Characterisation of heterogeneous solid surfaces by multiple probe, temperature-programmed inverse gas chromatography (IGC). A feasibility study
Adam W.
McMahon
*,
David G.
Kelly
and
Paul J.
McLaughlin
Department of Chemistry and Materials, Manchester Metropolitan University, Chester Street, Manchester, UK M1 5GD. E-mail: A.McMahon@MMU.AC.UK; Fax: 44 161 247 6357
First published on 29th November 2001
Abstract
A method is described for the characterisation of the surfaces of powdered solids by temperature programmed, multiple-probe, inverse gas chromatography. This modification of “traditional” inverse gas chromatography (IGC) allows rapid screening of solid surfaces to compare the adsorptive behaviour of pairs or sets of surfaces. This feasibility study examined the criteria for selection of a suitable probe set and approaches to the analysis and interpretation of the data generated. The technique was used to compare carbon black samples, chosen for their surface heterogeneity and the difficulty of surface characterisation using other techniques. The choice of a low surface area, methylated silica solid diluent and the elimination of its influence on the measurements is discussed. The probe set selected readily distinguished the carbon black samples and allowed tentative conclusions to be drawn regarding steric and electronic influences on probe-solid interactions.
Introduction
The study of solid surfaces and their interaction is an extremely complex field. Many fundamental investigations are directed towards the study of single crystal surface planes under ultra high vacuum (UHV) conditions. This approach yields valuable insights, yet is nonetheless challenged by complexities that even these idealised samples present.1,2 By contrast, in industrial applications inexpensive materials with highly heterogeneous surfaces are commonly used, in high temperature environments and in the presence of high pressures of gases of limited purity. There is, what is known as a ‘materials and pressure gap’ to bridge between the extremes represented by the fundamental studies and the practical applications. One technique, used since the early days of gas chromatography,3,4 that allows direct characterisation of powdered solids with heterogeneous surfaces is inverse gas chromatography (IGC). Interaction of the solid with a volatile probe compound determines the chromatographic retention characteristics of that probe. Isothermal retention time measurements at a series of temperatures allow the estimation of ΔHads3–6 for the probe compounds on that surface. Very low probe concentration measurements are used to operate in the ‘infinite dilution’ regime, limited by the sensitivity of available chromatographic detectors. Normally a flame ionisation detector would be used with a limit of detection of ∼10−13 g carbon s−1.7 At ‘finite’ dilution more of the adsorption isotherm can be explored by methods such as frontal analysis3 and Charmas and Leboda8 and Pyda et al.9,10 have used this concentration regime to investigate the less adsorptive sites.Although column packing and conditioning are not trivial the overall time-scale of preparation and chromatogram recording is reasonable. The method is inexpensive and allows surface-probe interactions to be studied in the presence of a background gas at high pressures, conditions that may be closer to those experienced in industrial applications of the solid.
The technique described in this work represents an extension of the ‘classical’ IGC approach to surface studies. A cocktail of probes is injected under temperature programmed conditions, to provide a chromatographic ‘fingerprint’ typifying each surface, thereby significantly increasing the yield of data from a single chromatogram. The probes are injected under conditions close to the ‘infinite dilution’ ideal. Elution temperature is related to ΔGads for the probe, at the most active sites, but the absolute value of this parameter cannot be derived from a single chromatographic run. However relative values of ΔGads for a series of probes can offer greater insight into surface behaviour than a few absolute values. We argue that whilst valuable insights can be gained from traditional IGC, including, for example, adsorption energy distribution functions,11,12 these data are hard-won and often difficult to interpret in terms of surface properties of commercial value. The method described in this paper produces retention data that are related to the fundamental thermodynamic and kinetic parameters of mass transfer but allow a rapid comparison of a range of probe-surface interactions, through a chromatographic ‘fingerprint’.
