Cyclodextrin catalysis of the pH-independent hydrolyses of acetals![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) †
†
Received
(in Cambridge, UK)
10th August 2000
, Accepted 12th October 2000
First published on 5th December 2000
Abstract
The spontaneous hydrolysis reactions of 2-(4-nitrophenoxy)tetrahydropyran (6a) and 2-(4-cyanophenoxy)tetrahydropyran (6b) are accelerated in the presence of α-cyclodextrin (α-CD), but are slowed by the addition of β-CD, γ-CD, or mono[2-O-(carboxymethyl)]-β-CD (β-CD-acid 5). The observed rate constants for the hydrolysis reactions in the presence of CD are consistent with the CD-catalysed reaction occurring from a 1∶1 complex of cyclodextrin with the substrate. In contrast, hydrolysis does not occur from the 1∶1 complex for reactions conducted at high pH values at which ionisation of the CD becomes important. The spontaneous hydrolysis reactions for both anomers of 2-deoxyglucopyranosylpyridinium salts are markedly accelerated by the addition of β-CD. In these reactions, the binding affinity of the β-CD cavity for the heteroaromatic ring of the pyridinium substrate increases in response to a decreasing positive charge on the ring moiety that occurs with approach to the transition state, and this results in catalysis.
Introduction
The discovery of cyclodextrins (CDs) by Villiers in the late 19th century![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 was soon followed by their preparation and isolation by Schardinger in the early 1900s.2 Since that time, continued scientific interest has led to the exploration and development of a variety of novel chemical applications for these cyclic oligosaccharides.3,4 The ability of these compounds to form inclusion complexes with hydrophobic guest molecules
1 was soon followed by their preparation and isolation by Schardinger in the early 1900s.2 Since that time, continued scientific interest has led to the exploration and development of a variety of novel chemical applications for these cyclic oligosaccharides.3,4 The ability of these compounds to form inclusion complexes with hydrophobic guest molecules![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4–6 highlights the potential for use of cyclodextrins and their derivatives as artificial enzyme systems.7 In view of their possible role as enzyme models, a vast array of derivatised cyclodextrins have been synthesized, thus expanding the cyclodextrin functionality in order to increase their catalytic potential.8
4–6 highlights the potential for use of cyclodextrins and their derivatives as artificial enzyme systems.7 In view of their possible role as enzyme models, a vast array of derivatised cyclodextrins have been synthesized, thus expanding the cyclodextrin functionality in order to increase their catalytic potential.8
With regard to biologically relevant hydrolysis reactions such as those involving esters, amides, acetals, and phosphate esters, most mechanistic studies in solution have employed either acidic![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9,10 or basic reaction conditions, undoubtedly because very few of these hydrolysis reactions have significant non-catalysed reaction rates. In general, acetal hydrolysis involves specific-acid catalysis, i.e., rapid, reversible protonation followed by rate-limiting unimolecular C–O bond cleavage.11 However, acetals that contain activated leaving groups can hydrolyse via a dissociative, uncatalysed mechanism. For example, 2-(4-aryloxy)tetrahydropyrans hydrolyse via both general-acid catalysed and spontaneous uncatalysed reaction pathways.12,13 Acetals that contain positively charged leaving groups can also hydrolyse without acid-catalysis.14
9,10 or basic reaction conditions, undoubtedly because very few of these hydrolysis reactions have significant non-catalysed reaction rates. In general, acetal hydrolysis involves specific-acid catalysis, i.e., rapid, reversible protonation followed by rate-limiting unimolecular C–O bond cleavage.11 However, acetals that contain activated leaving groups can hydrolyse via a dissociative, uncatalysed mechanism. For example, 2-(4-aryloxy)tetrahydropyrans hydrolyse via both general-acid catalysed and spontaneous uncatalysed reaction pathways.12,13 Acetals that contain positively charged leaving groups can also hydrolyse without acid-catalysis.14
The CD-catalysed hydrolysis of 2-(4-nitrophenoxy)tetrahydropyran has been reported by Siegel et al. who monitored the reaction rate constants in the presence of β-CD and its 2-, 3-, and 6-monophosphate derivatives.9 These authors reported that the 3-monophosphate is the most active CD derivative for the promotion of hydrolysis of the activated acetal 2-(4-nitrophenoxy)tetrahydropyran.9 Since this hydrolysis reaction was only monitored at pH 4.0, it is difficult to assess whether the CD-promoted rate acceleration originates from an acid-catalysed or from a spontaneous reaction pathway. In a separate report, Tee et al. showed that acid-catalysed hydrolysis of benzaldehyde dimethyl acetal is inhibited by α-CD, β-CD, hp-β-CD (hydroxypropyl-β-cyclodextrin), and γ-CD.10 In this case, ground-state binding of the guest molecule benzaldehyde dimethyl acetal to the cyclodextrin cavity is favourable, whereas the cationic transition state only binds weakly to the cyclodextrin cavity, resulting in an observed decrease in the hydrolytic rate constants in the presence of cyclodextrin.
