A fluorescent PEBBLE nanosensor for intracellular free zinc
James P. Sumnera, Jonathan W. Aylottb, Eric Monsona and Raoul Kopelman*a
aDepartment of Chemistry, University of Michigan, Ann Arbor, MI 48109-1055, USA. E-mail: kopelman@umich.edu; Fax: +1 734 936 2778; Tel: +1 734 764 7541
bAnalytical Science Group, Department of Chemistry, University of Hull, Hull, UK HU6 7RX. E-mail: J.W.Aylott@hull.ac.uk; Fax: +44 (0) 1482 466416; Tel: +44 (0) 1482 465459
First published on 4th December 2001
Abstract
The development and characterisation of a fluorescent optical PEBBLE (Probe Encapsulated By Biologically Localised Embedding) nanosensor for the detection of zinc is detailed. A ratiometric sensor has been fabricated that incorporates two fluorescent dyes; one is sensitive to zinc and the other acts as a reference. The sensing components are entrapped within a polymer matrix by a microemulsion polymerisation process that produces spherical sensors that are in the size region of 20 to 200 nm. Cellular measurements are made possible by the small sensor size and the biocompatibility of the matrix. The effects of reversibility, photobleaching and leaching have been examined, as well as the selectivity towards zinc over other cellular ions such as Na+, Ca2+, K+, and Mg2+. The dynamic range of these sensors was found to be 4 to 50 μM Zn2+ with a linear range from 15 to 40 μM. The response time for the PEBBLE is less than 4 s and the sensor is reversible. In addition, the nanosensors are photostable and leaching from the matrix, determined using a novel method, is minimal. These sensors are capable of real-time inter- and intra-cellular imaging and are insensitive to interference from proteins.
Introduction
Zinc is the second most abundant trace element in humans behind iron. Most of the zinc found in the body is bound to proteins such as carbonic anhydrase or zinc finger proteins. Zinc has been implicated in Alzheimer’s disease,1,2 Parkinson’s disease1 and as a neuromodulator.3 In addition, zinc has been found to perform structural functions in some enzymes and found to act as a Lewis acid in others.4 The amount of zinc can range throughout the body from nanomolar concentrations in the cytosol of certain cells to millimolar concentrations in some neuronal vesicles.5 However, the full extent of zinc’s purpose in the body remains unclear. Therefore improved analytical measurement techniques are required to further elucidate the role of zinc in vivo.The availability of zinc-sensitive dyes is scarce compared to those accessible for detecting calcium or magnesium. The most commonly used zinc dyes are quinoline derivatives, namely N-(6-methoxy-8-quinolyl)-p-toluene sulfonamide (TSQ) and (2-methyl-8-P-toluenesulfonamido-6-quinolyloxy) acetic acid (Zinquin). Both of these dyes are UV excitable, which can cause increased autofluorescence and damage in cellular work. In addition, they form both 1∶1 and 1∶2 zinc complexes, which can complicate calibrations.6 Other fluorescent probes for measuring zinc have been developed utilising enzymes and proteins. Some of the probes are based on ‘zinc finger’ proteins, but these are not viable options for use in cellular work6,7 because zinc finger proteins have a tendency to become oxidised and the experiments must be performed under inert conditions. Systems based on mutant carbonic anhydrase, combined with a fluorescent inhibitor as a means for signal transduction, have recently been described in the literature.8,9 Two common magnesium and calcium dyes, Mag-Fura-210 and Mag-Fura-5,11 have also been used to quantitate zinc. However, because these dyes are sensitive to calcium or magnesium ions, the reported experiments were performed in the absence of these ions and therefore cannot be applied to measurements in the cellular environment. Two fibre optic sensors for zinc determination based on 9-(1′,4′,7′,10′,13′- pentaazacyclopentadecyl) methylanthracene and a modified chitosan (an aminopolysaccharide) molecule have been reported. However, due to reversibility issues12 and the solvent system,13 their usefulness is limited. There have been some very recent literature reports describing the synthesis of new zinc fluorophores that show impressive results, but these dyes are not yet commercially available.14 One dye that is commercially available, Newport Green, has many advantages compared to previously described probes. It is excitable in the visible range (506 nm), shows a reversible response to zinc, exhibits minimal interference in the presence of cellular ions and is water soluble. The dye is based on a fluorescein derivative that utilises a di-(2-picoly)amine chelator. Newport Green has a limited sensitivity and dynamic range but has been used previously to measure low micromolar concentrations of zinc in cellular experiments.11,15 For these reasons it was chosen as the recognition dye used to quantitate zinc in this work.