Chromatographic theory
Under isothermal conditions, the net retention volume, VN, can be measured over a range of temperatures and the heat of adsorption for an individual probe determined.4 Where:In these expressions, ΔS and ΔH are the entropy and enthalpy of adsorption (isosteric at infinite dilution, in our experiments), R the gas constant, T the column temperature (K), ns the number of moles of probe in the adsorbed state. Isothermal retention data can yield the two unknowns ΔS and ΔH, if data are recorded at at least two temperatures. It must be assumed that ΔH and ΔS are independent of temperature in the regime of the measurements. With a linear temperature programme, retention is also related to ΔH and ΔS but the relationship is more complex as the carrier flow rate changes in a non-linear manner with temperature. Retention can be modelled as a staircase of sequential, isothermal probe migrations in small regions of the column ultimately culminating in elution. Each of these isothermal increments takes place in a different environment of pressure and flow. Harris and Habgood13 have shown that the flow rate, Fz, of carrier gas at any point z along the column is given by:
The ratio of probe-band velocity in the column to carrier velocity is given by the capacity factor k′ at infinite dilution. Where:
| k′ = βK = βe−ΔG/RT |
On this basis the probe band velocity is given by:
 is the flow rate at the column outlet expressed at temperature T0, T0 is the initial temperature of a temperature programme, e is the base of natural logarithms and Vm the retention volume for a non-retained substance. The retention time can be estimated by numerical integration of the expression over the series of isothermal increments which comprise the temperature programme. However, this requires an estimate of ΔH and ΔS. In isothermal IGC, the two unknowns are determined by running the chromatogram at two or more temperatures. The equivalent solution
in a temperature-programmed method would be to run the column at a series of different temperature ramps. Another unknown that masks the thermodynamic parameters is the phase ratio, β. Whilst the surface area of the solid can be estimated by, for example, BET methods, the surfaces of interest are mostly heterogeneous and the probe interacts mostly with the most active sites. An estimation of β then requires previous knowledge of the unknown sample and will differ between probes if they are adsorbed at a distinct set of sites.
is the flow rate at the column outlet expressed at temperature T0, T0 is the initial temperature of a temperature programme, e is the base of natural logarithms and Vm the retention volume for a non-retained substance. The retention time can be estimated by numerical integration of the expression over the series of isothermal increments which comprise the temperature programme. However, this requires an estimate of ΔH and ΔS. In isothermal IGC, the two unknowns are determined by running the chromatogram at two or more temperatures. The equivalent solution
in a temperature-programmed method would be to run the column at a series of different temperature ramps. Another unknown that masks the thermodynamic parameters is the phase ratio, β. Whilst the surface area of the solid can be estimated by, for example, BET methods, the surfaces of interest are mostly heterogeneous and the probe interacts mostly with the most active sites. An estimation of β then requires previous knowledge of the unknown sample and will differ between probes if they are adsorbed at a distinct set of sites.
Exploration of the theory clearly demonstrates the role of ΔHads and ΔSads in determining retention times and their values are clearly convoluted into the chromatographic pattern obtained. However their extraction from the data would require multiple chromatograms and they are clearly more readily determined by the accepted isothermal methods. The goal of the multiple probe, temperature programmed method is to rapidly generate a chromatogram that can be used to distinguish surfaces with different adsorptive properties. Further interpretation of the chromatograms will, at this stage, be limited to comparisons of relative retention and cannot readily be extended to the determination of fundamental thermodynamic parameters.
Experimental conditions
Chromatographic conditions
Chromatograms were recorded using a Pye 204 gas chromatograph equipped with a flame ionisation detector. Samples were packed into 1 m long 2 mm internal diameter glass columns and the packing material was held in place with plugs of silanized glass wool. A linear temperature programme was used starting at 60 °C and ending at 300 °C, with a temperature ramp of 10 °C min−1. The lower temperature was constrained by the instability of the oven at temperatures close to ambient and the need to stabilise the temperature rapidly to optimise measurement cycle time. The upper temperature was limited by the chromatographic systems used. Modern instrumentation allows routine extension of the upper limit to 450 °C and the lower limit to sub-ambient temperatures, however care must be taken to avoid irreversible thermal modification of the surface under study. The carrier gas was He (99.999%, Air Products) at a flow rate of 4 mL min−1.Headspace samples of individual probes or probe mixtures were taken from 2 mL sample bottles sealed with silicone rubber septa. Where necessary, headspace samples were further diluted with nitrogen in a 100 mL glass bottle fitted with a silicone rubber septum. Sample volumes and dilution factors were adjusted to maintain peak size within a factor of 10–100 of the detector detection limit. This was assumed to represent infinite dilution both because of the small quantities injected and the invariance of retention time with amount injected within this range. Injections onto the IGC columns were made using a 50 μL gas-tight syringe (Hamilton).
Adsorbent preparation
Two commercial non-graphitised carbon blacks were selected for this feasibility study. These materials were E460 (specific surface area 87 m2 g−1) and ETP (specific surface area 130 m2 g−1) from Cabot Corporation, (Leuven, Belgium) they will be referred to as CB-1 and CB-2 respectively. The finely divided carbon black materials under study were highly retentive and led to unacceptably long retention times for the probes of interest. The adsorbents were therefore presented mixed with an ‘inert’ diluent. The diluent chosen was a deactivated acid washed silica, Chromosorb G, AW DMCS, 100/120 mesh (Jones Chromatography) of low surface area (2 m2 g−1). The diluent served to allow the presentation of a constant sample surface area and to reduce the pressure differential across the column. The influence of the diluent is discussed in more detail below.Results and discussion
Selection of the probe set
The usefulness of a wide range of probes for the characterisation of carbon black materials was assessed. These were selected as volatile compounds with a wide range of functionalities. A subset of 25 of these probes that exhibited desirable chromatographic properties was identified and used in the characterisation of the carbon blacks, these are listed in Table 1. A reduced data set, consisting simply of the retention times expressed as retention indices for each probe has been used in this exploratory study.| Rohrschneider probes | McReynolds' probes |
|---|---|
| Benzene | Benzene |
| Ethanol | Butanol |
| 2-Butanone | Pentan-2-one |
| Nitromethane | Nitropropane |
| Pyridine | Pyridine |
| 2-Methyl-2-pentanol | |
| Iodobutane | |
| 2-Octyne | |
| Dioxane | |
| cis-Hydrindane |
Temperature programmed, multiple probe studies of chromatographic stationary phases are not new to chromatography. Analytical gas chromatographic stationary phases are marketed using McReynolds′ constants,15 which are chromatographic retention indices of a widely accepted probe set (an extension of the work of Rohrschneider16). A related method has been introduced by Grob17,18 that allows the effects of column ageing to be examined from the temperature programmed retention characteristics of a multiple probe set. This study attempts to extend these techniques to IGC characterisation of solid surfaces and to determine the characteristics required of a probe set.
It is useful to examine the rationale for the choice of probes used by McReynolds and Grob as they give insight into the probes that might prove more generally useful in IGC of solids. Both approaches use n-alkanes, which act as internal reference markers and counter the effects of variation of experimental parameters through the use of retention indices. The McReynolds′ probes are designed to assess dipole–dipole, dipole–induced dipole, dispersion, electron donor acceptor and proton donor acceptor interactions. However each probe interacts with the surface by more than one mechanism and careful interpretation is required. In the context of gas–solid chromatography where retention can frequently be very strong if not irreversible, it is unlikely that there are many instances where the McReynolds probe set could simply be used without modification. It is also important that the method should demonstrate a degree of reproducibility significantly tighter than the differences to be observed between samples.
Carbon blacks are composed of aggregates of near-spherical particles with diameters of a few tens of nanometers. Their surfaces have a graphite-like component, although there is a great deal more disorder than in a true graphite. There is also a degree of functionality at the surface that includes alcoholic/phenolic, carboxylic acid, ketone and lactone residues.19–23 The materials have a variety of applications and have the advantage that they have previously been characterised by IGC.24,25
n-Alkanes and McReynolds’ probes were examined for their suitability in carbon black surface characterisation. For the experimental conditions defined above, n-butane, n-pentane, n-hexane and n-heptane all eluted during the linear ramp of the temperature programme on the carbon black columns. Of the McReynolds’ probes, n-butyl iodide gave rise to an extremely broad peak for which retention time measurements were associated with large errors. n-Butyl chloride was therefore substituted. The alkyl amines and alcohols also gave excessively broad peaks, some with more than one maximum, features which were tentatively be ascribed to on-column reactions in the presence of the silica-based diluent.
The probe set contained members of homologous series, rather than being limited to a simple McReynolds set of different interaction types. In temperature programmed gas chromatography, members of homologous series generally elute at temperatures proportional to their carbon number. This linearity is seen as a useful diagnostic tool, both in terms of the relative slopes of plots for different homologous series and any deviation from linearity that may occur, possibly indicative of effects such as size exclusion.
