Development of a highly sensitive enzyme-linked immunosorbent assay for bisphenol A in serum
H.
Ohkuma
ac,
K.
Abe
c,
M.
Ito
c,
A.
Kokado
a,
A.
Kambegawa
b and
M.
Maeda
a
aSchool of Pharmaceutical Science, Showa University, 1-5-8 Hatanodai, Shinagawa, Tokyo, 142-8555, Japan
bKambegawa Lab., 3-8-5 Motoizumi, Komae, Tokyo, 201-0013, Japan
cBiochemical Research Lab., Eiken Chemical Co., Ltd., 1381-3 Shimoishigami, Ohtawara, Tochigi, 324-0036, Japan
First published on 7th December 2001
Abstract
4,4′-Isopropylidenediphenol, bisphenol A (BPA), was derivatized to BPA–carboxymethylether (BPA–CME), BPA–carboxypropylether (BPA–CPE) and BPA–carboxybutylether (BPA–CBE), and then linked to bovine serum albumin (BSA). The BPA–BSA conjugates were injected into female New Zealand White rabbits, which then generated six kinds of polyclonal antibodies. In addition, BPA and bisphenol B (BPB)–enzyme conjugates were derivatized to BPA–CME, BPA–CPE, BPA–CBE, BPA–carboxyphenylether (CPhE) and BPB–CPE, and then linked to horseradish peroxidase (HRP). An enzyme-linked immunosorbent assay (ELISA) was developed and the specificity of the antibodies was confirmed by comparison with pre-immune serum and by competitive assays using different dilutions of BPA standards. Although anti-BPA antibodies cross-reacted with BPB by more than 13.6% at all dilutions used, cross-reaction with phthalates and phenols occurred only less than 0.1%. The combination with the highest sensitivity was obtained using anti-BPA–CME–BSA antibody and BPA–CPhE–HRP conjugate. ELISA successfully detected BPA in human serum at concentrations as low as 0.3 ng mL−1, and over a measurable range of 0.3–100 ng mL−1. Recovery tests were carried out by adding BPA to three kinds of human serum, and ranged from 89.7 to 97.3%, from 85.4 to 94.9% and from 81.9 to 97.4%, respectively. The correlation between the results from ELISA and gas chromatography–mass spectrometry (GC-MS) for BPA in spiked serum was r2 = 0.990, indicating that the proposed method is a potential tool for screening a large number of human serum samples.
4,4′-Isopropylidenediphenol, bisphenol A (BPA), is used as a starting material for epoxy and polycarbonate resins, and as a stabilizer for polyvinyl chloride resin. Polycarbonate is widely used in polycarbonate baby bottles and in the epoxy resin coating of the lid and polyethylene terephthalate (PET), and it is thought that BPA has a close connection with endocrine disruption. Approximately 0.3 million tons of BPA are produced in Japan each year, and BPA occurs in the atmosphere of Japan at levels ranging from 2.9 to 3.6 ng m−3,1 which causes environmental and ecological concerns.2,3 Vom Saal4 reported that in pregnant mice dosed at 2 μg per kg of body weight with a level of 1/25 of the threshold limit value per day, the prostate of the male offspring was enlarged. Also, Tsutsumi et al.5reported that growth is promoted in mouse embryos.
The main methods used today for the determination of BPA are high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS). A number of workers have reported studies on the application of chromatographic methods.6–8 However, for these analyses, complicated pre-treatment, extraction and concentration is needed. Therefore, they are not suited for rapid analyses, and also require a large volume of sample. When dealing with serum, plasma, saliva and urine as the measurement media, it would be very difficult to measure from the limited quantity available. Therefore, in this work we have developed an enzyme-linked immunosorbent assay (ELISA) to answer these points.
Kobayashi et al.9 have reported an ELISA method that uses monoclonal antibodies. Kato et al.10 compared BPA levels in human urine as measured by ELISA and HPLC, and Nishii et al.11 reported the successful development of monoclonal antibodies that can be used in organic solvents. However, all the above mentioned analytical methods require a pre-treatment, extraction and concentration step as with the HPLC and GC-MS methods.
In this work we synthesized three kinds of immunogens, we developed polyclonal antibodies and synthesized various conjugates. These were then compared for their sensitivities. We prepared different polyclonal antibodies and conjugates, and examined the best combination for use in the ELISA.12,13 It was found that, anti-BPA–carboxymethylether (CME)–BSA antiserum and BPA–carboxyphenylether (CPhE)–horseradish peroxidase (HRP) conjugate were the best of all the antibodies and conjugates used in this study. With the proposed method, it is suggested that it is possible to measure BPA in serum directly. In particular, the proposed method gave excellent correlation with GC-MS. We have devised a simple method with a high selectivity and with a high precision. This simple method was found to be suitable for the determination of trace amounts of BPA in serum, which contains large amounts of protein and the other material.
Experimental
Materials and solutions
Nunc-Immuno modules microwell plates were supplied by Nalge-Nunc International, Denmark. BPA and the cross-reaction compounds were purchased from Dr. Ehrenstorfer GmbH, Germany, and Kanto Chemical Co., Tokyo, Japan. o-Phenylenediamine (OPD) was purchased from Wako Pure Chemical Industries Ltd., Osaka, Japan. Other chemicals used were of analytical grade.A 0.1 mol L−1 coating solution of carbonate buffer, pH 9.8, was filtered before use. A 0.05 mol L−1 post-coating solution of phosphate buffer (PB), pH 7.5, containing 3% saccharose (Wako Pure Chemical Industries Ltd.) and 0.05% BSA (Oriental Yeast Co. Ltd., Tokyo, Japan), was filtered before use. A 0.01 mol L−1 washing buffer of PB, pH 7.5, containing 0.15 mol L−1 sodium chloride and 0.05% Tween 20, was used. A 50 mmol L−1 conjugate dilution buffer of PB, pH 7.0, containing 0.9% sodium chloride, 1% BSA, 10% fetal bovine serum (FCS) (Bioserum Co.), 0.1% ProClin300 (Sigma Chemicals, St. Louis, MO), 500 units L−1 lactoperoxidase (Sigma Chemicals), and 0.13 mol L−1 lomefloxacin hydrochloride (Hokuriku Seiyaku Co. Ltd., Fukui, Japan) was filtered before use. The antibodies were diluted with the conjugate buffer. A 0.05 mol L−1 substrate dilution buffer of citric acid buffer, pH 4.1, containing 0.05% hydrogen peroxide and 0.1% propylene glycol, was filtered before use. A 5.96 mmol L−1 substrate solution of OPD was diluted with the substrate dilution buffer.
Instruments
The absorbance was measured with a Rainbow Thermo spectrophotometer (Tecan Austria GmbH, Salzburg, Austria). The GC-MS (Hewlett-Packard Japan, Ltd., Tokyo, Japan) analyses were carried out on an HP589 GC with an HP5972 MS.Synthesis of immunogens
BPA–CME was prepared from bromoacetic acid methyl ester. In the presence of 400 mg of potassium carbonate, 200 mg of BPA, Mw 228.3, was dissolved in 800 μL of N,N′-dimethylformamide (DMSO), 200 μL of bromoacetic acid methyl ester was added, and the reaction mixture was stirred for 20 min at 65 °C. Under the same conditions, two further amounts of BPA of 120 mg and 150 mg were dissolved in 600 μL of DMSO each, 150 μL of bromoacetic acid propyl ester was added to one and 150 μL of bromoacetic acid butyl ester to the other, and the reaction mixtures were stirred for 40 min and 60 min at 65 °C, respectively. The mixtures were completely extracted three times with ethyl acetate. Then, 3 mL of distilled water (DW) was added, the pH of each solution was set at 3.5 using 5 mol L−1 hydrochloric acid. Then, 3 mL of DW was added, the BPA–CME, BPA–CPE and BPA–CBE present in aqueous solution (adjusted to pH 2.0) were completely washed after addition. The organic phase was dried over anhydrous sodium sulfate. Analytical thin layer chromatography (TLC) of the organic phase developing solvent, chloroform, indicated the disappearance of the starting material spot, and