Novel catalysis of dendrimer-bound Pd(0) complexes: sterically steered allylic amination and the first application for a thermomorphic system
Tomoo
Mizugaki
,
Makoto
Murata
,
Masahiko
Ooe
,
Kohki
Ebitani
and
Kiyotomi
Kaneda
*
Department of Chemical Science and Engineering, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka, 560-8531, Japan.. E-mail: kaneda@cheng.es.osaka-u.ac.jp
First published on 13th December 2001
Abstract
Phosphinated dendrimer-bound Pd(0) complex catalysts show high stereoselectivity for allylic amination due to the surface congestion of dendrimers and can be easily recycled without loss of activity under thermomorphic conditions.
Dendrimers are novel macromolecules with monodispersed molecular weights, precisely determined cascade structures, and a specific number of the end groups.1 One of the promising applications of metallo-dendrimers is in catalysis.2 Organometallic dendrimers offer potential in building the gap between homogeneous and heterogeneous catalysis because of their structurally well-defined and specific number of active sites as well as their advantage of facile recovery by nano-filtration or solvent precipitation.3 The characteristic structures of dendrimers have been expected to enhance selectivities, but, there are few reports on positive catalytic effects due to the dendritic structures.4 Here, we report the synthesis of dendrimer-bound Pd(0) complexes and their unique catalysis for allylic substitution reactions.5 Furthermore, facile recovery of the dendritic Pd complexes can be achieved by the use of a thermomorphic system,6 which enables the temperature-induced phase separation of the homogeneous catalyst solution from a product phase. Thermomorphic behavior has also been reported in some catalytic systems, e.g. in a fluorous biphase by Horvath and Rabai7 and for soluble PNIPAM-supported metal complexes by Bergbreiter et al.6 To our knowledge, thermomorphic catalyst systems including dendrimer-bound metal complexes have not, as yet, been reported.
Double phosphinomethylation of primary amino groups on the 1st, 3rd, 4th and 5th generation of poly(propylene imine) dendrimers were carried out giving 4, 16, 32 and 64 chelate phosphines on the periphery (1a–d), respectively, as shown in Scheme 1.3b Treatment of the dendrimers 1a–d with PdCl2(PhCN)2 afforded dendrimer-bound PdCl2 complexes 2a–d. Subsequently, reduction of the Pd(II) dendrimers 2 with hydrazine monohydrate in the presence of two equivalents of PPh38 led to the formation of dendritic Pd(0) complexes 3a–d, respectively, with retention of the parent dendritic structures. These Pd complexes were characterized by 1H, 13C{1H}, 31P{1H} NMR, IR and XPS.† The 31P{1H} NMR spectrum of 2 did not show any signal for the residual free phosphine at δ –27 but only one singlet at δ 8, and elemental analysis of 2 gave a Pd∶P∶Cl ratio of 1∶2∶2, which strongly supports the complete complexation of the chelate phosphine to Pd in a cis form.4b After the reduction of 2, the above resonance at δ 8 was fully replaced by a new one around δ 27. From XPS analysis of 2, the band observed at 338 eV assignable to Pd 3d5/2 is comparable with that of a typical Pd(II) complex such as PdCl2(PPh3)2, and the band at 336 eV for dendritic Pd complex 3 is due to a Pd(0) species.8
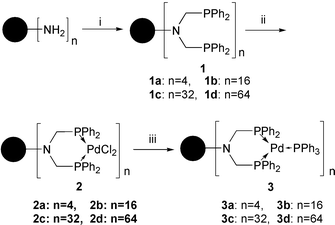 | ||
| Scheme 1 Preparation of dendritic Pd(II) complexes 2 and Pd(0) complexes 3. Reagents and conditions: i, HCHO, HPPh2, toluene, 60 °C; ii, PdCl2(PhCN)2, toluene, room temp.; iii, H2NNH2·H2O, PPh3, EtOH, room temp. | ||
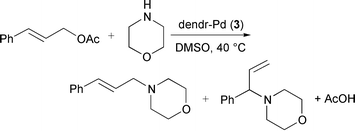
Catalytic performances of Pd(0) dendrimers 3 was examined in allylic substitution reactions of allylic acetates with amines [eqn. (1)].5‡ For example, the substitution of trans-cinnamyl acetate with morpholine catalysed by 3b smoothly proceeded to give the corresponding allylic amines in >99% yield (l∶b = 90∶10) within 30 min. The dendritic catalysts 3 were soluble in DMSO and it turned out to be the best solvent to study the catalytic reactions while CH2Cl2 and THF were not effective due to the low solubilities of the dendritic catalysts in these solvents. In the case of Pd(PPh3)4, DMSO, CH2Cl2 and THF were all good solvents. This substitution reaction using the dendritic catalysts could be also extended to include soft carbon nucleophiles and other allylic derivatives such as carbonates. van Leeuwen and coworkers reported that increased regioselectivity for the branched product was observed on going to the higher generation of a core functionalised dendritic Pd catalyst.9 In contrast, changing the generation of our surface functionalised dendritic catalysts did not affect the regioselectivity among linear and branched products.
The prominent catalysis of dendritic Pd complexes 3 was further investigated in the amination of cis-3-acetoxy-5-carbomethoxycyclohex-1-ene with morpholine. Notably, the stereoselectivity for a cis product increased with increasing the 3rd to the 5th generation of dendrimers as shown in Fig. 1: a high cis selectivity of 94% was obtained with 3d, while Pd(PPh3)4 as a typical monomeric Pd(0) catalyst led to a slight excess of cis product. The 1st and the 3rd generations of the dendritic catalysts 3a and 3b showed moderate cis selectivity, respectively. In order to clarify the origin of the stereoselectivity, 13C spin–lattice relaxation times (T1) of the dendritic ligands 1a–d were measured (Fig. 1).§T1 of peripheral phenyl groups on 1a–d decreased with increasing the generation of the dendrimers, and this phenomenon shows that the dendrimer surface becomes more congested for higher generations.10 In the case of 3d, attaining high stereoselectivity of the substitution reaction can be explained by steric steering of the nucleophilic attack to a surface (π-allyl)Pd intermediate, i.e. the active sites would be strongly shielded from the endo attack of the nucleophile. The moderate selectivity with 3a and 3b might be due to the loosely packed dendrimer surfaces, which would allow the partial dissociation of chelate phosphine ligands.11 Similar stereoselective performances in allylic aminations due to steric hindrance of solid supports have also been observed by Trost and others using polystyrene- and silica-bound Pd(0) complex catalysts.12
![Generation dependence on selectivity in the allylic amination of cis-3-acetoxy-5-carbomethoxycyclohex-1-ene with morpholine. Stereoselectivity for cis isomer was compared at 30% conversion of the substrate. Selectivity (%) = [(cis
− trans)/(cis + trans)] × 100. T1 values were calculated for the C4 carbon (128 ppm) in the phenyl groups of the corresponding dendrimers 1, respectively.](/image/article/2002/CC/b107452k/b107452k-f1.gif) | ||
| Fig. 1 Generation dependence on selectivity in the allylic amination of cis-3-acetoxy-5-carbomethoxycyclohex-1-ene with morpholine. Stereoselectivity for cis isomer was compared at 30% conversion of the substrate. Selectivity (%) = [(cis − trans)/(cis + trans)] × 100. T1 values were calculated for the C4 carbon (128 ppm) in the phenyl groups of the corresponding dendrimers 1, respectively. | ||
Recycling of dendritic metal complex catalysts was attempted by solvent precipitation3b or membrane filtration,3d which often results in some losses of the catalytic activities during the recovery and reuse processes. Fortunately, our dendritic Pd(0) catalysts 3 could be easily recycled by using a biphasic system of DMF and heptane and the system does not need any special procedures such as membrane filtration.¶ The two phases consisting of DMF and heptane became homogeneous when heated up to 75 °C, and then could be readily separated by cooling the reaction mixtures to room temperature. Extremely low solubility of the dendrimers 3 in the apolar heptane solvent meant the catalysts were completely transferred to DMF and the catalyst solution could be recycled after decantation of the heptane phase containing products. For example, in the allylic substitution of trans-cinnamyl acetate with dibutylamine, the high catalytic activity was retained during three reuse experiments: yields of the allylic amine in heptane phase were 66% (1st), 99% (2nd), 99% (3rd) and 99% (4th run), respectively.|| This is the first application of dendritic catalysts in a thermomorphic system.
