1D silver(I) complex of nitronyl nitroxide with strong spin–spin interaction through silver(I) ion
Deqing
Zhang
*a,
Liang
Ding
a,
Wei
Xu
a,
Huaiming
Hu
a,
Daoben
Zhu
*a,
Yuanhe
Huang
b and
Decai
Fang
b
aOrganic Solids Laboratory, Center for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100080, China. E-mail: dqzhang@infoc3.icas.ac.cn
bDepartment of Chemistry, Beijing Normal University, Beijing, 100875, China
First published on 4th December 2001
Abstract
A 1D silver(I) complex of nitronyl nitroxide was prepared and its structure was determined by X-ray diffraction analysis; magnetic studies indicate that the spin–spin interaction of nitronyl nitroxides through silver(I) ions along the chain are fairly strong (J/kb = −84 K).
Paramagnetic metal complexes of nitronyl nitroxides and imino nitroxides have been extensively studied,1 and large spin clusters, magnetic chains and even bulk ferromagnetism have resulted from these studies. Meanwhile, the research on diamagnetic metal complexes of imino nitroxides and semiquinones has also attracted great attention, since this is an interesting reversal on the more usual situation where paramagnetic metal ions show superexchange through a bridging ligand. For example, metal complexes with semiquinones2 show a variety of magnetic interactions depending on the metal ion and coordination geometry, and the magnitude of the magnetic interactions through diamagnetic ions depend strongly on the energy of dπ orbitals. Cu(I) complexes of imino nitroxides show fairly strong ferromagnetic interactions between imino nitroxides through the diamagnetic Cu+ ion,3 while Pd(II) complexes of imino nitroxides exhibit a substantial antiferromagnetic interaction through the Pd2+ ion.4 However, for discrete silver(I) complexes of imino nitroxides, the corresponding spin–spin interactions of imino nitroxides mediated by silver(I) ions have been found to be very small and even negligible in some cases.5 Up to now, to the best of our knowledge, no silver(I) complexes of nitronyl nitroxides have been described. Here, we report the synthesis, structure and magnetic property of a novel one-dimensional silver(I) complex (complex 1) of nitronyl nitroxide derived from m-N-methylpyridinium nitronyl nitroxide iodide (Scheme 1) and silver nitrate. A fairly strong antiferromagnetic interaction between nitronyl nitroxides was observed for complex 1.
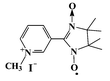 | ||
| Scheme 1 Chemical structure of m-N-methylpyridinium nitronyl nitroxide iodide. | ||
Complex 1 was prepared starting from m-N-methylpyridinium nitronyl nitroxide iodide6 and silver nitrate. After removal of the yellow precipitate, the solution was slowly evaporated to yield black crystals of complex 1.† Its structure was established by X-ray diffraction analysis.‡
Fig. 1 shows the asymmetric unit of complex 1 together with selected bond lengths and angles. There are two nitrate ions, one silver(I) ion and one m-N-methylpyridinium nitronyl nitroxide group (MPYNN), and thus the asymmetric unit is neutral as a whole. For the MPYNN unit, the bond lengths of N(1)–O(1) and C(7)–N(1) are almost identical to those of N(2)–O(2) and C(7)–N(2), respectively (see the caption of Fig. 1); the pyridinium and imidazoline rings are not coplanar, and they form a dihedral angle of 43.3°. Five oxygen atoms O(11), O(21), O(22), O(1) and O(2)i (not shown in Fig. 1) are coordinated to silver(I). The bond lengths of Ag(1)–O(1) and Ag(1)–O(2)i, which are formed between the silver(I) ion and nitronyl nitroxide units, are comparable to those of bonds linking silver(I) and nitrate ions [Ag(1)–O(11), Ag(1)–O(21) and Ag(1)–O(22)].
