Competing pathways for MgO, CaO, SrO, and BaO nanocluster growth
Fathi
Bawa
a and
Itai
Panas
b
aDepartment of Chemistry, Inorganic Chemistry, Göteborg University, S-412 96 Göteborg, Sweden
bDepartment of Environmental Inorganic Chemistry, Chalmers University of Technology, S-412 96 Göteborg, Sweden
First published on 18th December 2001
Abstract
A comparative structure and stability study is performed on a sequence of (MgO)n, (CaO)n, (SrO)n and (BaO)n clusters, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. The B3LYP functional is employed to seek preferred cluster growth pathways. Similar stabilities are found for two growth strategies, one producing rock-salt-like structures, and a second which employs stacks of hexagonal (MO)3 rings. Structures based on hexagonal rings are slightly preferred in the case of MgO and BaO, while for CaO and SrO the rock salt structural analogue prevails. The shift in isomer stability order is discussed in terms of packing of spheres. The small magnitude of the atomic size difference for oxygen and the corresponding metal atoms in the cluster implies preference for the rock salt structure. Larger |ΔRA|
result in a preference for hexagonal ring-based structures.
12. The B3LYP functional is employed to seek preferred cluster growth pathways. Similar stabilities are found for two growth strategies, one producing rock-salt-like structures, and a second which employs stacks of hexagonal (MO)3 rings. Structures based on hexagonal rings are slightly preferred in the case of MgO and BaO, while for CaO and SrO the rock salt structural analogue prevails. The shift in isomer stability order is discussed in terms of packing of spheres. The small magnitude of the atomic size difference for oxygen and the corresponding metal atoms in the cluster implies preference for the rock salt structure. Larger |ΔRA|
result in a preference for hexagonal ring-based structures.
1. Introduction
Characteristics of cluster growth are of major importance in the development of nano-technology and nano-catalysis. Magic numbers in the stability of metal clusters per se, and their reactivity dependence on shell closing, has long been the topic of a large number of studies.1–4 The sensitivity of any metal-cluster-based technology to oxidation by air is a serious drawback for these substances. This is why there is an increased interest in exploring the properties of oxide clusters, as these are trivially expected to display enhanced stability under oxidizing conditions. Now, the properties of this larger class of materials, are under investigation for e.g. future quantum dot and nanoscale catalyst applications.Various properties of complex oxides are presently exploited in combination with noble metals, such as platinum in car catalysts for e.g. NOx reduction and slip hydrocarbon oxidation. Lean-burn catalysis is one such particular case. In order to obtain low fuel consumption the engine is made to run under low fuel and oxygen-rich conditions. While this produces optimal combustion conditions, large amounts of NO(g) are emitted. These are oxidized catalytically on a Pt catalyst and collected on BaO(s)5,6 by solid nitrite/nitrate formation, the BaO(s) being precipitated on the porous alumina support of the catalyst. During subsequent relatively few fuel-rich cycles, NO2(g) is released from the oxide solid and reduced to N2(g). One key step in the lean-burn catalysis concept comprises the efficiency of the NOx storage material. At present BaO(s) is preferred due to the large surface to bulk ratio of its calcined precipitate. Possible future technology may employ nanometer-size oxide clusters, where this necessary prerequisite is automatically fulfilled. The purpose of this ongoing study is to determine how the shapes of nanometer-size clusters affect the reversible NOx storage and whether the exclusive position of BaO holds also at this length scale.
Comprehensive understanding of oxide cluster structures and stabilities is sought by means of electronic structure calculations. In a previous study, issues regarding convergence in the binding energy of (MgO)n and (CaO)n were addressed.7 It was demonstrated that the binding energy per formula unit for alkaline earth oxide clusters in the nanometer regime, displays an apparent convergence to that of an infinite (MO)n sheet, for which the discrepancy to the bulk value is some 20%. Here, in contrast, relative isomer stabilities for the (MgO)n, (CaO)n, (SrO)n, and (BaO)n, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 are addressed.
12 are addressed.
It is well known that (MgO)3 hexagonal ring structures are competitive building blocks in the growth of very small MgO clusters.8 Previous calculations have also suggested that the quasi-cubic rock salt slab shape is preferred for CaO nano-clusters.8–10 Here, the objective is to learn what are the magnitudes of the energies that discriminate isomers, whether magic numbers are expected in the stabilities of stoichiometric alkaline earth oxide clusters, and how the stability ordering changes among cluster growth pathways for the clusters of the four above-mentioned alkaline earth oxides.