The present report details the effects of various CDs on the hydrolysis of acetals that possess significant rates of spontaneous hydrolysis. In addition, substituted pyridinium and phenoxy leaving groups are employed in a study of the effects of leaving group charge on the CD-catalysed hydrolysis of acetals.
Results and discussion
The pathway utilised for the synthesis of mono-2-O-(carboxymethyl)-β-CD (5) is shown in Scheme 1. Selective protection of β-CD’s seven primary hydroxy groups with tert-butyldimethylsilyl groups was accomplished via the method of Pregel and Buncel.15 Subsequent treatment of the heptakis[6-O-(tert-butyldimethylsilyl)]-β-CD (2) with ethyl iodoacetate (1.2 equivalents) and Ag2O (2.5 equivalents) in DMF, and separation of the mixture by column chromatography, gave a 32% yield of the monoalkylated cyclodextrin. Removal of the silyl protecting groups was effected by treatment with BF3–OEt2 in CHCl3 (75% yield).16 Hydrolysis of 4 with aqueous NaOH gave β-CD-acid (5) in a yield of 91%. Given that the primary hydroxy groups of 2 are protected, it is expected that derivatisation of the compound will occur on a C-2 hydroxy group rather than on a non-reactive C-3 hydroxy group.17 Analysis of the 13C NMR spectrum of 5 indicates that substitution occurred on the C-2 position.18 Specifically, the occurrence of a large downfield chemical shift of C-2A and smaller upfield chemical shifts for C-3A and C-4A relative to those of the unsubstituted glucose units clearly indicates that substitution has occurred at the C-2 position.18,19![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡
‡
 |
| | Scheme 1 | |
The hydrolysis rate of 2-(4-nitrophenoxy)tetrahydropyran (6a) is accelerated in the presence of α-cyclodextrin, whereas upon addition of β-CD, β-CD-acid (5), or γ-CD, the hydrolytic rate is reduced. Shown in Fig. 1 is a representative example of the observed rate constants for the hydrolysis of 6a in the presence of α- and β-CD. Tables S1–S4 (supplementary data) list the complete kinetic data for the hydrolysis of 2-(4-nitrophenoxy)tetrahydropyran as a function of added CD concentration, while the corresponding kinetic data for the hydrolysis of 2-(4-cyanophenoxy)tetrahydropyran are given in Tables S5 and S6 (supplementary data).
 |
| | Fig. 1 Plot of the observed order rate constant kobsversus CD concentration for the hydrolysis of 6a in the presence of α-CD (•) and β-CD (■), pH = 10.6, T = 50 °C. Error limits are encompassed within the symbol diameter. The lines are the best non-linear least-squares fits through the data points.
| |
In the absence of cyclodextrin and at low buffer concentration, the hydrolysis of 2-(aryloxy)tetrahydropyrans can occur via an acid-catalysed (kH) pathway and an uncatalysed (kuncat) pathway [eqn. (1)].
| | |
k
hyd = kH[H+] + kuncat
|
(1)
|
The presence of cyclodextrin promotes the formation of a bound complex from which acetal hydrolysis can occur. Furthermore, since the first ionisation constant (pKa) for α- and β-cyclodextrin occurs around a pH value of 12–13 (C-2 OH group),3,4 the anionic form of these compounds can become kinetically important at high pH values.21 As the first-order rate constants for dissociation of guest molecules from within a cyclodextrin’s cavity are very large,22 the rate determining step for kcat (hydrolysis of the CD–guest complex) must involve bond cleavage at the acetal centre. Therefore, the full mechanistic scheme can be abbreviated to that shown in Scheme 2 where the acetal can react at pH values greater than 5 via one of three mechanistic pathways: (1) spontaneous hydrolysis kuncat; (2) CD-catalysed hydrolysis from the 1∶1 complex kcat; or (3) CD-catalysed hydrolysis from the ionised 1∶1 complex kcat−.
 |
| | Scheme 2 | |
The observed rate constants for hydrolysis (kobs) of an acetal that binds CD in a 1∶1 inclusion complex will adhere to eqn. (2), where k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat is the maximal rate constant in the presence of CD, khyd is the rate constant in the absence of CD, and K
′cat is the maximal rate constant in the presence of CD, khyd is the rate constant in the absence of CD, and K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d is the apparent dissociation constant of the 1∶1 complex.9
′d is the apparent dissociation constant of the 1∶1 complex.9![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) §
§
| |

|
(2)
|
The kinetic data given in Tables S1–S4 were fitted to eqn. (2), and the resulting constants k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat, khyd, and K
′cat, khyd, and K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d are listed in Tables 1–4. The derived values of khyd (Tables 1 and 2) were fitted to eqn. (1) to give estimates for the acid-catalysed (kH = 1.59 ± 0.05 M−1 s−1) and the spontaneous (kuncat = 1.115 ± 0.005 × 10−3 s−1) hydrolysis rate constants for 6a at 50 °C. These calculated values for kuncat and kH are very similar to the value of 1.175 × 10−3 s−1 (μ = 0.5, KCl) for kuncat and 2.17 M−1 s−1 (μ = 0.5, KCl) for kH measured at 50 °C by Fife and Brod.13
′d are listed in Tables 1–4. The derived values of khyd (Tables 1 and 2) were fitted to eqn. (1) to give estimates for the acid-catalysed (kH = 1.59 ± 0.05 M−1 s−1) and the spontaneous (kuncat = 1.115 ± 0.005 × 10−3 s−1) hydrolysis rate constants for 6a at 50 °C. These calculated values for kuncat and kH are very similar to the value of 1.175 × 10−3 s−1 (μ = 0.5, KCl) for kuncat and 2.17 M−1 s−1 (μ = 0.5, KCl) for kH measured at 50 °C by Fife and Brod.13
| pH |
103khyd/s−1 |
K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM ′d/mM |
103k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1 ′cat/s−1 |
| 3.50 |
1.614 ± 0.033 |
8.76 ± 1.45 |
4.23 ± 0.20 |
| 5.00 |
1.121 ± 0.020 |
11.31 ± 1.25 |
4.12 ± 0.17 |
| 9.50 |
1.089 ± 0.006 |
9.73 ± 0.25 |
4.56 ± 0.04 |
| 10.60 |
1.090 ± 0.008 |
9.61 ± 0.32 |
4.46 ± 0.05 |
| 11.70 |
1.112 ± 0.020 |
10.92 ± 0.85 |
4.73 ± 0.13 |
| 12.18 |
1.104 ± 0.022 |
11.99 ± 1.16 |
4.69 ± 0.17 |
| 12.40 |
1.122 ± 0.009 |
12.59 ± 0.65 |
4.21 ± 0.08 |
| 12.70 |
1.125 ± 0.004 |
14.19 ± 0.51 |
3.76 ± 0.05 |
| 12.88 |
1.091 ± 0.009 |
14.66 ± 1.22 |
3.25 ± 0.10 |
| 13.00 |
1.119 ± 0.004 |
17.50 ± 1.10 |
3.05 ± 0.07 |
| pH |
103khyd/s−1 |
K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM ′d/mM |
103k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1 ′cat/s−1 |
| 3.50 |
1.619 ± 0.013 |
7.31 ± 1.12 |
0.656 ± 0.063 |
| 5.00 |
1.199 ± 0.017 |
5.31 ± 1.51 |
0.692 ± 0.054 |
| 9.50 |
1.092 ± 0.004 |
4.31 ± 0.37 |
0.702 ± 0.011 |
| 10.60 |
1.096 ± 0.004 |
4.35 ± 0.33 |
0.698 ± 0.011 |
| 11.70 |
1.128 ± 0.003 |
4.77 ± 0.25 |
0.653 ± 0.008 |
| 12.18 |
1.130 ± 0.007 |
5.10 ± 0.50 |
0.586 ± 0.018 |
| 12.40 |
1.130 ± 0.004 |
5.42 ± 0.32 |
0.514 ± 0.014 |
| 12.70 |
1.118 ± 0.003 |
7.41 ± 0.28 |
0.337 ± 0.013 |
| 12.88 |
1.114 ± 0.005 |
7.70 ± 0.42 |
0.246 ± 0.020 |
| 13.00 |
1.128 ± 0.003 |
7.39 ± 0.24 |
0.270 ± 0.012 |
| pH |
103khyd/s−1 |
K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM ′d/mM |
103k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1 ′cat/s−1 |
| 3.50 |
1.451 ± 0.023 |
6.14 ± 1.54 |
0.681 ± 0.078 |
| 5.00 |
1.005 ± 0.014 |
8.66 ± 3.34 |
0.573 ± 0.073 |
| 9.50 |
1.048 ± 0.006 |
8.21 ± 1.42 |
0.651 ± 0.029 |
| 10.60 |
1.031 ± 0.008 |
9.48 ± 2.20 |
0.622 ± 0.043 |
| pH |
103khyd/s−1 |
K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM ′d/mM |
103k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1 ′cat/s−1 |
| 3.50 |
1.492 ± 0.035 |
1.66 ± 0.94 |
1.183 ± 0.038 |
| 5.00 |
1.039 ± 0.022 |
6.1 ± 3.8 |
0.710 ± 0.076 |
| 9.50 |
1.068 ± 0.007 |
14.1 ± 3.5 |
0.572 ± 0.066 |
| 10.60 |
1.066 ± 0.008 |
12.8 ± 3.5 |
0.588 ± 0.068 |
At elevated pH values, there is a sharp decrease in the calculated rate constants (k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat) for the CD-catalysed hydrolysis of 6a (Tables 1 and 2). Consequently, the derived values for k
′cat) for the CD-catalysed hydrolysis of 6a (Tables 1 and 2). Consequently, the derived values for k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat (Tables 1 and 2) were fitted to eqn. (3), where kcat is the maximal rate constant for the neutral cyclodextrin-catalysed hydrolysis of 6a, kcat− is the rate constant for the hydrolysis of the CD–acetal complex when the cyclodextrin is fully ionised, and Ka is the acid dissociation constant for the CD.
′cat (Tables 1 and 2) were fitted to eqn. (3), where kcat is the maximal rate constant for the neutral cyclodextrin-catalysed hydrolysis of 6a, kcat− is the rate constant for the hydrolysis of the CD–acetal complex when the cyclodextrin is fully ionised, and Ka is the acid dissociation constant for the CD.
| |

|
(3)
|
Non-constrained fits to eqn. (3) of the data listed in Tables 1 and 2 give values for kcat− that are equal to 0.0, within experimental error. As a result, constrained fits of the data were used in which kcat− was set equal to zero. The best non-linear leastsquares fits of the data presented in Tables 1 and 2 are shown in Figs. 2 and 3, respectively. From these analyses, the computed parameters are kcat = 4.55 ± 0.06 × 10−3 s−1 and pKa = 13.35 ± 0.05 for the α-CD-catalysed reaction, and kcat = 0.709 ± 0.011 × 10−3 s−1 and pKa = 12.74 ± 0.04 for the β-CD-catalysed reaction. Similarly, the calculated dissociation constants (K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d) were fitted to a standard pH-titration model [eqn. (4)], where Kd and Kd− are the dissociation constants for the 1∶1 inclusion complexes of 6a with, respectively, the neutral form of CD and the monoanionic form of CD.
′d) were fitted to a standard pH-titration model [eqn. (4)], where Kd and Kd− are the dissociation constants for the 1∶1 inclusion complexes of 6a with, respectively, the neutral form of CD and the monoanionic form of CD.
| |

|
(4)
|
 |
| | Fig. 2 Plot of calculated k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values versus pH for hydrolysis of 6a in the presence of α-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3). ′cat values versus pH for hydrolysis of 6a in the presence of α-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3).
| |
 |
| | Fig. 3 Plot of calculated k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values versus pH for hydrolysis of 6a in the presence of β-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3). ′cat values versus pH for hydrolysis of 6a in the presence of β-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3).
| |
The computed parameters are pKa = 13.0 ± 0.2, Kd = 9.7 ± 0.1 mM, and Kd− = 24 ± 5 mM for the α-CD-catalysed reaction and pKa = 12.7 ± 0.3, Kd = 4.3 ± 0.3 mM, and Kd− = 9.3 ± 1.4 mM for the β-CD-catalysed reaction.
The measured rate constant data for the hydrolysis of 6b as a function of CD concentration display the same trends as those seen for the hydrolysis of 6a. For instance, between pH values of 9.5 to 12.4 the rate constant in the absence of CD is independent of pH (kuncat = 1.243 ± 0.013 × 10−4 s−1) and the calculated value for k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat decreases at high pH values (Tables 5 and 6). Unfortunately, the measured rate constant data for the hydrolysis of 6b become ill-conditioned at high pH values, presumably as a result of the onset of base-promoted hydrolysis of the cyano (CN) group.
′cat decreases at high pH values (Tables 5 and 6). Unfortunately, the measured rate constant data for the hydrolysis of 6b become ill-conditioned at high pH values, presumably as a result of the onset of base-promoted hydrolysis of the cyano (CN) group.
| pH |
104khyd/s−1 |
K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM ′d/mM |
104k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1 ′cat/s−1 |
| 9.50 |
1.229 ± 0.004 |
15.5 ± 0.6 |
3.24 ± 0.05 |
| 10.60 |
1.223 ± 0.002 |
15.6 ± 0.3 |
3.18 ± 0.02 |
| 11.70 |
1.240 ± 0.014 |
15.1 ± 2.5 |
3.02 ± 0.16 |
| 12.18 |
1.235 ± 0.004 |
15.6 ± 0.9 |
2.84 ± 0.05 |
| 12.40 |
1.257 ± 0.011 |
15.4 ± 2.8 |
2.57 ± 0.13 |
| pH |
104khyd/s−1 |
K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM ′d/mM |
104k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1 ′cat/s−1 |
| 9.50 |
1.253 ± 0.006 |
6.40 ± 0.36 |
0.236 ± 0.022 |
| 10.60 |
1.252 ± 0.011 |
6.21 ± 0.61 |
0.252 ± 0.036 |
| 11.70 |
1.249 ± 0.011 |
5.87 ± 0.55 |
0.250 ± 0.034 |
| 12.18 |
1.229 ± 0.008 |
7.81 ± 0.58 |
0.158 ± 0.033 |
| 12.40 |
1.262 ± 0.001 |
8.50 ± 1.00 |
0.128 ± 0.057 |
Ground state binding
Cyclodextrins are able to form inclusion complexes with a wide range of compounds that are compatible in size with the dimensions of the cavity.5,6 The extent of complex formation also depends on the polarity of the guest molecule. In general, complex formation occurs as a result of an energetically favourable interaction of a relatively non-polar guest molecule with the hydrophobic cavity.5,6,23 As noted by several researchers, many simple phenyl derivatives bind more tightly to β- than to α-CD,5,6,10 and this difference in binding can be attributed to the cavity of α-CD being too constricted to allow complete encapsulation of the aryl ring.3,4 Similar observations were made in the present study: lower dissociation constants are observed for the binding of either 6a or 6b to β-CD, relative to their binding to α-CD (Kd, Table 7). In addition, binding to the CD of the neutral aromatic guest molecules 6a or 6b weakens upon ionisation of the CD to give a deprotonated hydroxy group (Kd−, Table 7). It is presumed that this effect results from the formation of a solvation shell around the alkoxide negative charge and as a consequence the binding of a hydrophobic group within the cavity becomes disfavoured.
Table 7 Calculated dissociation constants for the 1∶1 complexes of α-CD, β-CD, and β-CD-acid (5) binding with 6a, 6b, and their respective hydrolytic transition states
| |
6a
|
6b
|
| CD |
K
d/mM |
K
TS/mM |
K
d−/mM |
K
d/mM |
K
TS/mM |
|
Calculated by a non-linear least-squares fit to eqn. (4).
Calculated from the data at pH values 9.5–11.7 (Table 5).
Calculated from the data at pH values 9.5–11.7 (Table 6).
Calculated from the data at pH values 5.0–10.6 (Table 3).
Not determined.
|
| α-CD |
9.7 ± 0.1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
2.4 ± 0.1 |
24 ± 5![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
15.6 ± 0.3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
6.09 ± 0.14![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| β-CD |
4.3 ± 0.3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
6.8 ± 0.5 |
9.3 ± 1.4![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
6.2 ± 0.3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
31.8 ± 2.7![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
| β-CD-acid (5) |
8.6 ± 1.1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
15 ± 2![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
ND![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
ND![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
ND![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
Transition state binding
The transition state for the spontaneous reactions of 6a and 6b involves a simple C–O bond cleavage that generates an ion-pair as the first-formed intermediate (Scheme 3).13 The dissociation constant (KTS, Scheme 2) for binding of this transition state to the various cyclodextrins can be calculated according to eqn. (5).24Table 7 lists the derived dissociation constants for binding of the hydrolytic transition states for 6a and 6b to α-CD, β-CD, and β-CD-acid (5). Although the 2-(aryloxy)tetrahydropyrans can, in principle, bind into the CD cavity either “head first” or “head last” (Scheme 4), the calculated TS dissociation constants (Table 7) are consistent with the productive mode of binding being “head first” for the following reasons: (1) 4-nitrophenoxide binds tightly to both α-CD![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6
6![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ¶ and β-CD;25 and (2) binding of cations to the hydrophobic cavity of α-CD and β-CD is generally very weak.5,6 Thus, the CD-catalysed reaction cannot proceed via the “head last” binding mode to give the tetrahydropyranosyl oxacarbenium ion inside the cavity since this would yield much higher values for KTS. In addition, it would be expected that at high pH values this reaction mode would become more favourable as a hydroxy group on the CD rim becomes ionised. Hence, it appears that even at high pHs it is the aromatic ring that is bound in the cyclodextrin cavity, and complete inhibition of the acetal hydrolysis reaction occurs due to the energetic impediment to forming an aryloxide ion in close proximity to a CD-based alkoxide ion. This very unfavourable situation would result in the generation of a strong electrostatic repulsion.
¶ and β-CD;25 and (2) binding of cations to the hydrophobic cavity of α-CD and β-CD is generally very weak.5,6 Thus, the CD-catalysed reaction cannot proceed via the “head last” binding mode to give the tetrahydropyranosyl oxacarbenium ion inside the cavity since this would yield much higher values for KTS. In addition, it would be expected that at high pH values this reaction mode would become more favourable as a hydroxy group on the CD rim becomes ionised. Hence, it appears that even at high pHs it is the aromatic ring that is bound in the cyclodextrin cavity, and complete inhibition of the acetal hydrolysis reaction occurs due to the energetic impediment to forming an aryloxide ion in close proximity to a CD-based alkoxide ion. This very unfavourable situation would result in the generation of a strong electrostatic repulsion.
| |

|
(5)
|
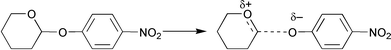 |
| | Scheme 3 | |
 |
| | Scheme 4 | |
Effect of an affixed CH2CO2− group
Since the attached carboxylic acid moiety of β-CD acid (5) has a pKa of 3.57 ± 0.02, all pH values greater than or equal to 5.0 will yield a CD that is greater than 96% ionised. Notwithstanding this fact, the data given in Tables 2 and 3 show that the attachment of this carboxylate group onto the secondary hydroxy face of β-CD gives rise to an insignificant change in the observed k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values for hydrolysis of 2-(4-nitrophenoxy)tetrahydropyran 6a. Although 6a binds approximately 2-fold more tightly to β-CD than it does to β-CD-acid, binding of the hydrolytic TS to β-CD and β-CD-acid displays a similar 2-fold difference. Therefore, cancellation of these two binding perturbations results in the observed k
′cat values for hydrolysis of 2-(4-nitrophenoxy)tetrahydropyran 6a. Although 6a binds approximately 2-fold more tightly to β-CD than it does to β-CD-acid, binding of the hydrolytic TS to β-CD and β-CD-acid displays a similar 2-fold difference. Therefore, cancellation of these two binding perturbations results in the observed k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values being essentially unchanged. This minor perturbation in k
′cat values being essentially unchanged. This minor perturbation in k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat arises from an almost exact cancellation of the changes in binding of 6a and its hydrolytic TS to both β-CD and β-CD-acid (Table 7).
′cat arises from an almost exact cancellation of the changes in binding of 6a and its hydrolytic TS to both β-CD and β-CD-acid (Table 7).
Hydrolysis of 2-deoxy-D-glucopyranosylpyridinium salts
Spontaneous hydrolysis of 7c.
Table S7 (supplementary data) lists the observed rate constants for hydrolysis of 7c as a function of pH. The hydrolytic rate constants for 7c are independent of pH, therefore, hydrolysis occurs without catalysis by either H3O+ or OH− at pH values between 4.4 and 10.8, and thus these reactions display an identical trend to those reported for 7a, 7b, 7d, 8a and 8b.26 In addition, Tables S8 and S9 (supplementary data) list the observed first-order rate constants as functions of temperature for the hydrolyses of 7b and 7c. The derived activation parameters for the hydrolyses of 7c and 7b are ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = 27.9 ± 0.2 kcal mol−1, ΔS
‡ = 27.9 ± 0.2 kcal mol−1, ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = +14.6 ± 0.7 cal mol−1 K−1, and ΔH
‡ = +14.6 ± 0.7 cal mol−1 K−1, and ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = 31.8 ± 0.5 kcal mol−1, ΔS
‡ = 31.8 ± 0.5 kcal mol−1, ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = +14.6 ± 1.5 cal mol−1 K−1, respectively. Therefore, the difference in the pH-independent hydrolytic rate constants for 7c and 7b originates from a variation in their individual activation enthalpies (27.9 ± 0.2 and 31.8 ± 0.5 kcal mol−1, respectively). In all likelihood this difference arises from a greater release of ground state strain energy for 7c that is caused by a severe steric interaction between the sugar and the quinoline moiety.
‡ = +14.6 ± 1.5 cal mol−1 K−1, respectively. Therefore, the difference in the pH-independent hydrolytic rate constants for 7c and 7b originates from a variation in their individual activation enthalpies (27.9 ± 0.2 and 31.8 ± 0.5 kcal mol−1, respectively). In all likelihood this difference arises from a greater release of ground state strain energy for 7c that is caused by a severe steric interaction between the sugar and the quinoline moiety.
Cyclodextrin-catalysed hydrolysis.
The observed rate constants (kobs) for hydrolyses of 7a–d and 8a, 8b as a function of α-CD, β-CD, β-CD-acid and γ-CD concentration are given in Tables S10–S13 (supplementary data), respectively. Plots of observed rate constant (kobs) versus CD concentration for these substrates 7a–d and 8a, 8b display no discernible curvature (for an example see Fig. 4). Therefore, under the conditions used in these experiments, binding to the various CDs must be weak. If it is assumed that a 10% deviation from linearity is detectable, then all dissociation constants for the CD-bound pyridinium salts (Kd) must be greater than 150 mM. The weak binding of the substrates 7a–d and 8a, 8b to the CD cavity is not too surprising given that, in general, binding of cations to the CD hydrophobic cavity is very weak. As an example, for the anilinium ion PhNH3+ and β-CD, the measured dissociation constant (Kd) is 440 mM.25,27
 |
| | Fig. 4 Plot of the observed order rate constant kobsversus CD concentration for the β-CD-catalysed hydrolysis of 7a (•) and 8a (■), pH = 7.0, T = 65 °C. Error limits are encompassed within the symbol diameter. The lines are the best linear least-squares fits through the data points.
| |
Due to the weak binding interactions between the various CDs and the glycosyl pyridinium salts, the hydrolytic rate constant data were analysed using eqn. (6), which contains the linear terms from the Taylor expansion of eqn. (2). Given in Tables 8–10 are the derived values for kuncat and (kcat − kuncat)/Kd calculated from the linear fits of kobsversus CD concentration. Also given in Tables 8–10 is the ratio of slope∶intercept obtained from the linear fits of [CD] versus reaction rate. This ratio can be expressed in several different ways, as is shown in eqn. (7). Clearly, the calculated data for β-CD and the single case for β-CD-acid show that these cyclodextrins catalyse the hydrolysis of 2-deoxy-α- and β-D-glucopyranosylpyridinium salts more efficiently than either α-CD or γ-CD. Unfortunately, the limited solubility of β-CD in water (1.85 g cm−3 at 25 °C),3 restricted the maximum concentration of β-CD used in these studies to 14.7 mM. The value of 43.4 M−1 for [(kcat/kuncat) − 1]/Kd calculated for the β-CD-catalysed hydrolysis of 7b requires that kcat/kuncat ≥ 7.5 as Kd ≥ 150 mM for these cations (vide supra).