the appearance of monocarboxyalkyl ether, methyl ether and ethyl ether spots. Those spots were dissolved in ethanol, and they were confirmed using a silica plate. A small amount of dicarboxyalkyl ether was removed using TLC. Then 400 μL of methanol was added, each solution was saponified using of 100 μL of 5 mol L−1 sodium hydroxide, and the reaction mixture was stirred overnight at room temperature. Then 3 mL of DW was added, the pH of each solution was set at below 2.0 using 5 mol L−1 hydrochloric acid. The mixtures were completely extracted three times with ethyl acetate, and then washed three times with DW. The organic phase was dried over anhydrous sodium sulfate. BPA–CME was recrystallized from benzene, and BPA–CPE and BPA–CBE were recrystallized from benzene–hexane, 1∶1.All compounds were analyzed and their identity was confirmed by at least three of the following methods: nuclear magnetic resonance (NMR), GC-MS, elementary analysis and melting point. BPA–CME: m.p. 110–115 °C from benzene–hexane; 1H-NMR (CDCl3) d: 1.60 (6H, s, 2CH3), 4.60 (2H, s, OCH2), 6.7–8.2 (8H, m, Ar); anal. calc. for C17H18O4: C, 71.31; H, 6.34, found: C, 71.28; H, 6.30. BPA–CPE: m.p. 88–95 °C from benzene–hexane; 1H-NMR (CDCl3) d: 1.61 (6H, s, 2CH3), 2.10 (2H, q, j = 6, OCH2CH2CH2–), 2.58 (2H, t, j = 7, –CH2CO), 4.02 (2H, g, j = 6, OCH2–), 6.7–7.2 (8H, m, Ar); anal. calc. for C19H22O4: C, 72.59; H, 7.05; found: C, 72.45; H, 7.08. BPA–CBE (semi-crystals): 1H-NMR (CDCl3) d: 1.61 (6H, s, 2CH3), 2.11 (4H, m, j = 6, OCH2CH2CH2CH2CO), 2.60 (2H, t, j = 7, CH2CO), 3.92 (2H, t, j = 6, OCH2), 6.8–7.2 (8H, m, Ar); anal. calc. for C20H24O4: C, 73.15; H, 7.37; found: C, 73.10; H, 7.40.
The haptens were conjugated covalently to the carrier protein BSA via amino bonds by the active ester method using N-hydroxysuccimimide (NHS) and a water soluble 1-ethyl-3-(3,4-dimethylaminopropyl) carbodiimide (EDC) as dehydrating agent. Twenty-five milligram of hapten were added to 100 μL of DMSO, 20 mg of NHS and 30 mg of EDC, and the reaction mixtures were stirred at room temperature for 1 h. The hapten was extracted with ethyl acetate, after addition of DW, and the organic phase was dried over anhydrous sodium sulfate. The solvent was then evaporated at reduced pressure, and 30 mg of the residue was suspended in 200 μL of DMSO. Fifty milligrams of BSA were dissolved in 100 μL of PBS, 50 μL of the above active ester solution were added to the BSA solution, and the reaction mixture stirred for 60 min at room temperature.14 The crude protein conjugate was purified by dialysis overnight at 4 °C. After freeze-drying, 5 mg of hapten–BSA was prepared in 500 μL of PBS, pH 7.2–7.4. Analytical ninhydrin color testing of the conjugates was carried out using 200 μL of the solutions. The molar density of the hapten on the carrier protein was obtained by calculating the amount of reduction with amino group per mole of BSA. As a result, BPA–CME–BSA, BPA–CPE–BSA and BPA–CBE–BSA (see Fig. 1) were 28, 26 and 25 per molecule, respectively.15,16
 | ||
| Fig. 1 Chemical structures of the immunogens and conjugates, BPA hapten and BPB hapten used in the present study. | ||
Production of polyclonal antibodies
Each immunogen was prepared by addition of one volume of BPA–BSA conjugate to one volume of saline. Six rabbits were given one footpad injection of the BPA–BSA conjugate (500 μg per rabbit) emulsified in complete Freund's adjuvant, followed by subcutaneous injection of the BPA–BSA conjugate emulsified in complete Freund′s adjuvant at 3 week intervals. A total of ten immunizations were given to each rabbit. Seven days after the last immunization, the six rabbits were sacrificed.Synthesis of hapten-labelled HRP
Three milligrams of BPA–CME or BPB–CPE were added to 50 μL of DMSO. Then, 3 mg of NHS and 5 mg of EDC were added, and the reaction mixture was stirred at 40 °C for 30 min. The hapten was extracted with ethyl acetate after addition of DW, and the organic phase was dried over anhydrous sodium sulfate. The solvent was then evaporated at reduced pressure, and 0.3 μmol L−1 of the residues was incubated in 75 nmol L-1 of HRP solvent, PB, for 60 min at room temperature. The crude hapten–enzyme conjugate was then purified on a PD-10 gel filtration column (Pharmacia Biotech Ab, Uppsala, Sweden). BPA–CPE, BPA–CBE and BPA–CPhE were also conjugated in the same manner.Preparation of second antibody coating plate
Microplates were coated by incubating for 48 h at 4 °C with 100 μL per well of 20 μg mL−1 of goat anti-rabbit IgG affinity purification (Eiken Chemical Co., Ltd.) in coating buffer. After washing, with 300 μL of post-coating solution, the plates were blocked by incubating overnight at 4 °C with 300 μL per well of post-coating solution. The plates were freeze-dried and stored at 4 °C until use.Competitive ELISA for BPA
A competitive ELISA using hapten-labelled HRP was used for the detection of BPA. The antibody selected was that with the best result from the IC50 test (the concentration that inhibits the assay by 50%) and with the lowest cross-reactivity. The hapten-labelled HRP selected was the best from the results of the IC50 and titer to antibody 1 (ab1) tests. To each microtiterplate well of the second antibody coating plate, 20 μ L of BPA standard (0.3–100 ng mL−1) in 100 mmol L−1 of PB (pH 7.5) or sample was added. Then 50 μL of 0.05 nmol L−1 hapten-labelled HRP (BPA–CPhE–HRP; dilution of 1∶40 000 in a conjugate dilution buffer), and 50 μL of anti-BPA antiserum (ab1; dilution of 1∶64 000 in an antibody dilution buffer) were added and mixed well, they were then incubated for 1 h at room temperature. After incubation, the plates were washed three times with 350 μL of washing buffer. After washing, 100 μL of OPD was added to each well and allowed to react for 30 min at room temperature. The enzymatic reaction was stopped by the addition of 100 μL per well of 1.5 mol L−1 H2SO4. The color intensities of the wells were read by a spectrophotometer at 492 nm.GC-MS analysis
Five hundred microliters of BPA standard or spiked serum were added to 2.5 mL of ethanol, and were mixed well. After centrifugation at 3000 rpm for 10 min, the mixture was enriched by reduced pressure, and 1 mL of 5 mol L−1 hydrochloric acid was added. The solution was extracted twice with ethyl acetate and collected in a glass bottle previously cleaned with hydrochloric acid. The solvent was evaporated at reduced pressure. Also, 100 μL of trimethylallylsilane (TMS) reagent was added, and the reaction mixture was stirred for 30 min at 80 °C. A 1 μL aliquot of the extract was injected into the gas chromatograph.17 The GC-MS parameters used were as follows: injector temperature, 200 °C; detector temperature, 280 °C; and oven temperature programmed from 60 °C (held for 2 min) to 280 °C (held for 3 min) at 10 °C min−1, remaining constant for 7 min. The selected ions of the compound for SIM mode operation were m/z 357 and 372. The concentration of BPA in the serum was calculated by the internal standard method.Results and discussion
Screening of polyclonal antibodies
The results are shown in Table 1. The screening of the polyclonal antibodies used was done by the IC50 parameter and cross-reactivity. The dosage level of the three synthesized immunogens was 0.5 mg mL−1 of emulsion. The corresponding antibodies were numbered as follows: BPA–CME–BSA, 1 and 2; BPA–CPE–BSA, 3 and 4; and BPA–CBE–BSA, 5 and 6, respectively. As a result, it was confirmed from ELISA that antibodies 1, 2 and 3 gave excellent high sensitivity. In particular, it was shown that ab1 was the best. Thus, we used ab1 to ascertain the optimum parameters for the assay performance.| Ab | IC50/ng mL−1 |
|---|---|
| a The antisera were evaluated by the proposed ELISA method. The immunogens are CME (abs 1 and 2), CPE (abs 3 and 4) and CBE (abs 5 and 6). The data shown were obtained with an added BPA concentration of 0–25 ng mL−1, an antiserum dilution of 1∶64 000 and a BPA–CPhE–HRP conjugate ratio of 1∶40 000. | |
| 1 | 5.4 |
| 2 | 6.2 |
| 3 | 6.5 |
| 4 | 10.2 |
| 5 | 10.1 |
| 6 | 7.1 |
Screening of HRP conjugate
The hapten-labelled enzymes examined were BPA–CME–HRP conjugate, BPA–CPE–HRP conjugate, BPA–CBE–HRP conjugate, BPA–CPhE–HRP conjugate and the site-heterologous derivative, BPB–CPE–HRP, conjugate. The results gave the detection limits increasing in the order BPA–CPhE–HRP conjugate < BPB–CPE–HRP conjugate < BPA–CBE–HRP conjugate < BPA–CPE–HRP conjugate < BPA–CME–HRP conjugate. Thus, we utilized ab1 and the BPA–CPhE–HRP conjugate to ascertain the parameters for the optimum assay performance (see Fig. 2). Therefore, the assay format used with this conjugate was a hapten-heterologous direct competitive format.18,19 | ||
| Fig. 2 Standard curve for the proposed ELISA, using a coating of anti-rabbit IgG goat antiserum and ab1 diluted 1∶64 000. The dynamic range for quantification is between 0.3 and 100 ng mL−1 using BPA–CPhE–HRP. The minimum detectable amount of BPA, defined as 95% B / B0−1 was 6.0 pg per well. Assays were carried out in triplicate in a microtiterplate using spiked concentrations of BPA from 0.3 to 100 ng mL−1. Type of conjugates : ●, BPA–CME–HRP×10 000; ▲, BPA–CPE–HRP×32 000; ■, BPA–CBE–HRP×5000; ○, BPB–CPE–HRP×45 000; □, BPA–CPhE–HRP×40000. | ||
Sensitivity and reproducibility
An assay was carried out using ab1 at a dilution of 1∶64 000 and BPA–CPhE–HRP conjugate at a dilution of 1∶40 000. As a result, the lower limits of detection were 0.3 ng mL−1 with an IC5 (the concentration that inhibits the assay by 5%) value of 6.0 pg per well, and the measurable range was from 0.3 to 100 ng mL−1. It was thought that the proposed method has a high sensitivity compared with conventional ELISA methods. The reproducibility of the standard curve was calculated from the absorbance. The relative standard deviations ranged from 1.0 to 2.6%, n = 10.Cross-reactivity
The cross-reactivity, the IC50, was tested with ab1. The cross-reactivity pattern for the antibody is shown in Table 2. After the data were normalized by B/B0−1 (%) transformation, B / B0-1 (%) = Aex/A0 × 100 (where A is the absorbance, A0 is the absorbance at a zero dose of hapten, and Aex is the absorbance at an excess of hapten), the BPA concentrations that caused 50% inhibition (IC50) were used to calculate the cross-reactivities according to formula: cross-reactivity (%) = (ng mL−1 of compound at IC50)/(ng mL−1 of cross-reacting BPA at IC50) × 100. As a result, ab1 was found to show a cross-reactivity to BPB of 13.6%. However, alkylphenol, phthalate ester and estrogen showed less than 0.1% cross-reactivity. This antibody was not subject to interference from phthalates and phenols. It was suggested that this would not affect the proposed method.| Compound | Ab 1 reactivity (%) (BPA=100%) |
|---|---|
| a BPA–diglycidylether methacrylate. | |
| Bispenol A | 100.0 |
| Bispenol B | 13.6 |
| Bis-GMAa | Below 0.1 |
| Diethylstilbestrol | Below 0.1 |
| Hexesterol | Below 0.1 |
| 17ß-Estradiol | Below 0.1 |
| 4,4′-Biphenyldiol | Below 0.1 |
| 4-n-Nonylphenol | Below 0.1 |
| 4-Propylphenol | Below 0.1 |
| 4-Hexyloxyphenol | Below 0.1 |
| 4-Pentylphenol | Below 0.1 |
| 4-Hexylphenol | Below 0.1 |
| 4-Heptylphenol | Below 0.1 |
| 2-tert-Butylphenol | Below 0.1 |
| 4-Dodecylphenol | Below 0.1 |
| 4-Butoxyphenol | Below 0.1 |
| Benzyl-n-butylphthalate | Below 0.1 |
| Di-n-butylphthalate | Below 0.1 |
Dilution reliability and recovery test
The dilution reliability of the proposed method was examined using normal human serum (data not shown). The correlation coefficient was r = 0.998. There was no deviation from linearity of the dilution plots. The results of the recovery tests for BPA are shown in Table 3. The recovery tests were carried out using three normal human sera, by addition of one volume of antigen to three volumes of serum. In the experiment, 1.56, 6.24 and 24.96 ng mL−1 of standard antigen were added to human serum 1, human serum 2 and human serum 3, respectively, n = 2. The results of the recovery tests were from 89.7% to 97.3%, from 85.4% to 94.9% and from 81.9% to 97.4% for the three sera, respectively. Therefore, it was suggested that the measurement of BPA in serum by the proposed method did not interfere in the antigen–antibody reaction. There was therefore no need for complicated extraction and concentrating procedures, and it was possible to make rapid and repeated measurements easily.| Sample | Added/ng mL−1 | Observed/ng mL−1 | Recovered/ng mL−1 | Recovery (%) |
|---|---|---|---|---|
| a The recovery test was carried out by the addition of one volume of antigen to three volumes of human serum (n = 2). | ||||
| Human serum 1 | 0.00 | 0.00 | ||
| 1.56 | 0.35 | 0.35 | 89.7 | |
| 6.25 | 1.52 | 1.52 | 97.3 | |
| 24.96 | 6.01 | 6.01 | 96.3 | |
| Human serum 2 | 0.00 | 0.78 | ||
| 1.56 | 1.15 | 0.37 | 94.9 | |
| 6.25 | 2.23 | 1.45 | 92.8 | |
| 24.96 | 6.11 | 5.33 | 85.4 | |
| Human serum 3 | 0.00 | 0.00 | ||
| 1.56 | 0.38 | 0.38 | 97.4 | |
| 6.25 | 1.28 | 1.28 | 81.9 | |
| 24.96 | 5.78 | 5.78 | 92.6 | |
Correlation with GC-MS
BPA was assayed by the proposed method and by GC-MS. The results are shown in Table 4. For samples 1 and 2, the assay was carried out using BPA spiked buffer solutions, and for samples 3–8, it was carried out using spiked sera. The correlation between the BPA values obtained by the two methods was y(GC-MS) = 1.25x(ELISA) - 1.67; r = 0.995, n = 8. The average values for ELISA and GC-MS methods were 17.9 and 20.6 ng mL1, respectively. In spite of being a direct measurement, the proposed method showed a good correlation with the extraction method.| Sample | ELISA/ng mL−1 | GC-MS/ng mL−1 |
|---|---|---|
| a The correlation test was carried out by the addition of one volume of antigen to four volumes of serum. The correlation between the BPA values obtained by the two methods was y(GC-MS) = 1.25x(ELISA) −1.67, r2 = 0.990 (n = 8). | ||
| 1 | 5.0 | 6.3 |
| 2 | 10.0 | 9.6 |
| 3 | 85.5 | 106.6 |
| 4 | 18.0 | 13.8 |
| 5 | 7.7 | 11.9 |
| 6 | 1.8 | 2.4 |
| 7 | 2.8 | 4.2 |
| 8 | 12.1 | 10.3 |
Application
Possible exposure to BPA from the high molecular compound material contained in dialysis packing and tubing is known. Therefore, we were alerted to the risk posed during artificial dialysis.BPA levels were measured in human sera from different sources: (1) after being allowed to stand for ten months at−20 °C, BPA levels in normal human sera, n = 100, were measured by the proposed method; (2) after being allowed to stand for five months at −20 °C, BPA levels in dialysis patients’ sera, n = 100, were measured by the same method. Many of the normal human sera measured, however, showed BPA levels of less than 0.3 ng mL−1. On the other hand, the BPA values in the dialysis patients’ sera gave significantly higher values, the average level being approximately eight-fold higher than for the normal human sera. The higher levels of BPA found in the dialysis patients was considered to be very significant. The results are shown in Fig. 3. We then compared the history of the patient’s dialysis with the BPA value. This experiment, however, did not suggest that the BPA values had a close correlation to the history of the dialysis.
