase the relative abundance of Bacteroidetes and Firmicutes, and decreased the relative abundance of Proteobacteria and Verrucomicrobia. At the level of genera, LP9010 could improve intestinal balance by decreasing the relative level of bacteria such as Akkermansia and increasing the relative level of beneficial bacteria such as Lactobacillus. Akkermansia muciniphila is an anticancer strain that degrades mucin in mucus.35 Plovier et al. found that a high-temperature resistant bacterial protein on the bacterial membrane of Akkermansia muciniphila could interact with Toll-like receptor 2 to inhibit the occurrence of obesity and promote intestinal barrier function.35 So far, a lot of evidence has shown that Akkermansia muciniphila is safe for humans,35 but numerous studies have found that Akkermansia muciniphila has different effects on acute and chronic colitis.2,28Akkermansia muciniphila had the function of alleviating bacterial dysregulation and intestinal inflammation for chronic colitis, but had the opposite effect for acute colitis, which led to a significantly shorter colonic length of mice and higher DAI score than the DSS group.2 Interestingly, LP9010 could promote the production of GABA and improve depressive symptoms by increasing the relative level of Parabacteroides, and the effects did not depend on the dose of LP9010 (Fig. 6C).
In addition, acute stress, such as DSS-induced colitis, would aggravate the intestinal barrier system disruption,26 resulting in bacterial metabolites, antigens and toxins and other harmful substances to interfere with the central and vagus nervous systems. From the perspective of inflammatory cytokines, LP9010 and Flu had similar therapeutic effects, which decreased the levels of pro-inflammatory cytokines (TNF-α and IL-1β), and increased the levels of anti-inflammatory cytokines (TGF-β). SCFAs are metabolites of intestinal flora, and butyric acid and propionic acid are important to regulate depression. Intragastric administration of LP9010 and Flu could increase the contents of SCFAs (Fig. 8). In contrast, in the DSS group, OFT and LDT experiments (Fig. 3) indicated the decrease of autonomous movement tendency and exploration desire of mice, and the significant reduction of the levels of neurotransmitters, especially NE, GABA, and DA (Fig. 4). Studies have shown that microorganisms were important sources of some neurotransmitters,28,34 and supplementation with GABA-producing Levilactobacillus brevis DPC6108 had the potential to ameliorate the metabolic and depression-like behavioral abnormalities associated with minor metabolic syndrome.34 However, as we mentioned above, more experiments are needed in the future to evaluate the causal relationship between SCFAs formation, remodeling of the intestinal microbiome, and neuroinflammatory responses during LP9010 treatment, which can be modulated in sterile mice.
5. Conclusions
In conclusion, intake of LP9010 lightened colitis, anxiety-like behavior, and social behavior disorders associated with DSS feeding. This was partly because it promoted anti-inflammatory cytokines, reduced proinflammatory cytokines, enhanced SCFAs production, and reorganized the gut microbiome. The possible link among gut microbiome, SCFAs and neuroinflammation can be the key to the MGB axis, although some underlying mechanisms remain elusive at this stage. This study provides a potential strategy for probiotics intervention to prevent colitis, and reveals a new role of probiotics in the treatment of neuropsychiatric disorders.Ethical statement
All animal experiments should be conducted in accordance with the procedures and permits approved by the Animal Ethics Review Committee of South China Agricultural University (SYXK 2019-0136), and in accordance with the Regulations of the People's Republic of China on the Management of Laboratory Animals.Conflicts of interest
All authors have no conflict of interest.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31771908), the Guangzhou City Science and Technology Program key project (201903010015), Natural Science Foundation of the Guangdong Province (2021A1515012451), and China Postdoctoral Science Foundation (2020M682717).References
- W. Qu, S. Liu, W. Zhang, H. Zhu, Q. Tao, H. Wang and H. Yan, Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: intestinal microbiota and gut microbiome function, Food Funct., 2019, 10, 5886–5897 RSC.
- J. H. Kuang, Y. Y. Huang, J. S. Hu, J. J. Yu, Q. Y. Zhou and D. M. Liu, Exopolysaccharides from Bacillus amyloliquefaciens DMBA-K4 ameliorate dextran sodium sulfate-induced colitis via gut microbiota modulation, J. Funct. Foods, 2020, 75, 104212 CrossRef CAS.
- J. Jalanka, J. Cheng, K. Hiippala, J. Ritari, J. Salojarvi, T. Ruuska, M. Kalliomaki and R. Satokari, Colonic Mucosal Microbiota and Association of Bacterial Taxa with the Expression of Host Antimicrobial Peptides in Pediatric Ulcerative Colitis, Int. J. Mol. Sci., 2020, 21, 6044 CrossRef CAS PubMed.
- B. Hu, S. Yu, C. Shi, J. Gu, Y. Shao, Q. Chen, Y. Li and R. Mezzenga, Amyloid-Polyphenol Hybrid Nanofilaments Mitigate Colitis and Regulate Gut Microbial Dysbiosis, ACS Nano, 2020, 14, 2760–2776 CrossRef CAS PubMed.