In conclusion dendrimer-bound Pd(0) complexes have been synthesised by reduction of dendritic Pd(II) complexes with hydrazine. They show high stereoselectivity for allylic amination ascribed to the surface congestion of the dendrimers. Employing a thermomorphic system makes it possible to efficiently recycle the dendritic catalysts. We expect that the above results could give a clue to more sophisticated catalyst designs of dendrimers for highly selective organic syntheses.
This work is supported by the Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (12750691 and 11450307). We are grateful to the Department of Chemical Science and Engineering, Graduate School of Engineering Science, Osaka University for scientific support via the ‘Gas-Hydrate Analyzing System (GHAS)’ and the Lend-Lease Laboratory System.
Notes and references
- Reviews and a book on dendrimers: (a) D. A. Tomalia, H. Baker, J. R. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J., 1985, 17, 117 Search PubMed; (b) F. Zeng and S. C. Zimmerman, Chem. Rev., 1997, 97, 1681 CrossRef CAS; (c) A. W. Bosman, H. M. Janssen and E. W. Meijer, Chem. Rev., 1999, 99, 1665 CrossRef CAS; (d) G. R. Newkome, C. N. Moorefield and F. Vögtle, Dendritic Molecules: Concepts, Synthesis, Perspectives,VCH, Weinheim, 1996. Search PubMed.
- (a) G. R. Newkome, E. He and C. N. Moorefield, Chem. Rev., 1999, 99, 1689 CrossRef CAS; (b) G. E. Oosterom, J. N. H. Reek, P. C. J. Kamer and P. W. N. M. van Leeuwen, Angew. Chem., Int. Ed., 2001, 40, 1828 CrossRef CAS; (c) D. Astruc and F. Chardac, Chem. Rev., 2001, 101, 2991 CrossRef CAS.
- (a) J. W. J. Knapen, A. W. van der Made, J. C. de Wilde, P. W. M. N. van Leeuwen, P. Wijkens, D. M. Grove and G. van Koten, Nature, 1994, 372, 659 CrossRef CAS; (b) M. T. Reetz, G. Lohmer and R. Schwickardi, Angew. Chem., Int. Ed. Engl., 1997, 36, 1526 CrossRef CAS dendritic catalysts for continuous flow membrane reactors, see:; (c) N. J. Hovestad, E. B. Eggeling, H. J. Heidbuchel, J. T. B. H. Jastrzebski, U. Kragl, W. Keim, D. Vogt and G. van Koten, Angew. Chem., Int. Ed., 1999, 38, 1655 CrossRef CAS; (d) D. de Groot, E. B. Eggeling, J. C. de Wilde, H. Kooijman, R. J. van Haaren, A. W. van der Made, A. L. Spek, D. Vogt, J. N. H. Reek, P. C. J. Kamer and P. W. N. M. van Leeuwen, Chem. Commun., 1999, 1623 RSC; (e) N. Brinkmann, D. Giebel, G. Lohmer, M. T. Reetz and U. Kragl, J. Catal., 1999, 183, 163 CrossRef CAS; (f) E. B. Eggeling, N. J. Hovestad, J. T. B. H. Jastrzebsky, D. Vogt and G. van Koten, J. Org. Chem., 2000, 65, 8857 CrossRef CAS.
- (a) C. Koellner, B. Pugin and A. Togni, J. Am. Chem. Soc., 1998, 120, 10274 CrossRef; (b) T. Mizugaki, M. Ooe, K. Ebitani and K. Kaneda, J. Mol. Catal. A: Chem., 1999, 145, 329 CrossRef CAS; (c) R. Breinbauer and E. N. Jacobsen, Angew Chem., Int. Ed., 2000, 39, 3604 CrossRef CAS; (d) L. Ropartz, R. E. Morris, D. F. Foster and D. J. Cole-Hamilton, Chem. Commun., 2001, 361 RSC.
- Reviews on allylic amination: (a) M. Johannsen and K. A. Jørgensen, Chem. Rev., 1998, 98, 1689 CrossRef CAS; (b) A. Heumann, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 1998, p. 251. Search PubMed.
- (a) D. E. Bergbreiter, Y-S. Liu and P. L. Osburn, J. Am. Chem. Soc., 1998, 120, 4250 CrossRef CAS; (b) D. E. Bergbreiter, P. L. Osburn, A. Wilson and E. M. Sink, J. Am. Chem. Soc., 2000, 122, 9058 CrossRef CAS.
- I. Horvath and J. Rabai, Science, 1994, 266, 72 CrossRef CAS.
- M. Terasawa, K. Kaneda, T. Imanaka and S. Teranishi, J. Organomet. Chem., 1978, 162, 403 CrossRef CAS.
- G. E. Oosterom, R. J. van Haaren, J. N. H. Reek, P. C. J. Kamer and P. W. N. M. van Leeuwen, Chem. Commun., 1999, 1119 RSC.
- (a) A. M. Nayler, W. A. Goddard III, G. E. Kiefer and D. A. Tomalia, J. Am. Chem. Soc., 1989, 111, 2339 CrossRef; (b) J. F. G. A. Jansen, E. M. M. de Brabander-van den Berg and E. W. Meijer, Science, 1994, 266, 1226 CrossRef CAS.
- Z. Csakai, R. Skoda-Fördes and L. Kollár, Inorg. Chim. Acta, 1999, 286, 93 CrossRef CAS.
- (a) B. M. Trost and E. Keinan, J. Am. Chem. Soc., 1978, 100, 7779 CrossRef CAS; (b) B. M. Trost and T. R. Verhoeven, J. Am. Chem. Soc., 1980, 102, 4730 CrossRef CAS; (c) J. E. Bäckvall, K. L. Granberg and A. Heumann, Isr. J. Chem., 1991, 31, 17 Search PubMed.
Footnotes |
| † Selected data: for 1b: 1H NMR (CDCl3): δ 7.16–7.33 (m, 320H, Ph), 3.50 (br, 54H, NCH2P), 2.76 (br, 32H, CH2NCH2P), 2.17–2.34 (br, 92H, CH2NCH2CH2, NCH2CH2CH2NCH2P), 1.42(br, 60H, NCH2CH2CH2N, NCH2CH2CH2CH2 N). 31P{1H} NMR (CDCl3): δ −27. For 2b: 31P{1H} NMR (DMSO-d6): δ 8. XPS: 343.4 eV (Pd 3d3/2), 338.3 eV (Pd 3d5/2). IR (CsI) 294 cm−1 (cis Pd–Cl). For 3b: 31P{1H} NMR (DMSO-d6): δ 27. XPS: 342.0 eV (Pd 3d3/2), 336.8 eV (Pd 3d5/2). |
| ‡ Typical reaction conditions: trans-cinnamyl acetate (1.5 mmol), morpholine (1.8 mmol), catalyst (5 μmol of Pd atoms), DMSO (5 mL), 40 °C, Ar atmosphere. |
| § Because of the low solubility of the dendrimers 3, T1 for the dendrimers 1 in place of 3 was measured by the inversion–recovery method. |
| ¶ Reaction conditions for the thermomorphic catalysis are as follows: dendritic catalyst (10 μmol of Pd atoms), DMF (1.5 mL), dibutylamine (1.8 mmol), triethylamine (1.5 mmol), heptane (3 mL), and trans-cinnamyl acetate (1.5 mmol) were added in a Schlenk type flask under Ar and stirred at 75 °C for 1 h. For reuse experiments, heptane, allylic acetate, a nucleophile and triethylamine were added subsequently after decantation of the heptane phase. These procedures were repeated three times. |
| || The relatively low yield for the fresh run was due to the distribution of allylic amine product in the DMF phase. No Pd leaching in the heptane phase was observed during recycle experiments according to ICP (detection limit is 0.1 ppm). |
| This journal is © The Royal Society of Chemistry 2002 |