We employ the B3LYP hybrid functional, which, in contrast to the LDA8,9 and AIPI10 studies, was previously shown to produce semi-quantitative binding energies for these systems7 for the (MgO)n and (CaO)n systems. In the present study, systematic features are extracted by addressing larger cluster sizes than were previously treated by means of LDA, and extending the class of systems addressed, to include also the (SrO)n and (BaO)n clusters.
2. Computational details
The B3LYP three-parameter hybrid exchange functional11 in conjunction with a combination of the Lee–Yang–Parr12 and Vosko–Wilks–Nusair13 correlation functionals, optimised on a training set, as implemented in the GAUSSIAN98 program package14 is employed throughout this study. Applicability is supported by comparison of predictions to those of ab initio 4th order Møller–Plesset perturbation theory MP4. Analysis of basis set effects on structures and stabilities were performed for (MgO)n and (CaO)n by optimising the cluster structures employing the 6-311G basis set, followed by a single-point calculation with the 6-311G(d) set, and finally the structures were optimised with the latter set. In the case of (SrO)n and (BaO)n, the Dunning full double-Z basis set was employed on oxygen,15 while the Stuttgart/Dresden effective core potentials (SDD ECP)16 were employed to replace the chemically inert core electrons by model potentials in order to reduce the computational effort, and efficiently describe the relativistic core contraction effects. It was previously shown that d-polarisation functions are important for getting the total binding energy right in the case of (CaO)n. This improved description turned out to have a much lesser effect on the relative stabilities of optimised isomer structures. Indeed, validation of the double-Z quality SDD ECP, was performed on (CaO)9 and (CaO)12 isomers (see below). Good agreements with the all electron predictions were obtained.All (MgO)n isomers (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) were checked and found to be true minima on the corresponding potential energy surface, by computing the vibrational frequencies. In the case of the (MgO)12 systems, only the spherical cluster was explicitly checked and found to be stable. The findings for (MgO)n were taken to suggest that the corresponding optimised structures of the Ca, Sr, and Ba cluster oxides also correspond to local energy minima.
9) were checked and found to be true minima on the corresponding potential energy surface, by computing the vibrational frequencies. In the case of the (MgO)12 systems, only the spherical cluster was explicitly checked and found to be stable. The findings for (MgO)n were taken to suggest that the corresponding optimised structures of the Ca, Sr, and Ba cluster oxides also correspond to local energy minima.
3. Results
3.(a) (MgO)4 and (CaO)4 isomers
This paragraph focuses on issues regarding validation, stability ordering and comparisons to previous studies. The applicability of the B3LYP hybrid functional was previously confirmed6 by comparing the binding energy obtained for (MgO)4 with that of MP4 perturbation theory. The hybrid DFT prediction agrees well with MP4 both with respect to absolute stabilities and stability ordering among the isomers considered (see Table 1, where a basis set investigation is also included).| (MO)n | Structure | B3LYPa6-311G(d) Opt | LDAb | MP4c6-311+G(2df) | LDAd6-31G** |
|---|---|---|---|---|---|
| a Present study. b Ref. 8. c Ref. 7. d Ref. 10. | |||||
| (MgO)4 | Slab | −27.04 | −32.12 | −27.70 | −33.60 |
| Octa-ring | −26.12 | −29.90 | −26.78 | −32.24 | |
| Ladder | −25.63 | −29.95 | −26.41 | — | |
| (CaO)4 | Slab | −32.37 | −40.20 | — | — |
| Ladder | −29.56 | −37.01 | — | — | |
| Octa-ring | −28.79 | −35.35 | — | — | |
The (MgO)4 and (CaO)4 isomers addressed here (see Fig. 1) have been studied previously by means of DFT, employing the local density approximation (LDA).8,10 It is gratifying to note that the stability orderings among isomers are the same for LDA as for B3LYP (cf.Table 1). This is in spite of the fact that the predicted binding energies deviate significantly. The latter is due to the well known tendency of LDA to overbind, as demonstrated here by comparing with the MP4 predictions in the case of (MgO)4. The excellent agreement between MP4 and B3LYP is stressed repeatedly. Consequences of faulty (MgO)n and (CaO)n cluster stabilities include erroneous conclusions regarding apparent convergence of the cluster binding energies to the bulk limit. This was discussed in ref. 7.