![The asymmetric unit of complex 1 together with selected bond lengths (Å) and angles (°): N(1)–O(1) 1.281(3), N(1)–C(7) 1.342(3), N(2)–O(2) 1.284(3), N(2)–C(7) 1.339(3), Ag(1)–O(1) 2.4452(19), Ag(1)–O(2)i 2.3706(19), Ag(1)–O(11) 2.459(2), Ag(1)–O(21) 2.480(2), Ag(1)–O(22) 2.540(2); N(2)–C(7)–N(1) 109.6(2), N(1)–O(1)–Ag(1) 120.74(14), N(2)–O(2)–Ag(1)i 123.51(15), O(1)–Ag(1)–O(11) 89.58(7), O(1)–Ag(1)–O(21) 125.91(7), O(11)–Ag(1)–O(21) 81.82(8), O(11)–Ag(1)–O(22) 108.19(7), O(21)–Ag(1)–O(22) 51.23(7) [symmetrical operation:ix, y − 1, z].](/image/article/2002/CC/b109354a/b109354a-f1.gif) | ||
| Fig. 1 The asymmetric unit of complex 1 together with selected bond lengths (Å) and angles (°): N(1)–O(1) 1.281(3), N(1)–C(7) 1.342(3), N(2)–O(2) 1.284(3), N(2)–C(7) 1.339(3), Ag(1)–O(1) 2.4452(19), Ag(1)–O(2)i 2.3706(19), Ag(1)–O(11) 2.459(2), Ag(1)–O(21) 2.480(2), Ag(1)–O(22) 2.540(2); N(2)–C(7)–N(1) 109.6(2), N(1)–O(1)–Ag(1) 120.74(14), N(2)–O(2)–Ag(1)i 123.51(15), O(1)–Ag(1)–O(11) 89.58(7), O(1)–Ag(1)–O(21) 125.91(7), O(11)–Ag(1)–O(21) 81.82(8), O(11)–Ag(1)–O(22) 108.19(7), O(21)–Ag(1)–O(22) 51.23(7) [symmetrical operation:ix, y − 1, z]. | ||
Atoms O2 and Ag1 shown in Fig. 1 are further linked to another silver(I) ion and MPYNN unit, respectively, and the structure is extended in this manner to generate a one dimensional chain as clearly indicated in Fig. 2. Interestingly, despite the steric hindrance the m-N-methylpyridinium ring and the nitrate ions are arranged on one side of the chain, which is very likely due to electrostatic attraction interactions among the m-N-methylpyridinium and the nitrate ions. The inter-chain arrangement of complex 1 is also shown in Fig. 2. Besides the short contacts among the hydrogen atoms of m-N-methylpyridinium ring and oxygen atoms of nitrate ions, no short interatomic distances are found among the neighboring chains. According to previous studies,7 for nitronyl nitroxide derivatives most of the spin density is distributed onto the atoms of the imidazoline ring, and the spin densities carried by other atoms, in particular hydrogen atom attached to m-N-methylpyridinium ring, are negligible. Thus, each of the one-dimensional chains in the crystal lattice of complex 1 can be treated independently from the magnetic point of view. Preliminary theoretical investigations were performed for a “dimer” of the asymmetrical unit.8 The calculation results show that the electron densities ρb for the bonds of silver(I) ions and oxygen atoms of nitronyl nitroxide units are only about 0.004, indicating that these bonds are not typical covalent ones but electrovalent bonds. The electron density distribution around the silver(I) ions are spherical, from which it can be inferred that only the s orbitals of silver(I) ions are involved in the formation of the bonds of silver(I) ions with oxygen atoms of MPYNN units and nitrate ions.
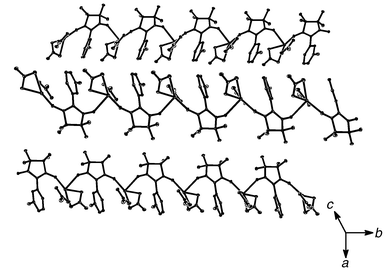 | ||
| Fig. 2 The 1D structure and inter-chain arrangement of complex 1. | ||
Fig. 3 shows a plot of the χTvs. T for complex 1, where χ is the molar magnetic susceptibility in terms of the asymmetric unit formula C13H19AgN5O8. The room temperature value of χT is 0.330 emu K mol−1, smaller than 0.375 emu K mol−1, the theoretically expected value for a one spin (S = 1/2) system. By lowering temperature, χT is reduced gradually from 300 to 100 K, and then it decreases more sharply reaching 0.014 emu K mol−1 at 5 K. Such temperature dependent behavior of χT indicates the presence of antiferromagnetic spin–spin interaction in complex 1.