Most of the quantitative measurements of zinc reported in the literature have been carried out by histochemical staining of cellular samples.16 This assay type of procedure may result in many artefacts such as organelles or proteins inadvertently affecting the dye by non-specific binding.17 In addition, the dyes are often toxic to their surrounding environment and therefore not appropriate for real-time in vivo monitoring.18 Zinc-selective ion-selective electrodes (ISE) have been reported19 but the size of ISEs will cause serious physical perturbation to cells. Thus far no viable zinc sensor that is reliable for real-time intracellular biological measurements has been established. Nanoparticle sensors are the most suitable for measurements inside living cells.20–23 The recent development of Probes Encapsulated By Biologically Localised Embedding (PEBBLE) has the advantage of protecting the dye from the cell and vice versa,17,21 resulting in a non-invasive monitoring technique that has been successfully applied to measurements inside cells. The dyes themselves are encapsulated in a protective polyacrylamide matrix. This encapsulation decreases the artefacts that result from erroneous bindings or toxic complications. Although initial studies of PEBBLE were conducted with a polyacrylamide matrix, new sensor designs include the matrices decyl methacrylate22 and sol–gel.23 The ability to utilise multiple matrices allows both hydrophobic and hydrophilic dyes to be encapsulated. Furthermore, PEBBLE are less than 200 nm in diameter, which allows for specific localisation within the cells that would otherwise be unlikely for conventional histochemical staining. Because of their small size, PEBBLE have a fast response time and impose a minimal physical perturbation on the cells.
In this paper we report the development of an optical nanosensor that is sensitive to zinc. It relies on the co-immobilisation of two fluorescent dyes in a polymer matrix to produce a ratiometric sensor. To achieve this, a zinc sensitive dye, Newport Green, was utilised, as well as a reference dye, Texas Red bound to dextran. The Texas Red–dextran is unaffected by changing zinc concentrations, which makes it an ideal reference dye. Because both dyes are hydrophilic, polyacrylamide was used as the sensor matrix. To yield nanometer-sized sensors, the dyes were encapsulated within the polyacrylamide matrix, prepared using a microemulsion polymerisation technique. Sensor characteristics, immunity to interference, and stability are detailed in this paper.
Experimental and methods
Reagents
Acrylamide, N,N-methylenebis(acrylamide), N,N,N′,N′-tetraethylmethylenediamine (TEMED), polyoxyethylene (4) lauryl ether (Brij 30), N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), potassium phosphate dibasic, and potassium dihydrogen phosphate were purchased from Aldrich (Milwaukee, WI, USA). Ammonium persulfate, dioctyl sulfosuccinate (AOT), and bovine serum albumin (BSA) fraction X were purchased from Sigma (St. Louis, MO, USA). Hexanes were purchased from Fisher (Fair Lawn, NJ, USA). Ethanol was purchased from Pharmco (Brookfield, CT, USA). Zinc sulfate was purchased from Mallinckrodt (St. Louis, MO, USA). Newport Green dipotassium salt and Texas Red dextran were purchased from Molecular Probes (Eugene, OR, USA). All solutions were prepared from 18 MΩ water purified by a Barnstead 1 Thermolyne Nanopure II system.Acrylamide PEBBLE preparation
The polymerisation procedure was as described previously24 with minor changes. The polymer solution contained 2.7 g acrylamide, 0.8 g methylenebis(acrylamide), and 9 mL of 10 mM phosphate buffer pH 7.2. A 2 mL portion of this polymer solution was added drop-wise to a deoxygenated solution that contained 43 mL of hexanes, 1.59 g AOT, and 3.08 g Brij 30. A 50 μL portion of Newport Green (1 mg mL−1) and 50 μL of Texas Red–dextran (25 mg mL−1) were added to the solution. The solution was stirred under argon throughout the duration of the preparation. To initiate the polymerisation, 30 μL of 10% (w/v) ammonium persulfate solution and 15 μL of TEMED were added. The solution was stirred at room temperature for two hours to assure complete polymerisation. The hexanes were removed by rotary evaporation and the remaining solution was transferred to an Amicon ultra-filtration cell (Millipore Corp., Bedford, MA, USA). The surfactant, unreacted monomers and dye molecules were separated from the sensors using a 100 kDa filter under 10 psi of pressure. The PEBBLE were washed with 500 mL ethanol. The PEBBLE suspension was then passed through a suction filtration system (Fisher, Pittsburgh, PA, USA) with an 0.02 μm filter membrane. Again the PEBBLE were rinsed with another 500 mL of ethanol. The particles were collected when dry.Optics
Fluorescence data from PEBBLE was taken on an Olympus IMT-II (Lake Success, NY, USA) inverted fluorescence microscope. The spectra were acquired using an Acton Corp. spectrograph and with a liquid nitrogen-cooled Princeton Instrument 1024 × 256 charged-coupled device array (CCD) (Trenton, NJ, USA). The PEBBLE were excited with a mercury arc lamp (Olympus, Melville, NY, USA). The resulting excitation and emission light were filtered using an Omega Curv-o-matic blue filter cube (module XF-19, excitation filter 450–490 nm, long pass emission filter, 520 nm). Sensor measurements were also performed on a Fluoro-Max 2 spectrofluorimeter (ISA Jobin Yvon-Spex, Edison, NJ, USA) with the excitation and emission slits set at 2 nm bandwidth.PEBBLE calibration and reversibility
To calibrate the zinc-sensitive PEBBLE, 30–40 mg of the PEBBLE were collected in a 20 mL scintillation vial. To this an aliquot of 5.1 mM MOPS buffer (pH 7.13) was added to produce a 3 mg PEBBLE mL−1 buffer solution. Three mL of this suspension was transferred into a cuvette. Spectra were collected using the fluorimeter by exciting the sample at 506 nm and recording the resulting emission from 520 to 630 nm. An aliquot of 20 mM zinc nitrate was added after each successive spectrum was collected, allowing a period of 10 s for the sample to equilibrate. The samples were continuously stirred by a magnetic stir bar and held at 22 °C by a temperature controller. The detection limit was calculated as described previously.25 Briefly, regression analysis was performed on the data to obtain a line in the form of y = mx + b. The detection limit, defined as the minimal concentration that is statistically different from the blank, was calculated as xm (where xm = (ym - b)/m) when ym = y1 + 3sB, where y1 is the intensity value of the first point without analyte and sB is the standard deviation of the blank. The reversibility of the sensors was obtained using the 3 mg mL−1 PEBBLE suspension prepared as described previously. The fluorimeter was set to monitor the peak wavelengths of Newport Green (530 nm) and Texas Red–dextran (600 nm) every second with a 0.1 s acquisition time. Additions of zinc nitrate and the chelator EGTA were used to oscillate the zinc concentration.PEBBLE interference
Solutions of biologically relevant ions such as Na+, K+, Mg2+, and Ca2+ were prepared. The experiment was conducted using the methodology as described for the zinc calibration, with the exception that the zinc solution was substituted with the respective ion solutions. An assay was also conducted to measure the interference due to non-specific binding of proteins to the fluorophore. Solid BSA (Fraction X) was dissolved in water to produce a 10% w/v solution. Aliquots of the BSA solution were added to either a 3 mg mL−1 PEBBLE suspension or a 125 nM Newport Green dye solution and the resulting fluorescence was monitored.PEBBLE ratiometric check and photostability
The PEBBLE’s ability to be ratiometric with changing illumination intensity was conducted on an inverted fluorescence microscope. A 3 mg mL−1 zinc sensor suspension was prepared as described above. The suspension was placed in a glass container on the microscope. Neutral density filters were used to modulate the excitation power. Aliquots of a 20 mM zinc nitrate solution were added and the experiment was repeated. A photobleaching study was conducted with a similar experimental set-up. The sample was under continuous illumination using an Argon Ion laser (488 nm) while the emission was collected using an avalanche photodiode and recorded every 0.4 s with a digital oscilloscope for more than an hour. The Newport Green emission was selected using a 535 ± 5 nm band pass filter, the Texas Red–dextran, a 610 nm long pass filter. The excitation illumination power was measured with a Fieldmaster Laser Power Meter (Coherent, Inc., Auburn, CA, USA).PEBBLE leaching
Leaching was examined using two methods. Firstly a suspension containing zinc-sensitive PEBBLE (7.7 mg mL−1 of Newport Green PEBBLE), MOPS (9.4 mM, pH 7.19), and BSA (10% w/v) was prepared. BSA was present in the suspension to act as a marker to determine the leaching of Newport Green from the PEBBLE because the protein causes a dramatic signal increase when in the presence of the free dye. Therefore if leaching occurred there would be a significant increase in fluorescence intensity of the suspension. A significant change in the suspension fluorescence was determined to be an increase greater than 5% (3 times the standard deviation of the blank). The absolute change in fluorescence of the suspension was compared to the change in the intensity observed with the free dye in buffer and in a 0.1% BSA solution. A calibration of intensity versus concentration for Newport Green dye was constructed (see Fig. 1). This graph contains two lines that intersect at the origin. A calculated 5% change in PEBBLE suspension fluorescence was correlated to the difference in fluorescence between the two lines constructed for the Newport Green dye. Once this value was correlated to the difference on the graph, the concentration of Newport Green that had leached out could be determined from the graph by extrapolation to the x-axis. This Newport Green concentration was then compared to the dye concentration in the PEBBLE so as to calculate the percent that leached out. The fluorescent emission of the Newport Green PEBBLE suspension was monitored at various time points over two days.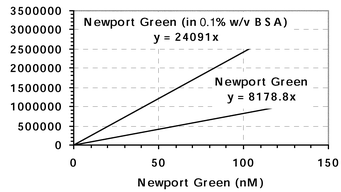 | ||
| Fig. 1 Plot of intensity versus dye concentration. This graph was used to calculate the amount of dye in the sensors. In addition, intensity increases observed in the leaching experiments were compared to the difference between the dye in BSA and in buffer, from this graph. | ||
A second leaching study was performed by preparing a 5 mg PEBBLE suspension in TRIS buffer (10 mM, pH 7.2), measuring the initial fluorescence, and transferring the suspension to stir in an Amicon Ultra filtration set-up, with a 5000 kDa cut-off membrane filter. Three mL of the suspension was then filtered, analysed, and then replaced back into the Amicon. Over the course of two days, the filtrate was analysed at various time intervals. The amount of leaching was ascertained by dividing the filtrate intensity by the initial PEBBLE intensity.
Particle sizing
Particles were first suspended from powder at a concentration of 15 mg PEBBLE per mL of a running buffer (400 ppm SDS, 200 ppm sodium azide, 100 ppm NaCl in ultra-pure water filtered to 0.1 μM) and sonicated for one-half to one hour, to reduce aggregation. Samples were filtered through a 1.6 μM glass fibre syringe filter (13 mm diameter, CF/A grade; Whatman) before injection into the 20 μL sample loop of an asymmetric field-flow fractionation (AFFF) liquid separation system (Consenxus, Germany). The output of the separation channel was directed into a multi-angle static light scattering instrument (Dawn EOS-Wyatt Technologies Corp., Santa Barbara, CA, USA) where fifteen detectors simultaneously measure the scattered intensity every 0.5 s. The multi-angle data was used at each time point to calculate the particle number density and volume within that sample slice (assuming the validity of the Raleigh–Gans–Debye approximation, i.e, spherical scatterers with an index of refraction close to that of the aqueous phase). This information was then accumulated into a histogram of differential mass fraction vs. rms radius for all identified sample peaks, without making any assumptions about the distribution of sizes present in the sample.Results and discussion
Calibration
Zinc sensitive polyacrylamide sensors have been prepared containing immobilised Newport Green, and Texas Red–dextran as a reference dye. The sensor response to zinc is shown in Fig. 2. The maximal percent increase in the ‘Intensity Ratio’ is 50%, which is less than observed with the Newport Green dye in solution (which had an increase of ∼250%). The data indicates a linear range between 15–40 μM Zn2+, with a detection limit of 4 μM. Although the sensor has a low affinity for zinc, the PEBBLE may be useful for neural analysis where zinc levels can reach micromolar concentrations. It remains unclear as to why there is a decrease in the response of the sensor compared to the naked dye. One possibility is that the matrix is reducing the sensitivity of the dye. | ||
| Fig. 2 A 3 mg mL−1 PEBBLE suspension calibrated with zinc nitrate. The samples were excited at 508 nm on the fluorimeter and the emission spectra were collected. From the emission spectra the ratios of intensities were collected. | ||
Reversibility
The reversibility of this system was tested using ethylene glycol-bis(beta-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA). EGTA, a common metal ion chelator, has a dissociation constant for zinc in the nanomolar range, thus it is orders of magnitude more tightly binding to zinc than Newport Green (Kd ≈ 1 μM). Fig. 3 shows that as the zinc ion binds to Newport Green encapsulated in the PEBBLE, the intensity of the dye increases and, conversely, as the zinc ion is removed by the chelator, EGTA, the fluorescence returns to its original state. The data indicates that the sensor configuration is reversible. In addition, it shows that the sensors respond to zinc ion concentrations on a time scale (<4 s) that makes them applicable to real time imaging. In addition to demonstrating the response and reversibility of the system, it also shows that the reference dye, Texas Red–dextran, is insensitive to the analyte ion. (Note: The gradual increase in the Newport Green emission is a result of successive qualitative additions of the zinc solution to the sample.) The apparent response of the reference dye to changes in zinc concentration is a result of the overlap in the emission peaks. Although the presence of FRET can not be discounted, fitting the emission curves to lorentzian profiles indicates that simple superposition, with no energy transfer, is consistent with the spectroscopic results. | ||
| Fig. 3 A 3 mg mL−1 PEBBLE suspension was tested with successive quantitative aliquots of 119 mM Zn(NO3)2 and 119 mM EGTA. The peak emissions of both dyes were monitored (530 nm Newport Green and 600 nm Texas Red–dextran) as the samples were excited at 508 nm. Once a steady baseline was reached an aliquot of zinc was added which produced an increase in Newport Green fluorescence. (There is also some increase in the Texas Red–dextran peak due to the intrinsic overlap of the dye peaks.) After the fluorescence stabilised, an aliquot of EGTA was added to remove the zinc ions, resulting in a decrease in Newport Green fluorescence. This procedure was repeated two more times. These experiments were conducted on a fluorimeter. | ||
Interference
Newport Green, either as the free dye or entrapped in the sensor matrix, exhibits little or no response to expected levels of possible cellular interferents such as Na+, K+, Mg2+, and Ca2+ as shown in Table 1. The experiments show that these ions will cause minimal or non-existent artefacts resulting from non-analyte signal in cellular measurements. Although Newport Green has good selectivity over intracellular ions, the dye itself is prone to artefacts resulting from non-specific binding of proteins, such as bovine serum albumin (BSA), as shown in Fig. 4. Monitoring the peak of Newport Green at 530 nm there is a substantial increase in the peak intensity with each successive addition of BSA. In addition to the increase of intensity, there is also a 4 nm shift in peak wavelength. The PEBBLE containing the Newport Green dye, however, are unaffected by the additions of BSA, which is confirmed by the peak wavelength remaining the same. As little as 0.02% BSA caused a major change in the Newport Green dye intensity (i.e. >200% increase) but the intensity of the Newport Green embedded in the sensor remained unchanged, even at BSA concentrations above 0.10%. This suggests that the BSA is unable to enter the PEBBLE and come into contact with the dye, so as to cause perturbation. This concept of a protective matrix is a prime advantage of the PEBBLE sensor compared to free dye, especially for cellular analysis. One of the drawbacks of fluorescence intracellular analysis is demonstrated in part by the fact that the dyes show no interference in neat (one analyte samples) but are subject to artificial fluorescent responses in a biological environment when a multitude of proteins and enzymes are present. The matrix of the PEBBLE eliminates this problem, implying that the in vitro calibrations would remain valid for measurements in an intracellular environment. Additionally, the cellular environment is protected from the toxicity of the dye by the matrix. Although, there has been no reported toxicity of Newport Green, the studies of its harmful properties are limited.15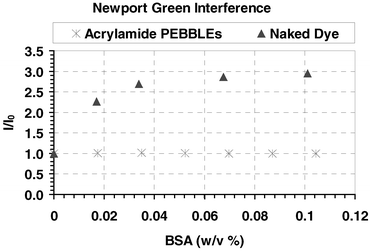 | ||
| Fig. 4 Peak emission intensity of Newport Green, 530 nm, monitored on a fluorimeter. Spectra were acquired after each successive aliquot of a 10% (w/v) BSA solution. The peak intensity ratio was plotted versus the BSA concentration. | ||
Ratiometric validation
One advantage obtained by designing a ratiometric sensor is that differences in excitation light intensity will not affect the ratio of the peak wavelengths. This is demonstrated in Fig. 5. Although the absolute intensity of fluorescent emission decreased with decreasing illumination power, the ratio of peak intensities, of Newport Green and Texas Red, remained constant. There is, however, a small deviation in the mean ratio at lower illumination power, as a result of the reduced signal to noise. It is evident, though, that the ratiometric method has substantial advantages over single dye fluorescence measurements. For example, fluctuations in the intensity of either a laser or arc lamp would complicate quantitative analysis for intensity-based measurements. The ratiometric PEBBLE eliminate the artefacts resulting from power fluctuations. | ||
| Fig. 5 A 10 mg mL−1 PEBBLE suspension in 10 mM TRIS buffer was monitored on a fluorescence microscope. Neutral density filters (1, 0.5 and 0.3) were used to attain specific excitation powers. An aliquot of zinc nitrate was added after the emission spectra were collected for each filter. From the emission spectra the intensity ratio was calculated from the maxima centred at 545 nm and 604 nm. | ||
Stability
The photostability of the polyacrylamide PEBBLE was studied. The experiment was conducted by constantly illuminating a PEBBLE suspension in a glass container with an argon ion laser. Checking the photobleaching under the listed intracellular conditions (∼1 W cm−2 of 488 nm laser light) demonstrates that performing over 1000 measurements on a cell with 0.1 s spectral acquisitions would result in <5% change in the intensity ratio. Some change was expected since the Newport Green dye molecule is a derivative of fluorescein and bleaches slightly faster than the Texas Red–dextran.The leaching of Newport Green from the PEBBLE was also studied. The experiment was performed using BSA as a qualitative marker of leaching. The fluorescence emission from a 0.1% w/v BSA solution containing zinc-sensitive PEBBLE (7.6 mg mL−1) was monitored for 2 d. If leaching of the Newport Green had occurred, then there would have been an observable red shift in wavelength and a concomitant increase in intensity. Over the period of two days there was no significant change (as defined as a change in intensity equal to 3 times the standard deviation of the blank, i.e. 5%) in the fluorescence emission of the BSA/PEBBLE suspension. From this we can ascertain that there was <3% leaching of the Newport Green from the PEBBLE. The amount of leaching was corroborated with the leaching data obtained by monitoring the PEBBLE filtrate through an Amicon Ultra-filtration device. From this data, the percent leaching of Newport Green from the PEBBLE was determined to be <7% after one week. The leaching of Texas Red–dextran was not an issue due to the large dextran molecule (10000 Mw) not being able to diffuse through the polyacrylamide matrix.
PEBBLE size
The sensor particles were determined to be 50–80 nm in diameter, based on data recorded using the AFFF-multi-angle static light scattering instrumentation and plotted in Fig. 6. There is an indication of some 160–170 nm particles; however, these larger particles are generally a result of aggregation. By increasing the time of sonication, there was a decrease in the observed signal resulting from the larger particles, and even if the sensor distribution has some bimodal character, a 0.1 μM membrane filter could be utilised to separate the two sizes. These 50–80 nm PEBBLE would cause little if any physical perturbation if they were inserted in even the smallest cells, i.e. a 10 μM neuron. Considering that the volume of 80 nm sensor particles are in the order of 2.6 × 10−4 μm3 and a neuron volume is about 520 μm3, one PEBBLE added would result in less than a 0.5 ppm change in volume. Although cellular measurements would require an ensemble of PEBBLE to produce an acceptable signal to noise, it would take more than 20000 PEBBLE to cause a 1% perturbation in cellular volume. However, previous studies suggest that only about 10–1000 PEBBLE need to be inserted into a cell for reliable measurements.21 These reported particle sizes are consistent with previously published literature.17,24 | ||
| Fig. 6 Plot of differential mass fraction of the Newport Green PEBBLE versus hydrodynamic radius. Size distribution was determined by asymmetric field flow fractionation followed by multi-angle static light scattering measurements on the separation channel output. The distribution shows that over 85% of the particles (by mass) are below 80 nm in diameter. | ||
Conclusion
This paper details the fabrication of the first zinc-sensitive polyacrylamide PEBBLE nanosensors and their characterisation. The polyacrylamide particles were synthesised using a microemulsion process that generated particles 50–80 nm in diameter. The sensors incorporated both Newport Green and Texas Red–dextran, resulting in a ratiometric sensor for zinc. The PEBBLE devices were shown to have a reversible response to zinc and are ratiometric over three decades of power. In addition, they are photostable at high illumination power. Also, dye leakage is small, as determined by a newly developed method. The advantage of entrapping the dye molecules inside a protective matrix was demonstrated by the non-response of the PEBBLE sensors to the non-specific binding of the protein BSA (in stark contrast to the free fluorescent dye). The advantage is multi-fold in as much as the sensor is not prone to artefacts from biological materials, such as non-specific binding of proteins, and conversely the possible toxic effects of the dye interacting with the cellular environment are eliminated. The nanosensors described have been shown to respond to zinc in a fast, reversible and reproducible manner. These zinc-sensitive PEBBLE may be utilised in neuron samples where the zinc concentration is believed to reach micromolar levels.Acknowledgements
The authors would like to thank Professor Martin Philbert, Murphy Brasuel, Edwin Park, and Terry Miller for useful discussions during the preparation of this manuscript. Supported by NIH Grant 2R01 GM 50300.References
- M. P. Cuajungco and G. J. Lees, Neurobiol. Dis., 1997, 4, 137 CrossRef CAS.
- C. J. Frederickson, S. W. Suh, D. Silva and R. B. Thompson, J. Nutr., 2000, 130, 1471S Search PubMed.
- D. W. Choi and J. Y. Koh, Annu. Rev. Neurosci., 1998, 21, 347 CrossRef CAS.
- S. C. Cunnane, Zinc: Clinical and Biochemical Significance, CRC Press Inc., Boca Raton, 1988. Search PubMed.
- J. Silvia and R. Williams, The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, Claredon Press, Oxford, 1991. Search PubMed.
