First examples of the catalytic asymmetric ring-opening of meso 1,2-dioxines utilising cobalt(II) complexes with optically active tetradentate Schiff base ligands: formation of enantio-enriched cyclopropanes
Thomas D.
Avery
,
Natalie F.
Jenkins
,
Marc C.
Kimber
,
David W.
Lupton
and
Dennis K.
Taylor
*
Department of Chemistry, Adelaide University, South Australia, Australia 5005. E-mail: dennis.taylor@adelaide.edu.au;; Fax: +61 8 8303 4358;; Tel: +61 8 8303 5494
First published on 14th December 2001
Abstract
The combination of chiral cobalt β-ketoiminato or cobalt salen complexes and meso 1,2-dioxines leads to catalytic asymmetric ring-opening affording enantio-enriched cis γ-hydroxy enones; subsequent capture by an ylide affords enantio-enriched cyclopropanes.
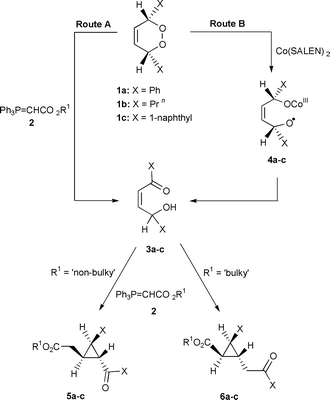
Functionalised cyclopropanes have proven to be exceedingly useful building blocks for the synthesis of natural and non-natural products.1 Although there exists many excellent ways for the construction of the cyclopropyl core,2,3 there is currently a deficiency in diastereoselectively efficient methods for the construction of diversely functionalised optically-pure cyclopropanes that contain greater than di-substitution. Our efforts have focussed on exploiting 1,2-dioxines 1 and stabilised phosphorus ylides 2 (e.g. R1 = Me, Bn, Butetc.) as precursors for the construction of diversely functionalised cyclopropanes 5 or 6, Scheme 1. The ylides act as a mild base inducing ring-opening of the 1,2-dioxine 1 in a regiochemical fashion to produce the isomeric cis γ-hydroxy enones 3, route A. Capture of this latter isomer by the ylide ultimately affords the observed cyclopropanes and is widely applicable to both symmetrical and non-symmetrical 1,2-dioxines.4,5 We have also shown that cobalt salen can be utilised as a catalyst, which accelerates the ring-opening process affording cis γ-hydroxy enones 3 through the intermediacy of species 4, route B.4,5 This latter process is currently restricted to the ring-opening of symmetrical 1,2-dioxines 1 as only one regioisomer of enone 3 can be formed.
 | ||
| Scheme 1 | ||
A logical extension to these latter findings was to investigate the use of cobalt(II) complexes with optically-active tetradentate Schiff base ligands. The hypothesis being that the chirality of the cobalt complex would induce enantioselectivity during the ring-opening process. We have previously shown that if the cis γ-hydroxy enone 3 is optically-pure then the resultant cyclopropanes formed on addition of ylide are also optically-pure.6 We therefore report here the first examples of a catalytic asymmetric ring-opening of meso 1,2-dioxines which relies on the use of chiral cobalt β-ketoiminato or cobalt salen complexes; subsequent capture by an ylide affords enantio-enriched cyclopropanes.7
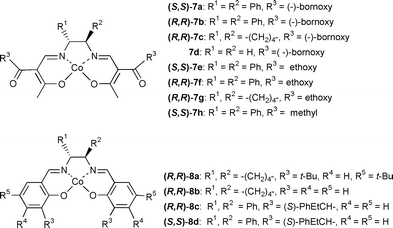
Three meso 1,2-dioxines (1a–c)4,5 and twelve chiral cobalt catalysts (7a–h and 8a–d) were chosen to be evaluated. It is worthy of mention that while cobalt catalysts (8a and b) are known, only the synthesis of the chiral ligands for some of the remaining catalysts have previously been reported.8 In particular, chiral cobalt β-ketoiminato complexes of types (7a–h) represent a new class of chiral cobalt complexes.