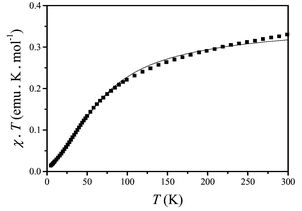 | ||
| Fig. 3 Plot of χT product vs. T for complex 1; the solid line represents the best theoretical fit (see text). | ||
As discussed above, the spin–spin interaction among the one-dimensional chains (inter-chain interaction) in the crystal lattice of complex 1 should be rather weak. Therefore, the experimental magnetic data was fitted to a one dimensional antiferromagnetic model with eqn. (1):9
| χ = (Ng2β2/kbT)(0.25 + 0.074975x + 0.075235x2)/(1.0 + 0.9931x + 0.172135x2 + 0.757825x3), x = |J|/kbT | (1) |
In summary, a novel silver(I) complex of nitronyl nitroxide with an extended 1D structure has been prepared and characterized. The spin–spin interaction of nitronyl nitroxides through the silver(I) ions along the chain is fairly strong with an exchange constant J/kb = −84 K.
The present research work was supported by Chinese Academy of Sciences, NSFC (29972044, 90101025) and the Major State Basic Research Development Program (G2000077500). The authors also thank Prof. Xianglin Jin of Peking University for his kind help on crystal structural analysis.
Notes and references
- See for example: (a) A. Caneschi, D. Gatteschi and P. Rey, Prog. Inorg. Chem., 1991, 39, 331 Search PubMed; (b) D. Luneau, F. M. Romero and R. Ziessel, Inorg. Chem., 1998, 37, 5078 CrossRef CAS.
- (a) G. A. Fox and C. G. Pierpont, Inorg. Chem., 1992, 31, 3718 CrossRef CAS; (b) C. W. Lange, B. J. Conklin and C. G. Pierpont, Inorg. Chem., 1994, 33, 1276 CrossRef CAS; (c) A. Ozarowski, B. R. McGarvey, A. El-Hadad, Z. Tian, D. G. Tuck, D. J. Krovich and G. C. DeFotis, Inorg. Chem., 1993, 32, 841 CrossRef CAS.
- H. Oshio, T. Watanabe, A. Ohto, T. Ito and U. Nagashima, Angew. Chem., Int. Ed. Engl., 1994, 33, 670 CrossRef.
- H. Oshio, A. Ohto and T. Ito, Chem. Commun., 1996, 1541 RSC.
- H. Oshio and T. Ito, Coord. Chem. Rev., 2000, 198, 329 CrossRef CAS and references therein.
- m-N-Methylpyridinium nitronyl nitroxide iodide was prepared according to the following reference: K. Awaga, T. Inabe, U. Nagashima, T. Nakamura, M. Matsumoto, Y. Kawabata and Y. Maruyama, Chem. Lett., 1991, 1777 Search PubMed.
- (a) A. Zheludev, V. Barone, M. Bonnet, B. Delley, A. Grand, E. Ressouche, P. Rey, R. Subra and J. Schweizer, J. Am. Chem. Soc., 1994, 116, 2019 CrossRef CAS; (b) T. Otsuka, T. Okuno, K. Awaga and T. Inabe, J. Mater. Chem., 1998, 8, 1157 RSC.
- The calculation was carried out on the basis of density functional theory (UB3LYP/Lanl2DZ with GAUSSIAN 98 program) in combination with crystal structural data.
- T. Otsuka, T. Okuno, K. Awaga and T. Inabe, J. Mater. Chem., 1998, 8, 1157 RSC and references therein.
Footnotes |
| † Yield, 40.9%; mp, 175–176 °C (decomp.); IR (KBr), 1384 (s), 1290 (w), 1215 (w), 1170 (w), 1137 (w) cm−1; Anal. Calc. for C13H19AgN5O8: C, 32.50; H, 3.99; N, 14.59. Found: C, 32.25; H, 3.95; N, 14.60%. |
| ‡ Crystal data: C13H19AgN5O8, M = 481.20, monoclinic, C2/c, a = 28.278(6), b = 7.0410(14), c = 18.894(4) Å, β =112.55(3)°, V = 3474.3(12) Å3, Z = 8, Dc = 1.840 g cm−3; F(000) = 1944, μ(Mo-Kα) = 1.216 mm−1. 16047 reflections of which 3956 are unique (Rint = 0.0204) were collected to a θ limit of 27.48° on a Rigaku RAXIS RAPID IP instrument at 293(2) K. The structure was solved by direct methods and refined by a least-squares matrix method. The final cycle of full-matrix least-square refinement was based on 3400 observed reflections [I > 2σ(I)] and 244 variable parameters and converged to R1 = 0.0292, wR2 = 0.0775. CCDC reference number 172993. See http://www.rsc.org/suppdata/cc/b1/b109354a/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2002 |
