Novel reactions of phosphorus(III) azides and isocyanates: unusual modes of cycloaddition with dipolarophiles and an unexpected case of ring expansion†
Sudha Kumaraswamy, Praveen Kommana, N. Satish Kumar and K. C. Kumara Swamy*
School of Chemistry, University of Hyderabad, Hyderabad-, 500046, A. P, India. E-mail: kckssc@uohyd.ernet.in; Fax: +91-40-3010120
First published on 3rd December 2001
Abstract
New modes of 1,3-dipolar cycloaddition are uncovered by the isolation of [CH2(6-t-Bu-4-Me-C6H2O)2]P{C(CO2Me)C(CO2Me)N[NP(N3)(OC6H2-6-t-Bu-4-Me)2CH2]N} (3) and [CH2(6-t-Bu-4-Me-C6H2O)2]P{C(CO2Me)C(CO2Me)C(O)N} (4) on treating [CH2(6-t-Bu-4-Me-C6H2O)2]P-X [X = N3 (1) and NCO (2)] with the dipolarophile MeO2CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me; compound 4 undergoes an unprecedented ring expansion upon addition of 2-(methylamino)ethanol to afford the spirocycle [CH2(6-t-Bu-4-Me-C6H2O)2]P{OCH2CH2N(Me)CH(CO2Me)CH(CO2Me)C(O)N} (5).
CCO2Me; compound 4 undergoes an unprecedented ring expansion upon addition of 2-(methylamino)ethanol to afford the spirocycle [CH2(6-t-Bu-4-Me-C6H2O)2]P{OCH2CH2N(Me)CH(CO2Me)CH(CO2Me)C(O)N} (5).
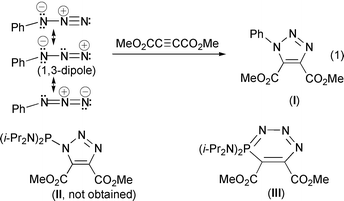
1,3-Dipolar cycloaddition reactions constitute a large class of synthetically useful processes.1 Organic azides (e.g. PhN3) are valuable substrates in such cycloadditions, and behave typically as 1,3-(N,N) dipoles towards dipolarophiles such as MeO2CC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me (dimethyl acetylenedicarboxylate, DMAD) [eqn. (1)].1a,2 On this basis, one could naively expect that treatment of a σ3,λ3-phosphorus azide [e.g. (i-Pr2N)2PN3] with DMAD would lead to the cycloaddition product II; however, the novel heterocycle III featuring a six-membered ring is formed by 1,4-(P,N) dipolar addition of the acetylene.3 It has also been shown that the classical reactivity of an organic functional group can be dramatically altered by the presence of a σ3,λ3-phosphorus
substituent.4
CCO2Me (dimethyl acetylenedicarboxylate, DMAD) [eqn. (1)].1a,2 On this basis, one could naively expect that treatment of a σ3,λ3-phosphorus azide [e.g. (i-Pr2N)2PN3] with DMAD would lead to the cycloaddition product II; however, the novel heterocycle III featuring a six-membered ring is formed by 1,4-(P,N) dipolar addition of the acetylene.3 It has also been shown that the classical reactivity of an organic functional group can be dramatically altered by the presence of a σ3,λ3-phosphorus
substituent.4In this context, two questions that arose in our mind were, (i) how general are the reactions leading to products such as III? (ii) how do the isoelectronic P(III) isocyanates, R2P–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O behave towards dipolarophiles? To address these questions, we chose the P(III) azide and isocyanate 1 and 2 respectively, which possess a sterically hindered eight-membered ring. The cyclic part contains two oxygens that could impart electronic effects different from those in (i-Pr2N)2PN3. Similar systems were employed by us previously to explore subtle aspects of penta- and hexa-coordinate phosphorus chemistry.5 Herein, we describe some interesting aspects of the reactivity of 1‡ and 2, that include
their unusual behaviour towards the dipolarophile MeO2CC
O behave towards dipolarophiles? To address these questions, we chose the P(III) azide and isocyanate 1 and 2 respectively, which possess a sterically hindered eight-membered ring. The cyclic part contains two oxygens that could impart electronic effects different from those in (i-Pr2N)2PN3. Similar systems were employed by us previously to explore subtle aspects of penta- and hexa-coordinate phosphorus chemistry.5 Herein, we describe some interesting aspects of the reactivity of 1‡ and 2, that include
their unusual behaviour towards the dipolarophile MeO2CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me (DMAD)§ and a novel ring expansion reaction (Scheme 1).
CCO2Me (DMAD)§ and a novel ring expansion reaction (Scheme 1).
 | ||
| Scheme 1 | ||
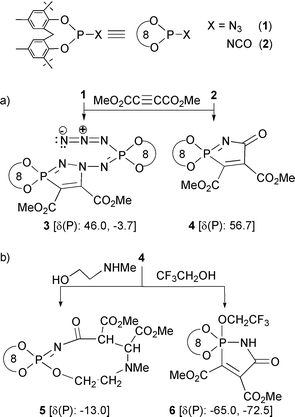
(i) Unlike an organic azide RN3 or (i-Pr2N)2PN3, our azide 1does not behave like a 1,3-(N,N) or 1,4-(P,N) dipole towards the dipolarophile DMAD. The reaction involves one molecule of acetylene and two molecules of azide resulting in the practically exclusive formation of the 1,3-(P,N) heterocycle 3 containing an appended phosphazenyl azide residue! It may be noted that 3 is obtained in high yield irrespective of whether DMAD is added to the azide or vice versa.
(ii) For the first time, a convincing demonstration of the 1,3-(P,C) dipolar nature of a P(III) isocyanate is provided by the quantitative formation of 4, in the reaction of 2 with DMAD. In contrast, it must be noted that treating an organic isocyanate R-NCO with DMAD could, logically, lead to 6-membered pyridone rings through a [2 + 2 + 2] cycloaddition, possibly via the unstable azetones.6
An unprecedented ring expansion (from five to nine membered) occurs upon addition of 2-(methylamino)ethanol to 4, to yield 5. A Michael-type [1,4] addition in which the amine attacks at the carbon adjacent to phosphorus is the key step; cleavage of the P–C bond occurs during subsequent attack by the hydroxy group on phosphorus. This reaction contrasts with the addition of 2,2,2-trifluoroethanol across the P=N bond of 4 resulting in the spirocyclic pentacoordinate phosphorane 6.
The molecular structures of 3–6 have been determined by X-ray crystallography; those of 3 and 5 are shown in Figs. 1 and 2.7¶ It can be noted that the formal P–N double bond at the spiro-phosphorus in 3 [P(2)–N(6) 1.599(4) and P(4)–N(12) 1.612(4) Å; second molecule of the asymmetric unit not shown in Fig. 1] is slightly longer than the formal single bond in H2NP[OC(CF3)2C(CF3)2O]2 [1.590(8) Å],8 thus posing an intriguing question on the nature of the P–N bond. A rationalization can be put forth by assuming some phosphonium character for the spiro-phosphorus atom;3 this would also explain the ready formation of the addition product 6 from 4 and CF3CH2OH.
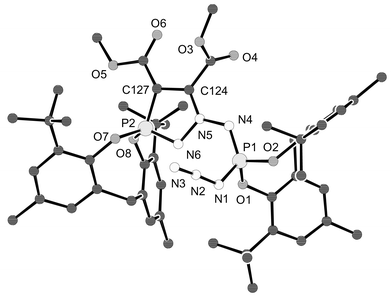 | ||
| Fig. 1 Molecular structure of 3·3/4CH3CN; the solvent, second molecule in the asymmetric unit and hydrogen atoms are omitted. Selected distances: P(1)–N(1) 1.664(4), P(1)–N(4) 1.541(4), P(1)–O(1) 1.563(3), P(1)–O(2) 1.538(3), P(2)–N(6) 1.599(4), P(2)–O(7) 1.583(3), P(2)–O(8) 1.586(3), P(2)–C(127) 1.720(5), N(1)–N(2) 1.192(6), N(2)–N(3) 1.117(7), N(4)–N(5) 1.394(5), N(5)–N(6) 1.387(5), N(5)–C(124) 1.312(5), C(124)–C(127) 1.412(6) Å. | ||
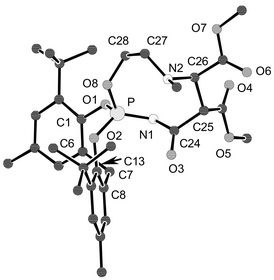 | ||
| Fig. 2 Molecular structure of 5·C6H5CH3; the solvent and hydrogen atoms are omitted. Selected distances: P–N(1) 1.550(2), P–O(2) 1.566(2), P–O(1) 1.573(2), P–O(8) 1.580(2), O(8)–C(28) 1.457(2), N(1)–C(24) 1.370(2), N(2)–C(27) 1.467(3), N(2)–C(26) 1.471(3), C(24)–C(25) 1.533(3), C(25)–C(26) 1.539(3), C(27)–C(28) 1.510(3) Å. | ||
Thus we have shown here that (i) the reaction of a P(III) azide with DMAD can lead to products other than that reported before and (ii) new heterocycles which are amenable for further exploration can be synthesised using P(III) isocyanates and DMAD. In this context, it may be noted that the isothiocyanate [CH2(6-t-Bu-4-Me-C6H2O)2]P-NCS also reacts with DMAD to give a heterocycle [via 1,3-(P,C) cycloaddition] which is similar to 4.|| This and related reactions are currently being investigated.
This work was supported by the Department of Science and Technology (DST), New Delhi. We also thank (i) the Council of Scientific and Industrial Research, New Delhi for fellowships to P. K. and N. S. K., (ii) Professor Dr M. Veith and Dr V. Huch for providing the X-ray data on compound 5, (iii) Dr R. Herbst-Irmer for help in the X-ray structural analysis of 3, and (iv) DST (New Delhi) for setting up a National Single Crystal Diffractometer facility at the University of Hyderabad.
Notes and references
- (a) F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry, Part A: Structure and Mechanisms and Part B: Reactions and Synthesis, Plenum Press, New York, 1990, pp. 635–640 and pp. 300-306 respectively Search PubMed; (b) A. Padwa, 1,3-Dipolar Cycloaddition Chemistry, vol. 1 and 2, Wiley Interscience, New York, 1984 Search PubMed; (c) A. Padwa, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1991, vol. 4 (volume ed. M. F. Semmelhack), pp. 1069–1168 Search PubMed; (d) J. Nakayama, A. Kimata, H. Taniguchi and F. Takahashi, Chem. Commun., 1996, 205 RSC; (e) K. Banert and F. Köhler, Angew. Chem., Int. Ed. Engl., 2001, 40, 174 CrossRef CAS; (f) J. S. Carey, J. Org. Chem., 2001, 66, 2526 CrossRef CAS.
- R. Huisgen, R. Knorr, L. Möbius and G. Szeimies, Chem. Ber., 1965, 98, 4014 Search PubMed.
- K. Bieger, J. Tejeda, R. Rèau, F. Dahan and G. Bertrand, J. Am. Chem. Soc., 1994, 116, 8087 CrossRef CAS.
- M. Granier, A. Baceiredo, M. Nieger and G. Bertrand, Angew. Chem., Int. Ed. Engl., 1990, 29, 1123 CrossRef.
- (a) M. A. Said, M. Pülm, R. Herbst-Irmer and K. C. Kumara Swamy, J. Am. Chem. Soc., 1996, 118, 9841 CrossRef CAS; (b) M. A. Said, M. Pülm, R. Herbst-Irmer and K. C. Kumara Swamy, Inorg. Chem., 1997, 36, 2044 CrossRef CAS; (c) S. Kumaraswamy, C. Muthiah and K. C. Kumara Swamy, J. Am. Chem. Soc., 2000, 122, 964 CrossRef CAS.
- (a) N. E. Shore, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 5 (volume ed. L. A. Paquette), p. 1155 Search PubMed; (b) M. A. Pericás, F. Serratosa, E. Valenti, M. Font-Altaba and X. Solans, J. Chem. Soc., Perkin Trans. 2, 1986, 961 RSC; (c) L. Gosez and J. Marchand-Brynaert in ref. 6(a), pp. 102–108; (d) F. P. Cossío, G. Roa, B. Lecea and J. M. Ugalde, J. Am. Chem. Soc., 1995, 117, 12306 CrossRef CAS.
- G. M. Sheldrick, SHELX-97, University of Göttingen, 1997 Search PubMed.
- W. Storzer, D. Schomburg and G. V. Röschenthaler, Z. Naturforsch. B, 1981, 36, 1071 Search PubMed.
- (a) R. Francke and G. V. Röschenthaler, Z. Anorg. Allgem. Chem., 1989, 572, 135 Search PubMed; (b) R. Francke, J. Heine and G. V. Röschenthaler, Phosphorus, Sulfur, and Silicon, 1990, 49/50, 377 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Platon diagrams with selected bond parameters for 4 and 6 and experimental details. See http://www.rsc.org/suppdata/cc/b1/b107087h/ |
| ‡ X-Ray structural data for this compound is available from the authors. |
| § The reaction of some P(III) isocyanates with carbonyl compounds are reported.9 |
¶ Single crystal X-ray data were collected on an Enraf-Nonius MACH3 (compounds 3, 4 and 6) or Stoe IPDS (compound 5) diffractometer at 293 K using Mo-Kα (λ = 0.71073 Å) radiation. The structures were solved by direct methods and refined by full-matrix least squares method with the SHELXL-97 program.7Crystal data: 3·3/4CH3CN, C107H136.5N13.5O16P4, M = 1991.68, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 14.616(4), b = 19.288(4), c
= 23.112(8), α = 114.26(2), β = 103.91(2), γ = 96.55(2), V = 5597(3) Å3, Z = 2, μ = 0.134 mm−1, data/restraints/parameters: 19610/132/1374. R indices (I > 2σ(I)): R1 = 0.0658, wR2 = 0.1651, CCDC number 157568. See http://www.rsc.org/suppdata/cc/b1/b107087h/ for crystallographic files in .cif or other electronic format. 4: C30H36NO7P, M = 553.57, monoclinic, space group P21/n, a = 10.308(3), b = 17.604(4), c = 16.464(3), β = 107.14(2), V = 2854.9(11) Å3, Z = 4, μ = 0.144 mm−1, data/restraints/parameters: 5012/0/362. R indices (I > 2σ(I)): R1 = 0.0515, wR2 = 0.1247. CCDC 157569. 5·C6H5CH3,
C40H53N2O8P, M = 720.81, triclinic, space group P1, a = 11.792(7), b = 12.580(7), c = 15.162(9), α = 110.66(6), β = 103.36(7), γ = 90.53(7), V = 2037(2) Å3, Z = 2, μ = 0.118 mm−1, data/restraints/parameters: 5850/0/480. R indices (I > 2σ(I)): R1 = 0.0423, wR2 = 0.1218. CCDC 157570. 6.CH3CN, C34H42F3N2O8P, M = 694.67, triclinic, space group P1, a = 9.455(3), b = 10.861(3), c = 19.023(5), α = 93.24(2), β = 91.41(2), γ = 107.85(2), V = 1854.6(9) Å3, Z = 2, μ = 0.138 mm−1, data/restraints/parameters: 6520/0/454. R indices
(I > 2σ(I)): R1 = 0.0605, wR2 = 0.1698. CCDC 157571. , a = 14.616(4), b = 19.288(4), c
= 23.112(8), α = 114.26(2), β = 103.91(2), γ = 96.55(2), V = 5597(3) Å3, Z = 2, μ = 0.134 mm−1, data/restraints/parameters: 19610/132/1374. R indices (I > 2σ(I)): R1 = 0.0658, wR2 = 0.1651, CCDC number 157568. See http://www.rsc.org/suppdata/cc/b1/b107087h/ for crystallographic files in .cif or other electronic format. 4: C30H36NO7P, M = 553.57, monoclinic, space group P21/n, a = 10.308(3), b = 17.604(4), c = 16.464(3), β = 107.14(2), V = 2854.9(11) Å3, Z = 4, μ = 0.144 mm−1, data/restraints/parameters: 5012/0/362. R indices (I > 2σ(I)): R1 = 0.0515, wR2 = 0.1247. CCDC 157569. 5·C6H5CH3,
C40H53N2O8P, M = 720.81, triclinic, space group P1, a = 11.792(7), b = 12.580(7), c = 15.162(9), α = 110.66(6), β = 103.36(7), γ = 90.53(7), V = 2037(2) Å3, Z = 2, μ = 0.118 mm−1, data/restraints/parameters: 5850/0/480. R indices (I > 2σ(I)): R1 = 0.0423, wR2 = 0.1218. CCDC 157570. 6.CH3CN, C34H42F3N2O8P, M = 694.67, triclinic, space group P1, a = 9.455(3), b = 10.861(3), c = 19.023(5), α = 93.24(2), β = 91.41(2), γ = 107.85(2), V = 1854.6(9) Å3, Z = 2, μ = 0.138 mm−1, data/restraints/parameters: 6520/0/454. R indices
(I > 2σ(I)): R1 = 0.0605, wR2 = 0.1698. CCDC 157571. |
|| Diethyl acetylenedicarboxylate (EtO2CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Et) afforded products similar to 3 and 4. The product of EtO2CC CCO2Et) afforded products similar to 3 and 4. The product of EtO2CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Et with 2 also underwent ring expansion and addition leading to compounds similar to 5 and 6. CCO2Et with 2 also underwent ring expansion and addition leading to compounds similar to 5 and 6. |
| This journal is © The Royal Society of Chemistry 2002 |