Planar and central chiral [2.2]paracyclophane-based N,O-ligands as highly active catalysts in the diethylzinc addition to aldehydes†
Stefan
Dahmen
a and
Stefan
Bräse
*b
aInstitut für Organische Chemie der Rheinisch-Westfälischen Technischen Hochschule Aachen, Professor-Pirlet-Strasse 1, D-52074, Aachen, Germany
bKekulé-Institut für Organische Chemie und Biochemie der Rheinischen Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, D-53121, Bonn, Germany. E-mail: braese@uni-bonn.de; Fax: +49 228 739608; Tel: +49 228 732653
First published on 29th November 2001
Abstract
The application of planar and central chiral [2.2]paracyclophane-based N,O-ligands in asymmetric diethylzinc additions to various aldehydes is described revealing an unusual substrate spectrum for the catalysts and their remarkably high activity.
The element of planar chirality plays an important role in many modern ligand systems.1–3 The combination of different individual chirotopic elements within the ligand molecule can lead to highly selective catalysts as for instance observed in various ferrocene derivatives2 and arene transition metal complexes.3 However, limited attention has been paid to planar chiral ligands derived from [2.2]paracyclophane4 and even fewer reports focus on planar and central chiral [2.2]paracyclophane-ligands and their application in asymmetric catalysis.5
During ligand screening employing various hydroxy imine, hydroxy ketimine and amino alcohol ligands based on the [2.2]paracyclophane backbone, we discovered that ketimines like 1 and 2 (Fig. 1) showed superior selectivity and activity in the diethylzinc addition to benzaldehyde and revealed cooperative effects arising from the combination of the different chirotopic elements of the ligands (chiral cooperativity). However, the ketimines 1 and 2 were first synthesised by Rozenberg and Belokon who used them for the resolution of the corresponding racemic hydroxy ketones.6 Here, we will report on the unusual substrate spectrum and the high activity of these ligands.
![Diastereomeric [2.2]paracyclophane-based ketimine ligands employed in the diethylzinc addition.](/image/article/2002/CC/b108347c/b108347c-f1.gif) | ||
| Fig. 1 Diastereomeric [2.2]paracyclophane-based ketimine ligands employed in the diethylzinc addition. | ||
The diethylzinc addition to aldehydes is one of the most extensively studied enantioselective catalytic reactions, particularly because of features like autoinduction and nonlinear effects.‡ However, of the approximately 600 ligands recently reviewed by Pu and Yu,7 only a small number (10 to 15) is currently capable of efficiently catalysing the diethylzinc addition to aliphatic and especially α-branched aliphatic aldehydes.
Initially, all four ligands were tested with benzaldehyde as standard substrate (Table 1, entries 1–4). The diastereomers 1 showed cooperative effects of the two chiratopic elements of the ligand structure with (Sp,S)-1 giving 85% ee (R) and the (Rp,S)-1 giving 65% ee (S). In contrast to that, the diastereomers 2 catalysed the addition with quasi equal selectivity for both enantiomers (82 vs. 83% ee, entries 3 and 4). The configuration of the chiral centre in the sidechain of the ligands 2 obviously does not influence the selectivity of the reaction. However, in both cases, the planar chirality of the ligand backbone determines the product configuration.
|
|
||||
|---|---|---|---|---|
| Entry | Aldehyde | Ligand | Yield [%]b | ee [%]c |
| a 0.5 mmol Aldehyde, 1.0 mmol diethylzinc (1 molar in hexane), 1 ml of toluene, 1% ligand, 0 °C, 12 h. b Determined by GC. c Determined by GC (Lipodex G: entries 1–4, 17–20; Chirasil-Dex: entries 5–13), by HPLC ((S,S)-Whelk-O 1: entries 14–16), or by GC of (1S)-camphanic ester (entries 21,22). d No toluene was used as co-solvent. | ||||
| 1 | Benzaldehyde | (Rp,S)-1 | 36 | 60 (S) |
| 2 | Benzaldehyde | (Sp,S)-1 | 100 | 85 (R) |
| 3 | Benzaldehyde | (Rp,S)-2 | 94 | 82 (S) |
| 4 | Benzaldehyde | (Sp,S)-2 | 100 | 83 (R) |
| 5 | 4-Chlorobenzaldehyde | (Sp,S)-1 | 100 | 88 (R) |
| 6 | 4-Chlorobenzaldehyde | (Rp,S)-1 | 0 | —d |
| 7 | 4-Methoxybenzaldehyde | (Sp,S)-1 | 98 | 86 (R) |
| 8 | 2-Methoxybenzaldehyde | (Sp,S)-1 | 100 | 55 (R) |
| 9 | 2-Methoxybenzaldehyde | (Rp,S)-2 | 100 | 41 (S) |
| 10 | 1-Naphthaldehyde | (Sp,S)-1 | 80 | 86 (R) |