The influence of the sample diluent
A major influence on retention will be the adsorbent surface area. In a comparison of surfaces the influence of surface area can be normalised by presenting the same surface area of sample to the probes. At the same time, chromatographic conditions of pressure profile and flow rate are ideally kept constant. These ideals can be approached by diluting the sample under investigation in a ‘non-retentive’ chromatographic packing material. Possible diluents are low surface area silicas such as acid washed diatomaceous earth (Chromosorb W-AW). These materials can be rendered even more ‘inert’ by surface modification, replacing polar surface silanols with methyl groups (such as Chromosorb G AW DMCS). Such materials are commercially available and have been used in this study. They have been chosen to have an ideal particle size for packed column chromatography, to be thermally stable up to and above 300 °C, to have relatively inert surfaces and a low surface area (2 m2 g−1). Many of the selected probes (see Table 2) elute at the dead time on Chromosorb G (are effectively un-retained) however a number of probes do show significant retention on columns filled only with the diluent and such effects must be taken into consideration and represent an important focus of this feasibility study.| Probea | bp/K | Probe | bp/K | ||
|---|---|---|---|---|---|
| a *Indicates those probes that showed a significant interaction with the diluent. | |||||
| 1 | Butane | 273 | 14 | Ethyl acetate * | 350 |
| 2 | Pentane | 309 | 15 | Nitropropane * | 404 |
| 3 | Hexane | 342 | 16 | 2,2-Dimethylbutane | 323 |
| 4 | Heptane | 371 | 17 | 2,3-Dimethylbutane | 331 |
| 5 | Cyclohexane | 354 | 18 | 2-Methylpentane | 335 |
| 6 | Benzene | 353 | 19 | Ethyl bromide * | 312 |
| 7 | Toluene | 384 | 20 | Thiophene | 357 |
| 8 | Acetone * | 329 | 21 | Dioxane * | 374 |
| 9 | Methyl ethyl ketone * | 353 | 22 | Propionitrile * | 370 |
| 10 | Pentan-2-one * | 378 | 23 | n-Butyl chloride * | 351 |
| 11 | Pinacolone * | 379 | 24 | Pyridine * | 388 |
| 12 | Diethyl ether * | 308 | 25 | Butyronitrile * | 389 |
| 13 | Acetonitrile * | 355 | |||
The carbon blacks examined in this study have significantly larger specific surface areas and significantly smaller particle sizes than the 100/120 mesh Chromosorb G AW DMCS. Each column contains approximately the same mass (and therefore surface area) of diluent, and if the appropriate dilution factor is used each column will contain the same surface area of the carbon black sample. The differences in the chromatographic behaviour of the columns are therefore due to their carbon black content and the effects of the diluent should be common to all columns prepared in this manner.
Data presentation and interpretation
A useful way of viewing the data is to plot retention on a particular stationary phase against boiling point, as in Fig. 1. In analytical chromatography, non-polar ‘boiling point’ columns give retention times almost proportional to boiling point (for linear temperature programmes). However strong interactions between the adsorbate and adsorbent distort this linear relationship. Linear relationships are generally observed for the n-alkanes whose adsorbate–adsorbate and adsorbate–adsorbent interactions are dominated by dispersion forces and dipole-induced dipole interactions. Stronger interactions can be seen as deviations from the n-alkane line. This can be put in quantitative terms by measuring the extent of the deviation. The strength of the interaction can be estimated by finding the carbon number of an n-alkane that would co-elute and that of an n-alkane of the same boiling point. The difference in carbon number is a measure of the strong interaction and provides useful insight into surface adsorptive behaviour. Care must be taken when interpreting these plots as the boiling process represents a phase transfer to the gas phase from a liquid phase that is different for each probe. In some instances, there may be a significant interaction such as hydrogen bonding in the liquid phase with well-known effects on boiling point. Desorption, on the other hand involves transfer to the gas phase from the same adsorbent in each case (although different adsorptive sites may be involved). | ||
Fig. 1
Probe retention temperatures on Chromosorb W-AW plotted against probe boiling point. ■ n-Alkanes (C4–C7); Δ McReynolds’ probes; × branched alkanes; ![[Cross with vertical bar through centre]](https://www.rsc.org/images/entities/char_e113.gif) nitriles; ◇ ketones; + other. nitriles; ◇ ketones; + other.
| ||
Instead, we have used a second sample as a reference column. Fig. 2 shows the retention of the probe set on the two carbon black samples (with diluent), Fig. 2a for CB1 and Fig. 2b for CB2. The n-alkanes: butane, pentane, hexane and heptane (indicated by filled squares) fall, as expected on a straight line. All the other probes lie below this line indicating that the surface has non-polar sites where the hydrocarbons are relatively strongly adsorbed. Not all the probes have been identified in this plot which simply illustrates the non-linear nature of the data, however the retention indices of each probe are given individually, below (see Table 3).
 | ||
Fig. 2
Probe retention temperatures on (a) CB-1 and (b) CB-2 plotted against probe boiling point. ■ n-Alkanes (C4–C7); Δ McReynolds’ probes; × branched alkanes; ![[Cross with vertical bar through centre]](https://www.rsc.org/images/entities/char_e113.gif) nitriles; ◇ ketones; + other. nitriles; ◇ ketones; + other.
| ||
| KI CB-1 | KI CB-2 | ΔKI | |
|---|---|---|---|
| McReynolds' probes: | |||
| Benzene | 599 | 550 | 49 |
| Pentan-2-one | 633 | 566 | 66 |
| Nitropropane | 675 | 540 | 136 |
| Pyridine | 732 | 641 | 91 |
| Dioxane | 602 | 547 | 55 |
| Alkanes: | |||
| 2,2-Dimethylbutane | 545 | 438 | 108 |
| 2,3-Dimethylbutane | 576 | 510 | 66 |
| 2-Methylpentane | 583 | 533 | 50 |
| Cyclohexane | 529 | 473 | 55 |
| Nitriles: | |||
| Acetonitrile | 601 | 428 | 174 |
| Propionitrile | 572 | 466 | 106 |
| Butyronitrile | 629 | 548 | 81 |
| Ketones: | |||
| Acetone | 526 | 419 | 107 |
| Pentan-2-one | 633 | 566 | 66 |
| Methyl ethyl ketone | 578 | 493 | 85 |
| Pinacolone | 622 | 491 | 131 |
| Other: | |||
| Ether | 524 | 480 | 44 |
| Ethyl bromide | 428 | 435 | −6 |
| Acetyl acetate | 616 | 527 | 89 |
| n-Butyl chloride | 595 | 565 | 29 |
| Thiophene | 613 | 598 | 15 |
| Toluene | 704 | 651 | 53 |
Fig. 3 shows the retention for the probes on CB-1 versus their retention on CB-2. This is a simple method for differentiating two materials. Identical columns should give identical retention and the data would lie on a diagonal line of slope 1 (indicated in the Figure). The influence of the diluent is removed as the same surface area is present on both columns. Difference in retention properties of the two surfaces lead to deviations from this line. A simple surface area effect would lead to a simple change in slope of the line. However the probe mixture presents a range of differences. It is immediately clear that all probes except acetonitrile are more strongly retained on column 2 than on column 1. However there is more information in the data that can reveal information about the surfaces and information about the suitability of the probe set for surface characterisation.
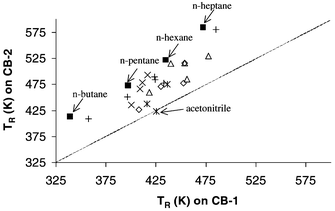 | ||
Fig. 3
Probe retention temperatures on CB-1 plotted against retention temperatures on CB-2. ■ n-Alkanes (C4–C7); Δ McReynolds’ probes; × branched alkanes; ![[Cross with vertical bar through centre]](https://www.rsc.org/images/entities/char_e113.gif) nitriles; ◇ ketones; + other. nitriles; ◇ ketones; + other.
| ||
Close examination of this plot reveals that the probes most distant from the line defined by the n-alkanes, are acetone, acetonitrile, proprionitrile and nitropropane. It is at this point that we must consider the effect of the diluent. Acetonitrile is the one probe that lies on the diagonal line of slope 1. The retention temperatures of acetonitrile on both the CB-1 and CB-2 columns are effectively the same as on the pure diluent. The influence of the carbon blacks on acetonitrile retention is negligible in this experiment. Of the other polar probes, proprionitrile shows the least difference in retention between the two columns however this retention temperature difference is 10 K, showing a significant influence of CB-1, presumably due to the alkyl moiety.
Returning to Fig. 2, it is clear that probe interaction with the carbon black surfaces is dominated by non-polar interactions. Another interesting feature of the plot is that branched-chain alkanes, cycloalkanes and the aromatics benzene and toluene lie below the n-alkane line being less well retained. This suggests that steric factors play a role with retention being dependent on how close the probe molecule can lie to the active site on the carbon black surface. 2,2-Dimethyl butane is the least strongly retained of the isomers and n-hexane the most strongly retained, indicating that steric factors are probably influencing retention and that their importance differs between the two phases. Probably for the same reason, the difference in cyclohexane retention between the phases is considerably less than that for n-hexane. It is expected that the most adsorptive sites are at ledges at the edge of graphitic planes. The higher porosity of CB-2 relative to CB-1 is indicated by their relative BET (N2) surface area and standard thickness surface area measurements.26 This porosity difference may contribute to the observed differences via selective penetration of the pores by alkanes, however further interpretation would at this stage be speculative.
In Table 3 the retention times of the probes have been expressed as Kovats indices (KI, i.e. the carbon number of the n-alkane that would co-elute with the probe, multiplied by 100). The differences in these indices between the two columns are shown in the third column as ΔKI values. The data suggest that the surface of CB-1 has a greater degree of functionality with more proton donor capability. This is indicated by the higher Kovats indices for all of these probes (except ethyl bromide, which is not a proton acceptor) on CB-1 and especially for the strong proton acceptor probe, pyridine. The branched and cyclic alkane probes highlight some interesting steric information. ΔHads is expected to increase, the closer the molecule can lie to the surface. 2,2 Dimethylbutane, and cyclohexane are least able to lie flat on the surface, with 2,3 dimethylbutane less restricted and 2 methylpentane able to lie flat and having the closest retention time to that of hexane. CB-1 is more retentive for all of these probes by at least 0.5 of an effective carbon number and by a whole carbon number for 2,2 dimethylbutane. This behaviour presumably reflects surface morphology, however further interpretation would be highly speculative.
The nitriles are seen to be retained well beyond their carbon numbers, the effect being strongest for acetonitrile. Retention is presumed to have two contributions, the major effect being polar interactions with polar surface sites (on the silica-based diluent). The minor effect is due to the alkyl chain interaction with graphitic regions on the carbon blacks. The difference in this effect between the two surfaces suggests that there are more strongly adsorbing non-polar sites per unit surface area on the CB-1 than CB-2. A similar effect is seen with the ketones. Despite the strong influence of the diluent on polar probe retention, Fig. 3 shows that the probes are nonetheless useful for sample characterisation and one sample whose retention is not influenced by the samples (acetonitrile) provides a useful reference point.
Overview
Multiple probe, temperature programmed IGC has been demonstrated as a valid method for rapid characterisation of solid surfaces. Two carbon blacks have been examined using the technique allowing a probe set to be developed, suited to related materials and approaches to data interpretation to be examined. It was found necessary to use a sample diluent to obtain a useful range of retention temperatures for the probe set. Some candidate probes were rejected due to ‘unsatisfactory’ chromatography. These probes would be valuable in the estimation of acid–base and hydrogen bonding type interactions and may well prove to be of value in the characterisation of other types of surface. However, despite using a reduced probe set the two surfaces were very clearly differentiated by the method.The diluent used, Chromosorb G-AW-DMCS, shows significant polarity and direct comparison of column retention temperatures was used to eliminate its effect. A better diluent would contribute a smaller surface area and be thermally stable up to the temperature limits of the chromatographic system used. Choice of diluent will depend on the nature of the surface under investigation.
The technique does not yield quantitative measures of thermodynamic adsorption parameters, however the data can be interpreted in terms of the relative retention of different probes. Surface heterogeneity must be borne in mind and the possibility that different probes interact with different surface sites cannot be excluded.
It can be concluded that it is useful to select probes to span the range of adsorptive interaction types defined by Rohrschneider and McReynolds. However, probes should be rejected if they are irreversibly adsorbed or react at the sample surface. The nature of the probe set can only be defined for a particular batch of sample types. Ideally the chromatography would be performed in the absence of a diluent. The use of a diluent, however, extends the applicability of the technique to highly adsorptive materials and to fine powders. The diluent should be selected to have minimum influence on retention, by minimising its surface area and the adsorptive activity of its surface.