- S. M. Matt, J. M. Allen, M. A. Lawson, L. J. Mailing, J. A. Woods and R. W. Johnson, Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice, Front. Immunol., 2018, 9, 1832 CrossRef PubMed.
- M. C. Dubinsky, I. Dotan, D. T. Rubin, M. Bernauer, D. Patel, R. Cheung, I. Modesto, M. Latymer and L. Keefer, Burden of comorbid anxiety and depression in patients with inflammatory bowel disease: a systematic literature review, Expert Rev. Gastroenterol. Hepatol., 2021, 1–13, DOI:10.1080/17474124.2021.1911644.
- Q. Li, L. Li, X. Niu, C. Tang, H. Wang, J. Gao and J. Hu, Probiotics alleviate depressive behavior in chronic unpredictable mild stress rat models by remodeling intestinal flora, NeuroReport, 2021, 32, 686–693 CrossRef CAS PubMed.
- O. Gawlik-Kotelnicka and D. Strzelecki, Probiotics as a Treatment for “Metabolic Depression”? A Rationale for Future Studies, Pharmaceuticals, 2021, 14, 384 CrossRef CAS PubMed.
- L. D. Kalischuk and A. G. Buret, A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease?, Am. J. Physiol.: Gastrointest. Liver Physiol., 2010, 298, G1–G9 CrossRef CAS PubMed.
- Y. Cheng, J. Liu and Z. Ling, Short-chain fatty acids-producing probiotics: A novel source of psychobiotics, Crit. Rev. Food Sci. Nutr., 2021, 1–31 Search PubMed.
- R. Xie, P. Jiang, L. Lin, J. Jiang, B. Yu, J. Rao, H. Liu, W. Wei and Y. Qiao, Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress, J. Psychiatr. Res., 2020, 122, 70–78 CrossRef PubMed.
- W. H. Liu, H. L. Chuang, Y. T. Huang, C. C. Wu, G. T. Chou, S. Wang and Y. C. Tsai, Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice, Behav. Brain Res., 2016, 298, 202–209 CrossRef CAS PubMed.
- Y. Sun, W. Geng, Y. Pan, J. Wang, P. Xiao and Y. Wang, Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan Kefir improves depression- like behavior in stressed mice by modulating the gut microbiota, Food Funct., 2019, 10, 925–937 RSC.
- J. F. Cryan, K. J. O'Riordan, C. S. M. Cowan, K. V. Sandhu, T. F. S. Bastiaanssen, M. Boehme, M. G. Codagnone, S. Cussotto, C. Fulling, A. V. Golubeva, K. E. Guzzetta, M. Jaggar, C. M. Long-Smith, J. M. Lyte, J. A. Martin, A. Molinero-Perez, G. Moloney, E. Morelli, E. Morillas, R. O'Connor, J. S. Cruz-Pereira, V. L. Peterson, K. Rea, N. L. Ritz, E. Sherwin, S. Spichak, E. M. Teichman, M. van de Wouw, A. P. Ventura-Silva, S. E. Wallace-Fitzsimons, N. Hyland, G. Clarke and T. G. Dinan, The microbiota-gut-brain axis, Physiol. Rev., 2019, 99, 1877–2013 CrossRef CAS PubMed.
- C. Long-Smith, K. J. O'Riordan, G. Clarke, C. Stanton, T. G. Dinan and J. F. Cryan, in Annual Review of Pharmacology and Toxicology, Vol. 60, ed. P. A. Insel, 2020, vol. 60, pp. 477–502 Search PubMed.
- B. Zhao, J. Wu, J. Li, Y. Bai, Y. Luo, B. Ji, B. Xia, Z. Liu, X. Tan, J. Lv and X. Liu, Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance, J. Agric. Food Chem., 2020, 68, 3963–3975 CrossRef CAS PubMed.
- P. De Rossi, E. Harde, J. P. Dupuis, L. Martin, N. Chounlamountri, M. Bardin, C. Watrin, C. Benetollo, K. Pernet-Gallay, H. J. Luhmann, J. Honnorat, G. Malleret, L. Groc, A. Acker-Palmer, P. A. Salin and C. Meissirel, A critical role for VEGF and VEGFR2 in NMDA receptor synaptic function and fear-related behavior, Mol. Psychiatry, 2016, 21, 1768–1780 CrossRef CAS PubMed.
- D. M. Liu, J. Guo, X. A. Zeng, D. W. Sun, C. S. Brennan, Q. X. Zhou and J. S. Zhou, The probiotic role of Lactobacillus plantarum in reducing risks associated with cardiovascular disease, Int. J. Food Sci. Technol., 2017, 52, 127–136 CrossRef CAS.
- Y. T. Fei, D. M. Liu, T. H. Luo, G. Chen, H. Wu, L. Li and Y. G. Yu, Molecular characterization of Lactobacillus plantarum, DMDL 9010, a strain with efficient nitrite degradation capacity, PLoS One, 2014, 9, e113792 CrossRef PubMed.
- K. Yao, D. M. Liu, M. H. Liang, C. S. Brennan and M. Brennan, Detection of nitrite degradation by Lactobacillus plantarum DMDL9010 through the anaerobic respiration electron transport chain using proteomic analysis, Int. J. Food Sci. Technol., 2021, 56, 1608–1622 CrossRef CAS.
- Y. Y. Huang, D. M. Liu, X. Z. Jia, M. H. Liang, Y. Z. Lu and J. S. Liu, Whole genome sequencing of Lactobacillus plantarum DMDL 9010 and its effect on growth phenotype under nitrite stress, LWT – Food Sci. Technol., 2021, 149, 111778 CrossRef CAS.
- Y. Y. Huang, J. J. Yu, Q. Y. Zhou, L. N. Sun, D. M. Liu and M. H. Liang, Preparation of yogurt-flavored bases by mixed lactic acid bacteria with the addition of lipase, LWT – Food Sci. Technol., 2020, 131, 109577 CrossRef CAS.
- Y. Y. Huang, M. H. Liang, L. N. Sun, C. S. Brennan and D. M. Liu, Effect of microencapsulation on morphology, physicochemical properties, and flavor profiles of solid yoghurt-flavored bases, Int. J. Food Sci. Technol., 2021, 56, 2565–2578 CrossRef CAS.
- J. S. Hu, Y. Y. Huang, J. H. Kuang, J. J. Yu, Q. Y. Zhou and D. M. Liu, Streptococcus thermophiles DMST-H2 Promotes Recovery in Mice with Antibiotic-Associated Diarrhea, Microorganisms, 2020, 8, 1650 CrossRef CAS PubMed.
- Z. Liu, L. Li, S. Ma, J. Ye, H. Zhang, Y. Li, A. T. Sair, J. Pan, X. Liu, X. Li, S. Yan and X. Liu, High-Dietary Fiber Intake Alleviates Antenatal Obesity-Induced Postpartum Depression: Roles of Gut Microbiota and Microbial Metabolite Short-chain Fatty Acid Involved, J. Agric. Food Chem., 2020, 68, 13697–13710 CrossRef CAS PubMed.
- S. Ding, W. Yan, J. Fang, H. Jiang and G. Liu, Potential role of Lactobacillus plantarum in colitis induced by dextran sulfate sodium through altering gut microbiota and host metabolism in murine model, Sci. China: Life Sci., 2021, 64, 1906–1916 CrossRef PubMed.
- R. M. Stilling, M. van de Wouw, G. Clarke, C. Stanton, T. G. Dinan and J. F. Cryan, The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis?, Neurochem. Int., 2016, 99, 110–132 CrossRef CAS PubMed.
- S. J. Yong, T. Tong, J. Chew and W. L. Lim, Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential, Front. Neurosci., 2020, 13, 1361 CrossRef PubMed.
- D. M. Gerhard, E. S. Wohleb and R. S. Duman, Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity, Drug Discovery Today, 2016, 21, 454–464 CrossRef CAS PubMed.
- K. Martinowich and B. Lu, Interaction between BDNF and serotonin: Role in mood disorders, Neuropsychopharmacology, 2008, 33, 73–83 CrossRef CAS PubMed.
- C. Bjorkholm and L. M. Monteggia, BDNF - a key transducer of antidepressant effects, Neuropharmacology, 2016, 102, 72–79 CrossRef PubMed.
- M. Guo and Z. Li, Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota, Food Funct., 2019, 10, 6873–6881 RSC.
- A. I. Petra, S. Panagiotidou, E. Hatziagelaki, J. M. Stewart, P. Conti and T. C. Theoharides, Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation, Clin. Ther., 2015, 37, 984–995 CrossRef CAS PubMed.
- E. Patterson, P. M. Ryan, N. Wiley, I. Carafa, E. Sherwin, G. Moloney, E. Franciosi, R. Mandal, D. S. Wishart, K. Tuohy, R. P. Ross, J. F. Cryan, T. G. Dinan and C. Stanton, Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome, Sci. Rep., 2019, 9, 16323 CrossRef CAS PubMed.
- H. Plovier, A. Everard, C. Druart, C. Depommier, M. Van Hul, L. Geurts, J. Chilloux, N. Ottman, T. Duparc, L. Lichtenstein, A. Myridakis, N. M. Delzenne, J. Klievink, A. Bhattacharjee, K. C. H. van der Ark, S. Aalvink, L. O. Martinez, M.-E. Dumas, D. Maiter, A. Loumaye, M. P. Hermans, J.-P. Thissen, C. Belzer, W. M. de Vos and P. D. Cani, A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice, Nat. Med., 2017, 23, 107–113 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |