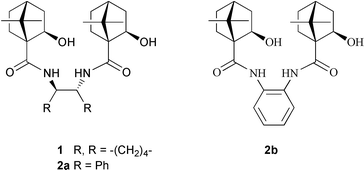
1,2-Diphenylethylenediamine linked chiral Ti(IV) complex—a new entry to the highly enantioselective silylcyanation of aliphatic and aromatic aldehydes†
Chun-Wei
Chang
,
Chun-
TzuYang
,
Chyuan-Der
Hwang
and
Biing-Jiun
Uang
*
Department of Chemistry, National Tsing Hua University, Hsinchu, Taiwan 300, Republic of China.. E-mail: bjuang@mx.nthu.edu.tw;; Fax: +886 3-5711082
First published on 13th December 2001
Abstract
Highly enantioselective silylcyanation of aliphatic and aromatic aldehydes was achieved by using a 1,2-diphenylethylenediamine linked chiral Ti(IV) complex as the catalyst.
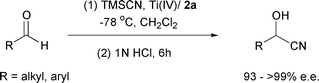
Enantiomerically pure cyanohydrins are versatile intermediates1,2 useful in the synthesis of a variety of natural and non-natural biologically active molecules owing to their potential for selective transformations given by the presence of a single asymmetric center bearing two reactive functionalities. Several efficient enzymatic2–8 and chemical methods2,9 have been developed for the preparation of chiral cyanohydrins and enantioselective addition of trimethylsilyl cyanide to aldehydes catalysed by chiral metal complexes is amongst the most successful approaches. Despite this, need exists for new chiral catalysts as most of the catalysts used for this purpose are either unstable or difficult to prepare, and sometimes cannot be easily recovered. Our earlier efforts in this area have led to the development of a chiral Ti(IV) catalyst with dihydroxy-trans-1,2-diamide 1 as the constituent which has promoted the enantioselective addition of trimethylsilyl cyanide to aromatic aldehydes in 94–97% ee and to aliphatic aldehydes in 87–97% ee.10 We have now addressed enantioselective silylcyanation using a new chiral catalyst, Ti(OiPr)4–diamide 2a, that efficiently promotes the addition of trimethylsilyl cyanide to both aromatic and aliphatic aldehydes with excellent enantioselectivity.
It is envisioned that, replacement of the cyclohexane ring with two vicinal phenyl groups in the chiral diamide moiety of 1 would result in a more gainful catalyst which can provide greater facial selectivity in the silylcyanation of the aldehydes. A relatively bulky phenyl group would increase the energy difference between the two diastereomeric transition structure orientations thereby enhancing the enantioselectivity. Chiral compound 2a with the desired structural and stereochemical features was prepared starting from (1R,2R)-(+)-1,2-diphenylethylenediamine. This diamine was submitted to condensation with (1S)-(+)-ketopinic acid chloride 311 in dichloromethane in the presence of triethylamine to furnish diketoamide 4a (Scheme 1). Subsequent reduction of 4a using L-selectride® in THF at −78 °C for 1 h and then at room temperature for 2 h gave 2a with the hydroxy group in the exo position.12 In conjunction with Ti(OiPr)4, chiral diamide 2a was shown to effect the enantioselective addition of trimethylsilyl cyanide to aldehydes 5 with a high degree of selectivity (Table 1).
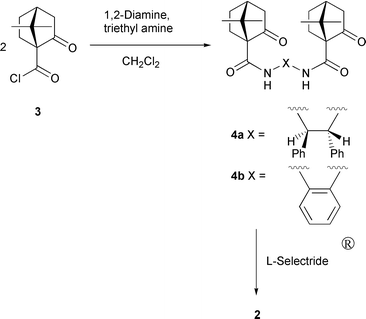 | ||
| Scheme 1 | ||
| Run | Aldehyde | Yield (%) | Ee (%) |
|---|---|---|---|
| a Determined by HPLC with chiracel OD column by converting into TBDMS ether for run 1 and as acetyl esters for runs 2–9. Absolute configuration was determined as S by the comparison of rotation with literature value5. b Percent conversions. | |||
| 1 | Benzaldehyde 5a | 87 | 93 |
| 2 | Cyclohexane carbaldehyde 5b | 90 | >99 |
| 3 | Valeraldehyde 5c | 92 | 97 |
| 4 | 2-Methylbenzaldehyde 5d | 80b | 94 |
| 5 | 3-Phenoxybenzaldehyde 5e | 78b | 95 |
| 6 | 4-Methoxybenzaldehyde 5f | 72b | 99 |
| 7 | 2-Naphthaldehyde 5g | 78b | 99 |
| 8 | (E)-Cinnamaldehyde 5h | 74b | 97 |
| 9 | 3-Phenylpropionaldehyde 5i | 73b | 97 |
Optimum results were obtained when the reaction was carried out at −78 °C in dichloromethane using the complex prepared from 16.5 mol% of 2a and 15 mol% of Ti(OiPr)4 in the presence of 4 Å molecular sieves.9a,13 Under these conditions, the desired cyanohydrin was isolated in high yield and the enantioselective induction achieved in both aromatic (>93% S) and aliphatic aldehydes (>97% S) was quite high after the hydrolysis (1 N HCl, rt 6 h) of the initial addition product. At the same time, the chiral diamide 2a was recovered in high yield. This compares favorably with other earlier reported organometallic processes used for such purpose. There is a pronounced enhancement of enantioselectivity in the reactions of aliphatic aldehydes when compared to our previously reported method.10
To ascertain the effect of the chirality of the diamide moiety of 2a on the extent of enantioselectivity, compound 2b lacking chirality in the diamide function was prepared from 1,2-phenylenediamine following the procedure used for 2a and employed as a constituent of catalyst in the silylcyanation of benzaldehyde. As expected, the enantioselectivity was significantly low with a maximum of 61% ee under optimum reaction conditions when the reaction was carried out at −78 °C in dichloromethane using the complex prepared from 16.5 mol% of 2b and 15 mol% of Ti(OiPr)4 in the presence of 4 Å molecular sieves. Apparently chirality in the diamide moiety is facilitating the higher enantioselectivity.
In conclusion, a new and efficient catalyst for enantioselective silylcyanation of aromatic and aliphatic aldehydes with excellent selectivity has been developed. The high degree of enantioselectivity coupled with the high stability and recovery rate of chiral component 2a of the catalyst constitutes the major improvement on existing methods.
We are grateful to the National Science Council, Republic of China for the finantial support of this work.
Notes and references
- (a) F. Effenberger, Angew. Chem., Int. Ed. Engl., 1994, 33, 1555 CrossRef; (b) H. Griengl, A. Hickel, D. V. Johnson, C. Kratky, M. Schmidt and H. Schwab, Chem. Commun., 1997, 1933 and references therein. Search PubMed.
- (a) For recent reviews see M. North, Synlett., 1993, 807 Search PubMed; (b) R. J. H. Gregory, Chem. Rev., 1999, 99, 3649 CrossRef CAS.
- (a) T. Ziegler, B. Horsch and F. Effenberger, Synthesis, 1990, 575 CrossRef CAS; (b) F. Effenberger, T. Ziegler and S. Forster, Angew. Chem., Int. Ed. Engl., 1987, 26, 458 CrossRef; (c) P. Zandbergen, J. Van der Linden, J. Brussee and A. Van der Gen, Synthetic Commun., 1991, 21, 1387 CAS; (d) J. Brussee, E. C. Roos and A. Ven der Gen, Tetrahedron Lett., 1988, 29, 4485 CrossRef CAS; (e) V. I. Ognyanov, V. K. Datcheva and K. S. Kyler, J. Am. Chem. Soc., 1991, 113, 6992 CrossRef CAS.
- Y. Lu, C. Miet, N. Kunesch and J. E. Poisson, Tetrahedron: Asymmetry, 1993, 4, 893 and references therein. Search PubMed.
- R. S. Ward, Tetrahedron: Asymmetry, 1995, 6, 1475 CrossRef CAS.
- F. Effenberger and S. Heid, Tetrahedron: Asymmetry, 1995, 6, 2945 CrossRef CAS.
- S. Forster, J. Roos, F. Effenberger, H. Wajant and A. Sprauer, Angew. Chem., Int. Ed. Engl., 1996, 35, 437 CrossRef.
- Topics in current chemistry, Vol 200, ed. W.-D. Fessner, 1999, pp. 203–226 and references therein. Search PubMed.
- (a) K. Narasaka, T. Yamada and H. Minamikawa, Chem. Lett., 1987, 2073 CAS; (b) M. Hayashi, T. Matsuda and N. Oguni, J. Chem. Soc., Chem. Commun., 1990, 1364 RSC; (c) M. Hayashi, Y. Miyamoto, T. Inoue and N. Oguni, J. Chem. Soc., Chem. Commun., 1991, 1752 RSC; (d) M. Hayashi, T. Inoue, Y. Miyamoto and N. Oguni, Tetrahedron, 1994, 50, 4385 CrossRef CAS; (e) M. Hayashi and N. Oguni, J. Synth. Org. Chem. Jpn, 1994, 488 Search PubMed; (f) M. Hayashi, Y. Miyamoto, T. Inoue and N. Oguni, J. Org. Chem., 1993, 58, 1515 CrossRef CAS; (g) Y. Z. Jiang, X. G. Zhou, W. H. Hu, L. J. Wu and A. Q. Mi, Tetrahedron: Asymmetry, 1995, 6, 405 CrossRef CAS; (h) C. Bolm, P. Muller and K. Harms, Acta Chem. Scand., 1996, 50, 305 Search PubMed; (i) W. D. Pan, X. M. Feng, L. Z. Gong, W. H. Hu, Z. Lin and Y. Z. Jiang, Synlett 1, 1996, 337 Search PubMed; (j) Y. Belokon, M. Flego, N. Ikonnikov, M. Moscalenko, M. North, C. Orizu, V. Tararov and M. Tasinazzo, J. Chem. Soc., Perkin Trans. 1, 1997, 1, 1293 RSC; (k) I. Iovel, Y. Popelis, M. Fleisher and E. Vukevics, Tetrahedron: Asymmetry, 1997, 8, 1279 CrossRef CAS; (l) C. Bolm and P. Muller, Tetrahedron Lett., 1995, 36, 1625 CrossRef CAS; (m) Y. Z. Jiang, L. Z. Gong, X. M. Feng, W. H. Hu, W. D. Pan. Z. Li and A. Q. Mi, Tetrahedron, 1997, 53, 14327 CrossRef CAS; (n) M. Wada, T. Takahashi, T. Domae, T. Fukuma, N. Miyoshi and K. Smith, Tetrahedron: Asymmetry, 1997, 8, 3939 CrossRef CAS; (o) Y. Belokon, M. Moscalenko, N. Ikonnikov, L. Yashkina, D. Antonov, E. Vorontsov and V. Rozenberg, Tetrahedron: Asymmetry, 1997, 8, 3245 CrossRef CAS; (p) V. I. Tararov, D. E. Hibbs, M. B. Hursthouse, N. S. Ikonnikov, K. M. A. Malik, M. North, C. Orizu and Y. N. Belokon, J. Chem. Soc., Chem. Commun., 1998, 387 RSC; (q) W. B. Yang and J. M. Fang, J. Org. Chem., 1998, 63, 1356 CrossRef CAS; (r) S. Kobayashi, Y. Tsuchiya and T. Mukaiyama, Chem. Lett., 1991, 541 CAS; (s) M. Mori, H. Imma and T. Nakai, Tetrahedron Lett., 1997, 38, 6229 CrossRef CAS; (t) Y. N. Belokon, N. S. Ikonnikov, M. Moscalenko, M. North, S. Orlova, V. Tararov and L. Yashkina, Tetrahedron: Asymmetry, 1996, 7, 851 CrossRef CAS; (u) Y. N. Belokon and M. North, J. Am. Chem. Soc., 1999, 121, 3968 CrossRef CAS; (v) Y. Hamashima, D. Sawada, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 1999, 121, 2641 CrossRef CAS; (w) Y. N. Belokon, M. North and T. Parsons, Org. Lett., 2000, 2, 1617 CrossRef; (x) Y. Hamashima, D. Sawada, H. Nogami, M. Kanai and M. Shibasaki, Tetrahedron, 2001, 57, 805 CrossRef CAS; (y) Y. N. Belokon, B. Green, N. S. Ikonnikov, M. North, T. Parsons and V. I. Taranov, Tetrahedron, 2001, 57, 771 CrossRef; (z) T. Ooi, T. Miura, K. Takaya, H. Ichikawa and K. Maruoka, Tetrahedron, 2001, 57, 867 CrossRef CAS.
- C.-D. Hwang, D.-R. Hwang and B.-J. Uang, J. Org. Chem., 1998, 63, 6762 CrossRef CAS.
- M. F. Haslanger and J. Heikes, Synthesis, 1981, 801 CrossRef CAS.
- W. Oppolzer, G. Bernardinelli and C. Chapuis, Tetrahedron Lett., 1984, 25, 5885 CrossRef CAS.
- H. Minamikawa, S. Hayakawa, T. Yamada and K. Narasaka, Bull. Chem. Soc., Jpn., 1988, 61, 4379 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: experimental section. See http://www.rsc.org/suppdata/cc/b1/b107867d/ |
| This journal is © The Royal Society of Chemistry 2002 |