The multiple probe temperature programmed approach to IGC, presented in this paper is ideally suited to the comparison of two or more surfaces expected to have similar properties. Batches of adsorbents could readily be screened to determine whether their behaviour lies outside an expected norm. On the basis of this feasibility study, further work on more diverse sample sets is envisaged using chemometric classification tools for data analysis.
Acknowledgements
The authors would like to thank the EPSRC for a PhD studentship (P. McLaughlin) and AEA Technology for the donation of equipment.References
- L. W. Bruch, M. W. Cole and E. Zaremba, Physical Adsorption: Forces and Phenomena, Oxford Science Publications, Clarendon Press, Oxford, 1997 Search PubMed.
- G. A. Somorjai, Chem. Rev., 1996, 96, 1223 CrossRef CAS.
- J. R. Conder and C. L. Young, Physicochemical Measurements by Gas Chromatography, Wiley, Chichester, 1979 Search PubMed.
- S. A. Greene and H. Pust, J. Phys. Chem., 1958, 62, 55 CrossRef CAS.
- N. A. Katsanos, A. Lycourghiotis and A. Tsiatsios, J.Chromatogr. Sci., 1971, 9, 9.
- N. A. Katsanos, R. Thede and F. Roubani-Kalantzopoulou, J. Chromatogr., A, 1998, 795, 133 CrossRef CAS.
- C. F. Poole and S. K. Poole, Chromatography Today, Elsevier, Amsterdam, 1991 Search PubMed.
- B. Charmas and R. Leboda, J. Chromatogr., A, 2000, 886, 133 CrossRef CAS.
- M. Pyda and G. Guiochon, Langmuir, 1997, 13, 1020 CrossRef CAS.
- M. Pyda, B. J. Stanley, M. Xie and G. Guiochon, Langmuir, 1994, 10, 1573 CrossRef CAS.
- H. Balard, Langmuir, 1997, 13, 1260 CrossRef CAS.
- N. A. Katsanos, E. Iliopoulou, F. Roubani-Kalantzopoulou and E. J. Kalogorirou, Phys. Chem.B, 1999, 103, 10228 Search PubMed.
- W. E. Harris and H. W. Habgood, Programmed Temperature Gas Chromatography, John Wiley & Sons, New York, 1966 Search PubMed.
- L. R. Snyder, Principles of Adsorption Chromatography, Edward Arnold Publishers, London, 1968 Search PubMed.
- W. O. McReynolds, J. Chromatogr. Sci., 1970, 8, 685 CAS.
- L. Rohrschneider, J. Chromatogr. Sci., 1973, 11, 160 CAS.
- K. Grob, G. Grob and K. Grob, J. Chromatogr., 1978, 156, 1 CrossRef CAS.
- K. Grob, G. Grob and K. Grob, J. Chromatogr., 1981, 219, 13 CrossRef CAS.
- E. Papirer, R. Lacroix and J.-B. Donnet, Carbon, 1996, 34(12), 1521 CrossRef CAS.
- B. Sahouli, S. Blacher, F. Brouers, H. Darmstadt, C. Roy and S. Kaliaguine, Fuel, 1996, 75(10), 1244 CrossRef CAS.
- H. Darmstadt, C. Roy, S. Kaliaguine, Xu Guoying, M. Auiger, A. Tuel and V. Ramaswamy, Carbon, 2000, 38, 1279 CrossRef CAS.
- H. Naono, M. Shimoda, N. Morita, M. Hakuman, K. Nakai and S. Kondo, Langmuir, 1997, 13, 1297 CrossRef CAS.
- J. B. Donnet and E. Custodero, Carbon, 1992, 30(5), 813 CrossRef CAS.
- E. Papirer, E. Brendle, F. Ozil and H. Balard, Carbon, 1999, 37, 1265 CrossRef CAS.
- M. Domingo-Garcia, F. J. Lopez-Garzon, C. Moreno-Castilla and M. J. Pyda, Phys. Chem. B, 1997, 101(41), 8191 Search PubMed.
- J. M. Pena, N. S. Allen, M. Edge, C. M. Liauw, F. Santamaria, O. Noiset and B. Valange, J. Mater. Sci., 2001, 36, 2885 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |