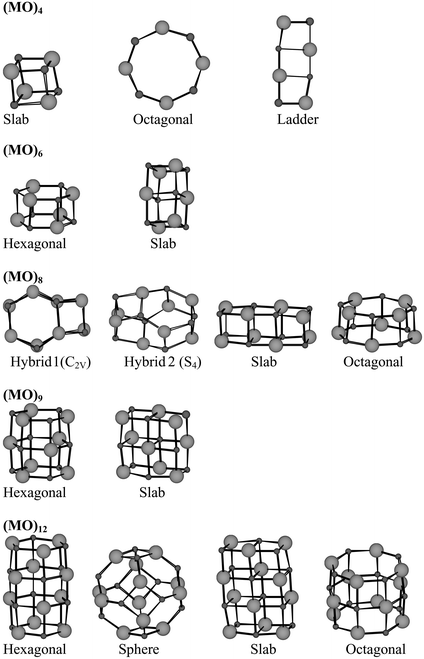 | ||
Fig. 1 Schematic description of the (MO)n isomers, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 6, 8, 9, and 12 clusters considered in the present study. 4, 6, 8, 9, and 12 clusters considered in the present study. | ||
The sensitivity of the stabilities and ordering to the choice of basis set among the n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 isomers, is displayed in Fig. 2a. The cube-like slab-shaped structure is found to be the most stable one in all cases and for both compounds. However, disagreement as to the second most stable structure is observed, i.e. it is the octagonal ring in the case of (MgO)4 and the ladder structure in the case of (CaO)4.
4 isomers, is displayed in Fig. 2a. The cube-like slab-shaped structure is found to be the most stable one in all cases and for both compounds. However, disagreement as to the second most stable structure is observed, i.e. it is the octagonal ring in the case of (MgO)4 and the ladder structure in the case of (CaO)4.
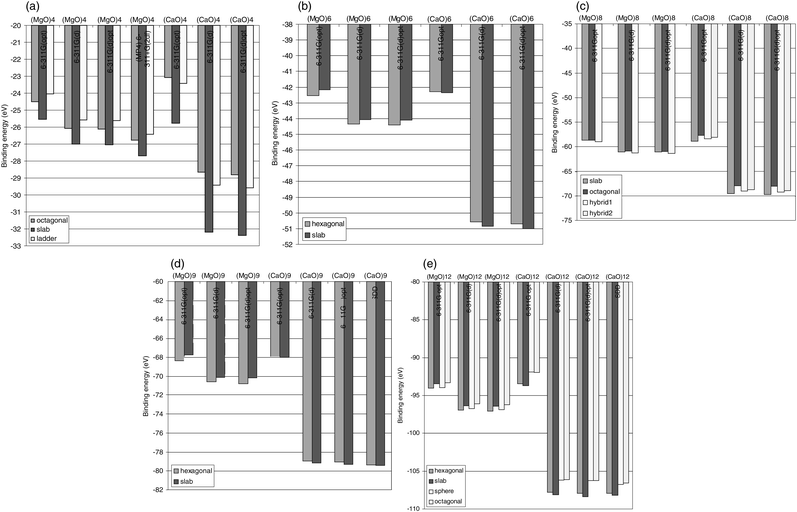 | ||
| Fig. 2 (a) Stability ordering dependence on basis set for (MgO)4 and (CaO)4 clusters. The B3LYP electron correlation description in case of (MgO)4 is validated by comparing to MP4 results. Stability ordering dependence on basis set, (b) for (MgO)6 and (CaO)6, (c) for (MgO)8 and (CaO)8 (d) (MgO)9 and (CaO)9, (e) and for (MgO)12 and (CaO)12 clusters. Energies are in eV. | ||
3.(b) Comparison of (MgO)n and (CaO)n isomer stabilities, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) n
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 12
12
The quest for understanding of the growth of small oxide clusters must start with a comparative study among isomers in order to extract information regarding their relative importance. In the case of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, three different isomers were studied, two different basic structures were investigated in the case of n
4, three different isomers were studied, two different basic structures were investigated in the case of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and n
6 and n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, and four were considered in the cases of n
9, and four were considered in the cases of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and n
8 and n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. The particular isomer structures, referenced in Fig. 2(a)–(e), are depicted in Fig. 1. The stabilities are presented in Tables 2 and 3.
12. The particular isomer structures, referenced in Fig. 2(a)–(e), are depicted in Fig. 1. The stabilities are presented in Tables 2 and 3.
| Binding energy | ||||
|---|---|---|---|---|
| (MgO)n | Structure | 6-311G Opt | 6-311G(d) | 6-311G(d) Opt |
| (MgO)4 | Slab | −25.52 | −27.01 | −27.04 |
| Octa-ring | −24.51 | −26.09 | −26.12 | |
| Ladder | −24.05 | −25.59 | −25.63 | |
| (MgO)6 | 2-Hexa-ring stack | −42.55 | −44.35 | −44.40 |
| Slab | −42.18 | −44.06 | −44.11 | |
| (MgO)8 | Hybrid | −58.99 | −61.22 | −61.34 |
| Slab | −58.72 | −61.04 | −61.11 | |
| 2-Octa-ring stack | −58.66 | −60.88 | −60.99 | |
| (MgO)9 | 3-Hexa-ring stack | −68.36 | −70.61 | −70.80 |
| Slab | −67.76 | −70.14 | −70.18 | |
| (MgO)12 | 4-Hexa-ring stack | −94.06 | −96.93 | −97.05 |
| Slab | −93.45 | −96.34 | −96.42 | |
| Sphere | −93.98 | −96.76 | −96.90 | |
| 3-Octa-ring stack | −93.32 | −96.11 | −96.21 | |
| Binding energy | ||||
|---|---|---|---|---|
| (CaO)n | Structure | 6-311G Opt | 6-311G(d) | 6-311G(d) Opt |
| (CaO)4 | Slab | −25.76 | −32.21 | −32.37 |
| Ladder | −23.42 | −29.43 | −29.56 | |
| Octa-ring | −23.07 | −28.64 | −28.79 | |
| (CaO)6 | Slab | −42.34 | −50.84 | −51.01 |
| 2-Hexa-ring stack | −42.28 | −50.57 | −50.70 | |
| (CaO)8 | Slab | −58.89 | −69.48 | −69.73 |
| Hybrid(1) | −58.37 | −69.04 | −69.23 | |
| Hybrid(2) | −58.09 | −68.71 | −68.93 | |
| 2-Octa-ring stack | −57.66 | −67.89 | −68.08 | |
| (CaO)9 | Slab | −67.98 | −79.15 | −79.33 |
| 3-Hexa-ring stack | −67.97 | −78.96 | −79.06 | |
| (CaO)12 | Slab | −93.71 | −108.14 | −108.38 |
| 4-Hexa-ring stack | −93.43 | −107.77 | −107.91 | |
| 3-Octa-ring stack | −91.97 | −106.15 | −106.24 | |
| Sphere | −91.90 | −106.16 | −106.25 | |
In the case of (CaO)n there is a consistent preference for slab-shaped structures throughout. But the stability difference between the most stable and the second most stable structure is always less than 0.5 eV, which must be deemed small considering the binding energy to be of the order of 8–10 eV per CaO unit in the clusters. Thus, nano-particles composed of CaO are not likely to take exclusively micro-NaCl structures. Particularly interesting in this context are the (CaO)8 hybrid structures made from the (CaO)6 double-hexagonal-ring structure in conjunction with a (CaO)2 dimer attached to one side of the former (see Fig. 1 and Fig. 2(c)). Two such hybrids, displaying a stability difference of 0.3 eV, are determined, such that all vibrational frequencies come out real. Whereas a slab section and double-hexagonal-rings section can be resolved in hybrid1 (of C2V point group symmetry), the more stable hybrid2 displays S4 symmetry. The latter reflects the fact that the slab and ring subunits have lost their structural integrities.
Making the same isomer comparison for the (MgO)n systems, as was done for (CaO)n, the hexagonal ring-based structures come out more stable, in agreement with pervious studies.8,9 Again though, the stability difference is comparatively small, i.e. less than 0.6 eV, where in some cases the slab structure is not even the second most stable structure. Two peculiarities are emphasized. Firstly, only the hybrid2 structure exists for (MgO)8, i.e. the quasi-spherical structure where the hexagonal double-ring and (MgO)2 subunits cannot be resolved. Secondly, in the case of (MgO)12, a spherical cluster shape stands out as the second most stable structure. The latter can be understood based on the facts that the most stable structure is a stack of four (MgO)3 subunits, and that the sphere has the four hexagonal rings on the tetrahedral axes. In contrast to the present study, the AIPI method9 predicts this spherical cluster to be the more stable one.
3.(c) Beyond CaO—(SrO)n and (BaO)n
In subsection 3.(b) it was learned that hexagonal rings are slightly more stable than the slab-shaped structures in the case of (MgO)n, whereas the opposite is true in the case of (CaO)n. Consequently, in the present subsection, we ask whether this possible trend continues, i.e. towards increased relative stability of the slab structures relative to the hexagonal.The SDD ECP were employed in the descriptions of the Sr and Ba atoms. In order to validate the applicability of the SDD ECP, the structures and stabilities of the (CaO)9 and (CaO)12 isomers were recalculated with the SDD ECP description of the Ca core. The results are displayed in Fig. 2(d) and (e), respectively. Both absolute energies and stability ordering are qualitatively reproduced by the SDD ECP description, while the 6-311G(d) stability differences of 0.27 and 0.47 eV are reduced to 0.09 and 0.27 eV for the n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and n
9 and n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 clusters respectively (see Table 4).
12 clusters respectively (see Table 4).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 12 is tested by comparing to the all electron 6-311G(d) description. The binding energies of (SrO)n and (BaO)n, n
9, 12 is tested by comparing to the all electron 6-311G(d) description. The binding energies of (SrO)n and (BaO)n, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 9, 12 are included in the table
6, 9, 12 are included in the table
| Binding energy | |||
|---|---|---|---|
| (MO)n | Structure | SDD ECP | AE 6-311G(d) Opt |
| (CaO)9 | Slab | −79.44 | −79.33 |
| 3-Hexa-ring stack | −79.35 | −79.06 | |
| (CaO)12 | 3-Octa-ring stack | −106.57 | −106.24 |
| Slab | −108.19 | −108.38 | |
| 4-Hexa-ring stack | −107.92 | −107.91 | |
| Sphere | −106.75 | −106.25 | |
| (SrO)6 | 2-Hexa-ring stack | −47.74 | |
| Slab | −47.89 | ||
| (SrO)9 | 3-Hexa-ring stack | −73.96 | |
| Slab | −74.10 | ||
| (SrO)12 | 3-Octa-ring stack | −98.83 | |
| Slab | −100.67 | ||
| 4-Hexa-ring stack | −100.39 | ||
| Sphere | −99.22 | ||
| (BaO)6 | 2-Hexa-ring stack | −50.63 | |
| Slab | −50.28 | ||
| (BaO)9 | 2-Hexa-ring stack | −76.88 | |
| Slab | −76.77 | ||
| (BaO)12 | 3-Octa-ring stack | −102.28 | |
| Slab | −103.79 | ||
| 4-Hexa-ring stack | −103.82 | ||
| Sphere | −103.14 | ||
Given this, we employ the SDD ECP description for SrO and BaO clusters, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 9, 12, and compare the stabilities of the slab and ring based structures. The results are displayed in Fig. 3 and 4, and in Table 4, where the former repeats the results for (MgO)12 and (CaO)12 and adds the (SrO)12 and (BaO)12 results for the same isomers. Whereas the overall patterns of the latter two compounds are similar to those of the former, we note in the case of (BaO)12 the negligible difference in stability between the slab and hexagonal ring-based isomers. In order to investigate this further, the two-rings stack and three-rings stack were included in the investigation. In Fig. 4 it is seen that for (SrO)6 and (SrO)9, the two
structural types are very close in energy, while a slight preference for the slab structure is observed for (SrO)12. This is in contrast to the (BaO)6 and (BaO)9 stability orderings, where a slight preference for the ring-based structures is found, while (BaO)12 displays the same stability for the two isomers. Based on these observations it can be concluded that there is no trend towards preference for the slab shape with increasing atomic number on the metal, for the nano-sized alkaline earth oxide clusters.
6, 9, 12, and compare the stabilities of the slab and ring based structures. The results are displayed in Fig. 3 and 4, and in Table 4, where the former repeats the results for (MgO)12 and (CaO)12 and adds the (SrO)12 and (BaO)12 results for the same isomers. Whereas the overall patterns of the latter two compounds are similar to those of the former, we note in the case of (BaO)12 the negligible difference in stability between the slab and hexagonal ring-based isomers. In order to investigate this further, the two-rings stack and three-rings stack were included in the investigation. In Fig. 4 it is seen that for (SrO)6 and (SrO)9, the two
structural types are very close in energy, while a slight preference for the slab structure is observed for (SrO)12. This is in contrast to the (BaO)6 and (BaO)9 stability orderings, where a slight preference for the ring-based structures is found, while (BaO)12 displays the same stability for the two isomers. Based on these observations it can be concluded that there is no trend towards preference for the slab shape with increasing atomic number on the metal, for the nano-sized alkaline earth oxide clusters.
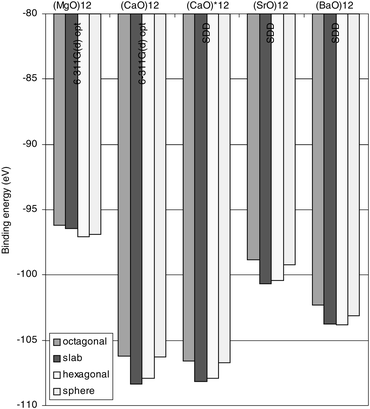 | ||
Fig. 3 Binding energies for (MO)12 isomers, M![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Mg, Ca, Sr, and Ba. (MgO)12 and (CaO)12 are computed with the 6-311G (d) set. Validation of the SDD ECP was done by recomputing the (CaO)12 cluster (cf.
(CaO)12*). The (SrO)12 and (BaO)12 are both described by the SDD ECP. Energies are in eV. Mg, Ca, Sr, and Ba. (MgO)12 and (CaO)12 are computed with the 6-311G (d) set. Validation of the SDD ECP was done by recomputing the (CaO)12 cluster (cf.
(CaO)12*). The (SrO)12 and (BaO)12 are both described by the SDD ECP. Energies are in eV. | ||
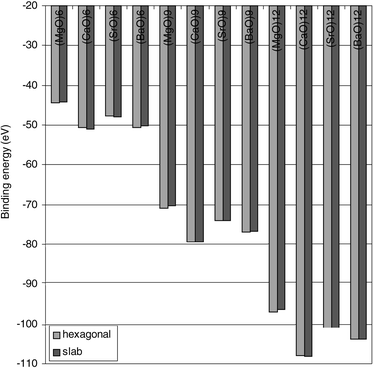 | ||
| Fig. 4 Comparison of the slab-shape and hexagonal-ring-stacks stabilities. Note the small differences in isomer stabilities and the switch in relative stabilities between the two structures when going e.g. from (MgO)6 to (CaO)6 and from (SrO)6 to (BaO)6. Energies are in eV. | ||
4. Conclusion
An outstanding result of the present study is the similar stabilities of the hexagonal-ring-based structures and the rock-salt-like slab-shaped isomers. While this observation is important as such and contradicts the exclusive nature of the latter structural shapes proposed previously,17–19 it is noteworthy how the stability ordering changes as the metal atomic number increases among the alkaline earth elements. In the case of (MgO)n, the hexagonal-ring-based structure is evidently the more stable one. Going to (CaO)n, the situation is reversed, in that the slab structure prevails. For (SrO)n, the slab structure is still the preferred one but to a lesser extent. Finally, addressing (BaO)n the trend towards relative stabilization of the ring based structures appears to restore the ordering found for (MgO)n.In some cases, small differences in cluster stabilities are observed, and possible sensitivity to zero point vibration effects comes to mind. The largest effects are expected for the (MgO)n clusters, which display the smallest reduced masses. For a selected set of clusters, such corrections on the relative stabilities among cluster pairs were investigated. Negligible effects on the relative stability were found in all cases, e.g. 2.7 meV ((MgO)6; hexagonal versus slab), 11.2 meV ((MgO)8; octagonal versus Hybrid I), and 5.7 meV ((MgO)9; hexagonal versus slab), when comparing to the numbers in Table 2, which were not corrected for this effect.
It is the topological equivalence of the two structural forms, that renders the hexagonal rings stack and the slab structures similar stabilities for small clusters. Deformation along one of the directions orthogonal to the rings stack transforms the former into the latter. Based on this, discrimination between the two structural forms cannot be conclusive, if based on experimental knowledge of abundance of masses alone. Indeed, the interconversion between the two becomes an important stabilizing deformation, which can be taken to explain the increased endurance of particular stoichiometric clusters.
It is difficult to propose an unambiguous qualitative wavefunction-based cause for the detailed stability ordering and reordering among cluster isomers. The differential stabilities are simply too small for any such understanding. Rather, the systematic empirical observations suggest that the simplest explanation of the gradual change is the increasing radii R of the alkaline earth elements. The metal radii for Mg, Ca, Sr, and Ba are 1.60, 1.97, 2.15, and 2.17 Å, respectively, whereas the radii for the corresponding M2+ cations are 0.65, 0.97, 1.15 and 1.35 Å.20 The atomic and ionic radii of O and lattice O2− are 0.66 and 1.40 Å, respectively. Having said this, the assignment of a radius to an atom in a molecule or a cluster is an ambiguous task. Still, any such defined size of a metal ion in an oxide cluster would be determined by the magnitude of the strongly anisotropic electrostatic field at this site, in conjunction with the electronegativity of the oxygen atoms in their electropositive surrounding.
In a previous study7 it was noted that the M–O bond distances vary strongly within any particular nano-cluster. These large variations are independent of isomer conformation and apply locally, so that each M displays a broad distribution of nearest-neighbour distances in the different directions, as exemplified in Fig. 5 for the slab-shaped (MO)9 cluster. Discriminating between the ring and slab-based isomer structures is precisely the ring, which becomes an (MO)3 rectangle in the slab isomers, i.e. the ring stacking direction produces bond distances which are very similar for the two aggregation pathways.
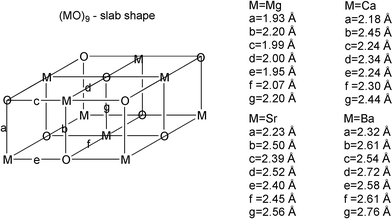 | ||
| Fig. 5 M–O bond distance variations in the slab-shaped (MO)9 cluster. Note particularly the difference between the a and b values. | ||
It is inferred from the above results that large absolute differences in anion–cation radii favour the hexagonal ring-based structures, while similar ionic radii make the slab structures the preferred choice. The cause for this is sought in simple packing arguments. Spheres of similar radii are best packed in the quasi-cubic rock-salt-type structures. In contrast, if the intermediate quasi-ionic radii found in the clusters are sufficiently dissimilar, then preference for structures based on hexagonal rings is found. The quasi-ionic nature of the metal oxygen bonds is reflected in the charges that come out approximately ±q, and 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) q
q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 from the Mulliken population analysis. The rectangular rings produce tension in the quasi-molecular M–O chemical bonds, while the octagonal-ring-structures become too open, thus penalizing the ionic interactions, making the hexagonal (MO)3 substructure a competitive
option.
1.1 from the Mulliken population analysis. The rectangular rings produce tension in the quasi-molecular M–O chemical bonds, while the octagonal-ring-structures become too open, thus penalizing the ionic interactions, making the hexagonal (MO)3 substructure a competitive
option.
To summarize, the stability orderings among a number of alkaline earth oxide cluster isomers (MO)n, M![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Mg Ca, Sr, Ba, and 4
Mg Ca, Sr, Ba, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 have been determined by means of the B3LYP hybrid density functional. Among the candidate structures, the hexagonal ring based isomers and the slab shapes were found to display similar stabilities. Stacks of hexagonal (MO)3 rings were found to be the slightly preferred growth strategy among the (MO)6, (MO)9, and (MO)12 isomers, for the MgO and BaO clusters. In contrast, the slab structures were slightly preferred for the CaO and SrO clusters. An explanation based on packing arguments was proposed, i.e. ion pairs with large absolute anion–cation radii differences |ΔRi| produce regular hexagonal-ring-based
structures, while small |ΔRi| prefer slab structures. These conceptual results may find use in modelling studies of initial oxide growth on alkaline earth metals, where the early oxide morphology displays a complex nature, which may indeed find its origin in the existence of two nearly equivalent initial growth strategies at low temperatures, whereas rapid interconversion is expected at elevated temperatures due to their similar stabilities.
12 have been determined by means of the B3LYP hybrid density functional. Among the candidate structures, the hexagonal ring based isomers and the slab shapes were found to display similar stabilities. Stacks of hexagonal (MO)3 rings were found to be the slightly preferred growth strategy among the (MO)6, (MO)9, and (MO)12 isomers, for the MgO and BaO clusters. In contrast, the slab structures were slightly preferred for the CaO and SrO clusters. An explanation based on packing arguments was proposed, i.e. ion pairs with large absolute anion–cation radii differences |ΔRi| produce regular hexagonal-ring-based
structures, while small |ΔRi| prefer slab structures. These conceptual results may find use in modelling studies of initial oxide growth on alkaline earth metals, where the early oxide morphology displays a complex nature, which may indeed find its origin in the existence of two nearly equivalent initial growth strategies at low temperatures, whereas rapid interconversion is expected at elevated temperatures due to their similar stabilities.
Ongoing investigations include a comparative study of the relative stabilities of surface carbonates, nitrites, nitrates, sulfites and sulfates formed on the class of nano-sized systems considered here, using the corresponding bulk surface properties for NOx storage,6 as reference.
Acknowledgements
Support from the Swedish Research Council is gratefully acknowledged.References
- E. M. Geusic, M. D. Morse and R. E. Smalley, J. Chem. Phys., 1985, 82, 590 CrossRef.
- M. D. Morse, E. M. Geusic, J. R. Heath and R. E. Smalley, J. Chem. Phys., 1985, 83, 2293 CrossRef CAS.
- I. Panas, P. Siegbahn and U. Wahlgren, in The Challenge of d and f Electrons, ed. R. D. Salahub and M. C. Zerner, Toronto, 1988, ch. 9, Search PubMedACS Symposium Series, American Chemical Society, Washington DC, 1989..
- I. Panas, J. Schüle, P. Siegbahn and U. Wahlgren, Chem. Phys. Lett., 1988, 149, 265 CrossRef CAS.
- N. Takahashi, H. Shinjoh, T. Iijima, T. Suzuki, K. Yamazaki, K. Yokota, H. Suzuki, N. Miyoshi, S. Matsumoto, T. Tanizawa, T. Tanaka, S. Tateishi and K. Kasahara, Catal. Today, 1996, 27 CrossRef CAS.
- P. Broqvist, I. Panas, E. Fridell and H. Persson, J. Phys. Chem. B Search PubMed.in press.
- F. Bawa and I. Panas, Phys. Chem. Chem. Phys., 2001, 3, 3042 RSC.
- M. J. Malliavin and C. Coudray, J. Chem. Phys., 1997, 106, 2323 CrossRef CAS.
- A. Aguado and J. M. Lopez, J. Phys. Chem. B, 2001, 104, 8398 CrossRef CAS.
- S. Veliah, R. Randey, Y. S. Li, J. M. Newsam and B. Vessal, Chem. Phys. Lett., 1995, 235, 53 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785 CrossRef CAS.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, M. C. Strain, J. C. Burant, R. E. Stratmann, S. Dapprich, K. N. Kudin, J. M. Millam, A. D. Daniels, G. A. Petersson, J. A. Montgomery, V. G. Zakrzewski, K. Raghavachari, P. Y. Ayala, Q. Cui, K. Morokuma, J. B. Foresman, J. Cioslowski, J. V. Ortiz, V. Barone, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, A. Nanayakkara, M. Challacombe, C. Y. Peng, J. P. Stewart, C. Gonzalez, M. Head-Gordon, P. M. W. Gill, B. G. Johnson and J. A. Pople, Gaussian98, Gaussian, Inc., Pittsburgh, PA, 1998. Search PubMed.
- T. H. Dunning, Jr. and P. J. Hay, in Modern Theoretical Chemistry, ed. H. F. Schaefer, III, Plenum Press, New York, 1976, p. 1. Search PubMed.
- T. Leininger, A. Nicklass, H. Stoll, M. Dolg and P. Schwerdtfeger, J. Chem. Phys., 1996, 105, 1052 CrossRef CAS.
- P. J. Ziemann and A. W. Castleman, Phys. Rev. B, 1991, 44, 6488 CrossRef CAS.
- P. J. Ziemann and A. W. Castleman, Jr., J. Chem. Phys., 1991, 94, 718 CrossRef CAS.
- P. J. Ziemann and A. W. Castleman, Jr., Z. Phys. D, 1991, 20, 97 CAS.
- M. C. Day and J. Selbin, Theoretical Inorganic Chemsitry, Reinhold, New York, Chapman and Hall, London, 1962, ch. 4. Search PubMed.
| This journal is © the Owner Societies 2002 |