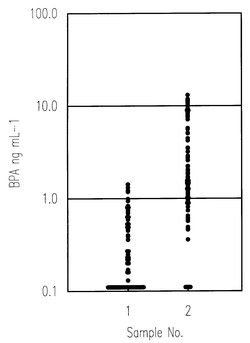 | ||
| Fig. 3 Detection of BPA levels in human sera. Sample 1, normal human serum (n = 100). Sample 2, dialysis patients’ serum (n = 100). | ||
The purpose of this paper was the establishment of a methodology. Therefore, it was examined based on the evaluation of a general ELISA method. This involved application to actual serum samples. However, correlation between the proposed ELISA method and GC-MS was not possible using the dialysis patients’ sera because of the need for sample pre-treatment prior to analysis by GC-MS. This required 25–50 times the amount of sample compared with that required for the proposed ELISA, and they were very valuable samples. It was also not possible to confirm from the results the influence of any matrix effect. However, the convenience of the proposed method seems to be an advantage. At this time, there is a problem for the future: to investigate a more detailed case, it is hoped that this work is studied by various other professionals.
It has been shown that a large number of determinations is possible, the cost of the reagents is lower, and the assay procedure is simpler than with other methods, such as GC-MS and HPLC. As a means of evaluating safety to humans, the proposed method can contribute to the determination of BPA.
Acknowledgements
We thank H. Takei and A. Anazawa, Tokyo Health Service Association, for their cooperation in the GC-MS measurements, and MT. T. Sasahara and Dr. H. Kojima, Yokohama Asahichuou General Hospital, for providing the dialysis patients’ sera used in these studies.References
- T. Kamiura, Y. Tajima and T. Nakahara, J. Environ. Chem., 1997, 7, 275 Search PubMed.
- K. L. Howdeshell, A. K. Hotchkiss, K. A. Thayer and J. G. Vandenbergh, Nature, 1999, 6755, 763.
- A. V. Krishnan, P. S. Starhis, F. Permuth, L. Tokes and D. Feldman, Endocrinology, 1993, 132, 2279 Search PubMed.
- F. S. Vom Saal, J. Anim. Sci., 1989, 67, 1824 Search PubMed.
- Y. Takai, O. Tsutsumi, H. Hirai, A. Fujimoto, A. Suenaga, Y. Kamei, M. Hyakueda, T. Yano and Y. Taketani, in Program and Abstracts, The 51st Annual Meeting of Japan Society of Obstetrics and Gynecology, Tokyo, April, 1999, p. S300.
- U. Berger, M. Oehme and L. Girardin, Fresenius' J. Anal. Chem., 2001, 3692, 115 Search PubMed.
- S. Podzimek and A. Kastanek, J. Appl. Polym. Sci., 1999, 7410, 2432 CrossRef.
- Summerfield, A. Goodson and I. Cooper, Food Addit. Contam., 1998, 157, 818.
- A. Kobayashi, Y. Goda, K. Fukuda, S. Fujimoto, M. Ike and M. Fujita, in Program and Abstracts, The 1st Annual Meeting of Japan Society of Endocrine Disrupters Research, Kyoto, December, 1998, p. 21.
- Y. Usuki, M. Kondo, H. Oguri, T. Kodaira, I. Kato and N. Yanaihara, Bio Clinica, 2000, 15, 55 Search PubMed.
- S. Nishii, K. Matsui, T. Ishibashi and Y. Kawamura, in Program and Abstracts, The 61st Annual Debate of Analytical Chemistry Society of Japan, Nagaoka, May, 2000, p. 135.
- A. Kokado, K. Hosono, K. Ito, H. Arakawa and M. Maeda, in Program and Abstracts, The 120th Annual Meeting of Japan Pharmaceutical Society, Gifu, March, 2000, p. 179(4).
- H. Ohkuma, K. Abe, A. Kokado, A. Kambegawa and M. Maeda, in Program and Abstracts, The 5th Annual Meeting of the Immunochemical Society of Japan, Kobe, June, 2000, p. 21.
- J. F. S. Ferreira and J. Janick, Phytochemistry, 1996, 41, 97 CrossRef CAS.
- R. McGrath, Anal. Chem., 1972, 49, 95 CAS.
- H.-J. Horstmann, Anal. Chem., 1979, 96, 130 CAS.
- M. Del Olmo, A. Gonzalez-Casado, N. A. Navas and J. L. Vilchez, Anal. Chim. Acta, 1997, 3461, 87 CrossRef CAS.
- K. Kothari and M. R. A. Pillai, J. Radioanal. Nucl. Chem., 1998, 231, 77 CAS.
- T. Niwa, K. Fujita, T. Kasuya, S. Ikegawa and J. Goto, Anal. Sci., 1996, 12, 565 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