| 11 | 1-Naphthaldehyde | (Rp,S)-2 | 66 | 77 (S) |
| 12 | 3,5-Dimethoxybenzaldehyde | (Sp,S)-1 | 98 | 84 (R) |
| 13 | 3,5-Dimethoxybenzaldehyde | (Rp,S)-1 | 0 | — |
| 14 | 3,5-Dibenzyloxybenzaldehyde | (Sp,S)-1 | 95 | 81 (R) |
| 15 | 3,5-Dibenzyloxybenzaldehyde | (Rp,S)-1 | 0 | - |
| 16 | a-Methylcinnamaldehyde | (Sp,S)-1 | 100 | 86 (R) |
| 17 | Cyclohexanecarbaldehyde | (Rp,S)-1 | 100 | 96d (S) |
| 18 | Cyclohexanecarbaldehyde | (Sp,S)-1 | 100 | 95d (R) |
| 19 | Cyclohexanecarbaldehyde | (Rp,S)-2 | 92 | 99d (S) |
| 20 | Cyclohexanecarbaldehyde | (Sp,S)-2 | 97 | 86d (R) |
| 21 | Pivalaldehyde | (Sp,S)-1 | 97 | 98d (R) |
| 22 | Pivalaldehyde | (Sp,S)-2 | 100 | 98d (R) |
The ligands were also tested with several other aromatic and aliphatic substrates (entries 5–22). For nearly all aromatic substrates, the selectivities obtained with (Sp,S)-1 were in the range of 81–85% ee. Only ortho-substituted benzaldehydes are recognised on a lower level (55% ee, entry 8). The mismatched diastereomer (Rp,S)-1 delivers for all aromatic substrates much lower yields and selectivities. In some cases, the difference in substrate recognition between the two diastereomers of 1 is so high that no product can be found for reactions carried out with (Rp,S)-1 (Table 1, entries 6,13,15).
However, for aliphatic and especially α-branched aliphatic aldehydes, which are usually considered as poor substrates for the majority of catalysts, the selectivities rise into the high 90's (Table 1, entries 17–22). In addition, the mismatched diastereomer (Rp,S)-1 now delivers slightly better results than (Sp,S)-1 (Table 1, entry 17). In the case of cyclohexanecarbaldehyde, the results obtained with ligand 1 are even surpassed by (Rp,S)-2 giving 99% ee (entry 19). For pivalaldehyde, very high selectivities are obtained also.
Because of their ability to form dimeric complexes, which results for example in the occurrence of nonlinear effects, usually ligands for diethylzinc additions have to be employed on a fairly high loading level (5 to 10%).8 Only very few ligands have been reported to work effectively on a 1 to 2% level.
The paracyclophane-based ligands, however, can be employed on a 0.5% level without loss of selectivity. At 0.1% catalyst loading of ligand (Sp,S)-1, the selectivity towards benzaldehyde and cyclohexanecarbaldehyde drops only by 2 to 3%. And even at 0.05%, very resonable results are obtained (91% ee for cyclohexancarbaldehyde and 80% ee for benzaldehyde, respectively). Obviously, the decrease in ee value is only dependent on the catalyst loading and completely independent of the substrate used (Fig. 2).
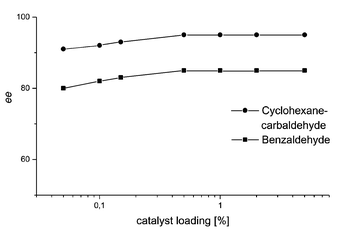 | ||
| Fig. 2 Dependency of the ee value of the diethylzinc addition product on catalyst loading using ligand (Sp,S)-1. | ||
The conversion is at 0.1% catalyst loading virtually complete after 16 h. Even at 0.05% catalyst loading, 92% (benzaldehyde) and 75% (cyclohexanecarbadehyde) of product are obtained. This corresponds to substrate-to-catalyst ratios (s/c) of 1840 (benzaldehyde) and 1500 (cyclohexanecarbaldehyde) respectively. These are, to the best of our knowledge, the highest activities so far observed in asymmetric diethylzinc additions.
In conclusion, we have demonstrated that [2.2]paracyclophane-based N,O-ligands can be valuable ligands in the diethylzinc addition especially to α-branched aliphatic aldehydes. Additionally, they reveal remarkably high activity allowing them to be employed on a 0.5 mol% scale without decrease of selectivity, which makes them some of the most efficient ligands for certain substrate classes.
We would like to thank the Deutsche Forschungsgemeinschaft (SFB 380, BR1750/2) and the Fonds der Chemischen Industrie for financial support.
Notes and references
- W.-P. Deng, S.-L. You, X.-L. Hou, L.-X. Dai, Y.-H. Yu, W. Xia and J. Sun, J. Am. Chem. Soc., 2001, 123, 6508 CrossRef CAS.
- For reviews, see: Ferrocenes, ed. A. Togni and T. Hayashi, VCH, Weinheim, 1995 Search PubMed; C. J. Richards and A. J. Locke, Tetrahedron: Asymmetry, 1998, 9, 2377 Search PubMed; A. Togni, N. Bieler, U. Burckhardt, C. Köllner, G. Pioda, R. Schneider and A. Schnyder, Pure Appl. Chem., 1999, 71, 1531 Search PubMed; L.-X. Dai, X.-L. Hou, W.-P. Deng, S.-L. You and Y.-G. Zhou, Pure Appl. Chem., 1999, 71, 1401 CrossRef CAS.