Table 1 contains examples of the formation of optically-enriched cyclopropane 5a9 utilizing 1,2-dioxine 1a, catalyst 7a (5 mol%) and the benzyl ester ylide.10 The results confirm our hypothesis that the chirality of the cobalt complex would induce enantioselectivity during the ring-opening process of meso 1,2-dioxines. Additionally, it can be seen that the solvent of choice for this particular ring-opening process from those currently trialled is THF. The effect of temperature on observed ee in both CH2Cl2 (entries 1–5) and THF (entries 11–14) was also probed and revealed that ee is maximized for the ring-opening of 1a by the catalyst 7a at ca. −15 to −20 °C. Based on these initial observations it is now possible to highlight the practical potential of this new catalytic asymmetric cyclopropanation reaction under semi-optimized conditions. Thus, a solution of 1,2-dioxine 1a 0.5 g in THF 20 mL and a solution of catalyst 7a 120 mg in THF 5 mL were equilibrated at −15 °C for 0.5 h after which time the two solutions were combined whilst maintaining the temperature at −15 °C. The rearrangement of 1a into 3a was monitored by TLC and when complete, (0.5 h) benzyl ester ylide 1.0 g added and the reaction mixture allowed to attain ambient temperature overnight. Workup and measurement of ee resulted in cyclopropane 5a being isolated in 78% yield and displaying a 76% ee.
| Entry | Solvent | Temp./°C) | Ee (%)b | Entry | Solvent | Temp./°C) | Ee (%)b |
|---|---|---|---|---|---|---|---|
| a Carried out utilising 20 mg 1a in the appropriate solvent (1 mL) at the specified temperature. The catalyst 7a (5 mol% with respect to 1,2-dioxine concentration) was then added and the ring-opening monitored by TLC. After complete rearrangement the benzyl ester ylide 1 equiv. was added and the mixture allowed to attain ambient temperature overnight. Cyclopropane 5a was then isolated by column chromatography. b Determined according to ref. 9. | |||||||
| 1 | CH2Cl2 | 20 | 38 | 8 | CCl4 | 20 | 34 |
| 2 | CH2Cl2 | 0 | 48 | 9 | Acetone | 20 | 52 |
| 3 | CH2Cl2 | −10 | 56 | 10 | CH3CN | 20 | 52 |
| 4 | CH2Cl2 | −20 | 72 | 11 | THF | 20 | 68 |
| 5 | CH2Cl2 | −40 | 60 | 12 | THF | 0 | 74 |
| 6 | Et2O | 20 | 34 | 13 | THF | −15 | 76 |
| 7 | Toluene | 20 | 34 | 14 | THF | −40 | 72 |
A diverse range of chiral cobalt β-ketoiminato complexes 7a–h and cobalt salen catalysts 8a–d were prepared next in order to probe in which quadrant(s) sterics and/or chiral group positioning is important on observed ee, Table 2. In addition, these catalysts were examined for a range of di-alkyl and di-aryl meso 1,2-dioxines 1a–c in order to probe the generality of this transformation. Overall, the results summarised within Table 2 indicate that useful enantioselectivities can be incorporated for a wide range of chiral cobalt β-ketoiminato catalysts 7a–h (entries 1–12) and that the catalytic asymmetric cyclopropanation is widely applicable to a range of meso 1,2-dioxines. Comparison of entries 1 and 4 indicate that chirality located within the northern hemisphere of these β-ketoiminato complexes is important in inducing the high enantioselectivities. Moreover, analysis of the use of chiral cobalt salen catalysts 8a–d (entries 13–20) also reveals that these catalysts induce useful ee’s into the cyclopropanation sequence. Indeed, the matched salen catalyst 8d led to an impressive 89∶11 enantiomeric ratio when the reaction was carried out in THF at 4 °C (entry 20).
| Entry | 1,2-Dioxine | Catalyst | Ee (%)b | Entry | 1,2-Dioxine | Catalyst | Ee (%)b |
|---|---|---|---|---|---|---|---|
| a Reactions were carried out utilising 20 mg of 1,2-dioxine in CH2Cl2 (1 mL) at 20 °C except where noted. The appropriate amount of catalyst (entries 1–3, 7.5 mol%; entries 4–12, 10 mol%; entries 13–20, 5 mol% with respect to 1,2-dioxine concentration) was then added and the ring-opening monitored by TLC. After complete rearrangement the benzyl ester ylide 1 equiv. was added and the mixture left overnight. Cyclopropanes 5a–c were then isolated by column chromatography and the isolated yields varied between 70–88%. b Determined according to ref. 9. c Performed in THF. d Performed at 4 °C. | |||||||
| 1 | 1a | 7a | 44 | 11 | 1b | 7h | 30 |
| 2 | 7b | 34 | 12 | 1c | 7a | 44 | |
| 3 | 7c | 46 | 13 | 1a | 8a | 30 | |
| 4 | 7d | 14 | 14c | 34 | |||
| 5 | 7e | 46 | 15 | 8b | 38 | ||
| 6 | 7f | 46 | 16c | 44 | |||
| 7 | 1a | 7g | 42 | 17 | 8c | 34 | |
| 8 | 7h | 46 | 18 | 8d | 50 | ||
| 9 | 1b | 7a | 34 | 19c | 64 | ||
| 10 | 7e | 32 | 20cd | 78 | |||
A full study encompassing a wide range of catalyst types is the focus of current studies and will be reported in full shortly along with a model depicting how the enantioselectivity is induced.
This work was supported by the Australian Research Council.
Notes and references
- See for example: H. W. Lin and C. T. Walsh, ‘Biochemistry of the Cyclopropyl Group’. In The Chemistry of the Cyclopropyl Group, ed. S. Patai and Z. Rappoport, Wiley, New York, 1987, Chapter 16 Search PubMed; J. Martel, ‘The Development and Manufacture of Pyrethroid Insecticides’. In Chirality in Industry, ed. A. N. Collins, G. N. Sheldrake and J. Crosby, Wiley, Chichester, 1992, Chapter 4 and references cited therein Search PubMed; J. R. Falck, B. Mekonnen, J. Yu and J. Y. Lai, J. Am. Chem. Soc., 1996, 118, 6096 Search PubMed; A. G. M. Barrett and J. Kasdorf, J. Am. Chem. Soc., 1996, 118, 11030 Search PubMed.
- For recent examples of the direct carbene transfer (both stoichiometric and catalytic) from a diazo precursor to an olefin utilizing transition metals see: S. E. Denmark, B. L. Christenson, S. P. O’Conner and N. Murase, Pure Appl. Chem., 1996, 68, 23 Search PubMed; V. K. Singh, A. DattaGupta and G. Sekar, Synthesis, 1997, 137 CAS; T. Ichiyanagi, M. Shimizu and T. Fujisawa, Tetrahedron, 1997, 53, 9599 CrossRef CAS; H. M. L. Davies and S. A. Panaro, Tetrahedron Lett., 1999, 40, 5287 CrossRef CAS.
- For examples of the Michael addition of nucleophiles to α,β-unsaturated ketones and esters followed by intramolecular cyclisation see: M. Calmes, J. Daunis and F. Escale, Tetrahedron: Asymmetry, 1996, 7, 395 Search PubMed; A.-H. Li, L.-X. Dai and V. K. Aggarwal, Chem. Rev., 1997, 97, 2341 CrossRef CAS.
- T. D. Avery, T. D. Haselgrove, T. J. Rathbone, D. K. Taylor and E. R. T. Tiekink, Chem. Commun., 1998, 333 RSC; T. D. Avery, D. K. Taylor and E. R. T. Tiekink, J. Org. Chem., 2000, 65, 5531 CrossRef CAS.
- T. D. Avery, B. W. Greatrex, D. K. Taylor and E. R. T. Tiekink, J. Chem. Soc., Perkin Trans. 1, 2000, 1319 RSC; T. D. Avery, G. Fallon, B. W. Greatrex, S. M. Pyke, D. K. Taylo and E. R. T. Tiekink, J. Org. Chem., 2001, in press. Search PubMed.
- F. N. Palmer and D. K. Taylor, J. Chem. Soc., Perkin Trans. 1, 2000, 1323 RSC.
- The overall concept is by no means limited to cyclopropane formation. We will describe shortly the formation of enantio-enriched lactones which is also reliant on the catalytic asymmetric ring-opening of meso 1,2-dioxines by these chiral cobalt catalysts..
- (a) For general procedures to prepare the β-ketoiminato ligands see: T. Nagata, K. Imagawa and T. Yamada, Inorg. Chim. Acta, 1994, 220, 283 Search PubMed; K. D. Sugi, T. Nagata, T. Yamada and T. Mukaiyama, Chem.Lett., 1997, 493 CrossRef CAS; T. Nagata, K. Imagawa, T. Yamada and T. Mukaiyama, Chem. Lett., 1994, 1259 CrossRef CAS; (b) For general procedures to prepare cobalt salen catalysts see: T. Fukuda and T. Katsuki, Tetrahedron, 1997, 53, 7201 Search PubMed; R. Irie, K. Noda, Y. Ito, N. Matsumoto and T. Katsuki, Tetrahedron: Asymmetry, 1991, 2, 481 CrossRef CAS.
- Racemic 5a has previously been characterized, see ref. 4. The ee was determined by dissolving cyclopropane 5a (5 mg) in a 1∶4 benzene-d6∶CCl4 solution and employing the chiral shift reagent europium tris[3-(heptafluoropropylhydroxymethylene)-(+)-camphorate] complex. The benzyl protons at ca. δ 5.2 ppm displayed baseline separation..
- It should be noted that a wide range of bulky and non-bulky ester ylides including chiral ester ylides can be employed. See refs. 4 and 5 for examples..
| This journal is © The Royal Society of Chemistry 2002 |