- E. Kimura and T. Koike, Chem. Soc. Rev., 1998, 27, 179 RSC.
- G. K. Walkup and B. Imperiali, J. Am. Chem. Soc., 1997, 119, 3443 CrossRef CAS.
- R. B. Thompson, W. O. Whetsell, B. P. Maliwal, C. A. Fierke and C. J. Frederickson, J. Neurosci. Methods, 2000, 96, 35 CrossRef CAS.
- R. B. Thompson, B. P. Maliwal and C. A. Fierke, Anal. Chem., 1998, 70, 1749 CrossRef CAS.
- C. L. Cheng and I. J. Reynolds, J. Neurochem., 1998, 71, 2401 CAS.
- L. M. T. Canzoniero, D. M. Turetsky and D. W. Choi, J. Neurosci., 1999, 19, D1 Search PubMed.
- N. Ertas, E. U. Akkaya and O. Y. Ataman, Talanta, 2000, 51, 693 CrossRef CAS.
- Y. Kurauchi, R. Hayashi, N. Egashira and K. Ohga, Anal. Sci., 1992, 8, 837 Search PubMed.
- T. Hirano, K. Kikuchi, Y. Urano, T. Higuchi and T. Nagano, Angew. Chem., Int. Ed., 2000, 39, 1052 CrossRef CAS; T. Hirano, K. Kikuchi, Y. Urano, T. Higuchi and T. Nagano, J. Am. Chem. Soc., 2000, 122, 12399 CrossRef CAS.
- B. Lukowiak, B. Vandewalle, R. Riachy, J. Kerr-Conte, V. Gmyr, S. Belaich, J. Lefebvre and F. Pattou, J. Histochem. Cytochem., 2001, 49, 519 Search PubMed.
- C. J. Frederickson, Int. Rev. Neurobiol., 1989, 31, 145 Search PubMed.
- H. A. Clark, S. L. R. Barker, M. Brasuel, M. T. Miller, E. Monson, S. Parus, Z. Y. Shi, A. Song, B. Thorsrud, R. Kopelman, A. Ade, W. Meixner, B. Athey, M. Hoyer, D. Hill, R. Lightle and M. A. Philbert, Sens. Actuators, B, 1998, 51, 12 CrossRef.
- C. J. Frederickson, E. J. Kasarskis, D. Ringo and R. E. Frederickson, J. Neurosci. Methods, 1987, 20, 91 CrossRef CAS.
- S. K. Srivastava, V. K. Gupta and S. Jain, Anal. Chem., 1996, 68, 1272 CrossRef; R. Dumkiewicz, C. Wardak and S. Zareba, Analyst, 2000, 125, 527 RSC.
- M. Qhobosheane, S. Santra, P. Zhang and W. H. Tan, Analyst, 2001, 126, 1274 RSC; T. Vo-Dinh, B. M. Cullum and D. L. Stokes, Sens. Actuators, B, 2001, 74, 2 CrossRef; K. P. McNamara, T. Nguyen, G. Dumitrascu, J. Ji, N. Rosenzweig and Z. Rosenzweig, Anal. Chem., 2001, 73, 3240 CrossRef CAS.
- H. A. Clark, M. Hoyer, S. Parus, M. A. Philbert and M. Kopelman, Mikrochim. Acta, 1999, 131, 121 CrossRef CAS.
- M. Brasuel, R. Kopelman, T. J. Miller, R. Tjalkens and M. A. Philbert, Anal. Chem., 2001, 73, 2221 CrossRef CAS.
- H. Xu, J. W. Aylott, R. Kopelman, T. J. Miller and M. A. Philbert, Anal. Chem., 2001, 73, 4124 CrossRef CAS.
- C. Daubresse, C. Grandfils, R. Jerome and P. Teyssie, J. Colloid Interface Sci., 1994, 168, 222 CrossRef CAS.
- G. L. Long and J. D. Winefordner, Anal. Chem., 1983, 55, A712 CrossRef.
| This journal is © The Royal Society of Chemistry 2002 |