- C. Bolm and K. Muniz, Chem. Soc. Rev., 1999, 28, 51 RSC.
- S. Banfi, A. Manfredi, F. Montannari, G. Pozzi and S. Quici, J. Mol. Catal. A, 1996, 113, 77 CrossRef CAS; Y. Belokon, M. Moscalenko, N. Ikonnikov, L. Yashkina, D. Antonov, E. Vorontsov and V. Rozenberg, Tetrahedron: Asymmetry, 1997, 19, 3245 CrossRef; P. J. Pye, K. Rossen, R. A. Reamer, N. N. Tsou, R. P. Volante and P. J. Reider, J. Am. Chem. Soc., 1997, 119, 6207 CrossRef CAS; K. Rossen, P. J. Pye, A. Maliakal and R. P. Volante, J. Org. Chem., 1997, 62, 6462 CrossRef CAS; P. J. Pye, K. Rossen, R. A. Reamer, R. P. Volante and P. J. Reider, Tetrahedron Lett., 1998, 39, 4441 CrossRef CAS; U. Wörsdörfer, F. Vögtle, M. Nieger, M. Waletzke, S. Grimme, F. Glorius and A. Pfaltz, Synthesis, 1999, 597 CrossRef; U. Wörsdörfer, F. Vögtle, F. Glorius and A. Pfaltz, J. Prakt. Chem., 1999, 341, 445 CrossRef CAS; D. S. Masterson, T. L. Hobbs and D. T. Glatzhofer, J. Mol. Catal. A: Chem., 1999, 145, 75 CrossRef CAS; D. S. Masterson and D. T. Glatzhofer, J. Mol. Catal. A: Chem., 2000, 161, 65 CrossRef CAS; M. J. Burk, W. Hems, D. Herzberg, C. Malan and A. Zanotti-Gerosa, Org. Lett., 2000, 2, 4173 CrossRef CAS; C. Bolm and T. Kühn, Synlett, 2000, 6, 899; V. I. Rozenberg, D. Y. Antonov, R. O. Zhuravsky, E. V. Vorontsov, V. N. Khrustalev, N. S. Ikonnikov and Y. N. Belokon, Tetrahedron: Asymmetry, 2000, 11, 2683 CrossRef CAS; S. Tanji, A. Ohno, I. Sato and K. Soai, Org. Lett., 2001, 3, 287 CrossRef CAS.
- A. H. Vetter and A. Berkessel, Tetrahedron Lett., 1998, 39, 1741 CrossRef CAS; X.-L. Hou, X.-W. Wu, L.-X. Dai, B.-X. Cao and J. Sun, Chem. Commun., 2000, 1195 RSC; X.-W. Wu, X.-L. Hou, L.-X. Dai, J. Tao, B.-X. Cao and J. Sun, Tetrahedron: Asymmetry, 2001, 12, 529 CrossRef CAS.
- V. Rozenberg, T. Danilova, E. Sergeeva, E. Vorontsov, Z. Starikova, K. Lysenko and Y. Belokon, Eur. J. Chem., 2000, 3295 Search PubMed.
- For reviews, see: K. Soai and S. Niwa, Chem. Rev., 1992, 92, 8333 Search PubMed; L. Pu and H.-B. Yu, Chem. Rev., 2001, 101, 757.
- The formation of dimeric complexes is a phenomenon commonly observed for amino alcohol ligands in diethylzinc addition reactions. Formation of hetero-dimeric complexes, which are often more stable than the homo-dimeric complexes leads to the occurrence of nonlinear effects. As the catalytically active species is a monomeric zinc alkoxide, often times high catalyst loadings have to be employed to circumvent catalyst deactivation by dimer formation. For reviews on nonlinear effects in asymmetric catalysis, see: C. Girard and H. B. Kagan, Angew. Chem., Int. Ed., 1998, 37, 2922 Search PubMed; M. Avalos, R. Babiano, P. Cintas, J. L. Jiménez and J. C. Palacios, Tetrahedron: Asymmetry, 1997, 8, 2997 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: synthesis, NMR data, optical rotation and chiral analysis. See http://www.rsc.org/suppdata/cc/b1/b108347c/ |
| ‡ General procedure for the addition of diethylzinc to aldehydes: Under a dry argon atmosphere, the ligand was dissolved in 1.0 mL of dry toluene at rt and 1.0 mL of a 1 M solution of diethylzinc in hexane was added. After stirring for 30 min., the resulting yellow solution was cooled to 0 °C, the aldehyde was added and the solution was kept at this temperature for 12 h. The solution was quenched with 1 M HCl and filtered over a short plug of silica to remove the inorganic salts. |
| This journal is © The Royal Society of Chemistry 2002 |