| |

|
(6)
|
| |

|
(7)
|
Table 8 Calculated constants for the hydrolysis of 7a–d and 8a, 8b in the presence of α-CD, pH = 7.0,aμ = 0.5 M (KCl)
| |
[104 (kcat − kuncat)/Kd]/M−1 s−1 |
104kuncat/s−1 |
(1/KTS −1/Kd)/M−1 |
T/°C |
|
Phosphate buffer; [buffer]total = 10.0 mM.
|
|
7a
|
81.1 ± 4.5 |
27.31 ± 0.03 |
2.97 ± 0.16 |
65.0 |
|
7b
|
3.22 ± 0.38 |
1.623 ± 0.003 |
1.98 ± 0.23 |
75.0 |
|
7c
|
9.1 ± 4.1 |
0.998 ± 0.027 |
9.1 ± 4.1 |
45.0 |
|
7d
|
1.73 ± 0.44 |
1.047 ± 0.002 |
1.65 ± 0.48 |
65.0 |
|
8a
|
12.0 ± 1.1 |
4.212 ± 0.008 |
2.85 ± 0.26 |
65.0 |
|
8b
|
2.01 ± 0.67 |
1.499 ± 0.004 |
1.34 ± 0.44 |
90.0 |
Table 9 Calculated constants for the hydrolysis of 7a–d and 8a, 8b in the presence of β-CD, pH = 7.0,aμ = 0.5 M (KCl)
| |
[104 (kcat − kuncat)/Kd]/M−1 s−1 |
104kuncat/s−1 |
(1/KTS −1/Kd)/M−1 |
T/°C |
|
Phosphate buffer; [buffer]total = 10.0 mM.
Values for β-CD acid.
Extrapolated value for 65 °C is 4.3.
|
|
7a
|
712 ± 9 |
27.30 ± 0.06 |
26.1 ± 0.3 |
65.0 |
7a![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
728 ± 11 |
26.70 ± 0.08 |
27.3 ± 0.4 |
65.0 |
|
7b
|
15.9 ± 0.7 |
0.366 ± 0.005 |
43.4 ± 2.0 |
65.0 |
|
7b
|
49.5 ± 0.6 |
1.634 ± 0.004 |
30.3 ± 0.4 |
75.0 |
|
7b
|
156 ± 4 |
6.032 ± 0.025 |
25.9 ± 0.7 |
85.0 |
|
7c
|
24.2 ± 1.3 |
1.943 ± 0.009 |
12.5 ± 0.7 |
35.0 |
|
7c
|
81.4 ± 1.6 |
9.950 ± 0.010 |
8.18 ± 0.16 |
45.0 |
|
7c
|
201 ± 16 |
33.36 ± 0.11 |
6.03 ± 0.48![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
55.0 |
|
7d
|
24.7 ± 0.5 |
1.058 ± 0.003 |
23.3 ± 0.5 |
65.0 |
|
8a
|
107.2 ± 1.0 |
4.219 ± 0.006 |
25.4 ± 0.2 |
65.0 |
|
8b
|
40.5 ± 0.5 |
1.502 ± 0.004 |
27.0 ± 0.3 |
90.0 |
Table 10 Calculated constants for the hydrolysis of 7b and 7c in the presence of γ-CD, pH = 7.0,aμ = 0.5 M (KCl)
| |
[104 (kcat − kuncat)/Kd]/M−1 s−1 |
104kuncat/s−1 |
(1/KTS −1/Kd)/M−1 |
T/°C |
|
Phosphate buffer; [buffer]total = 10.0 mM.
|
|
7b
|
7.23 ± 0.77 |
1.571 ± 0.01 |
4.60 ± 0.49 |
75.0 |
|
7c
|
18.6 ± 3.3 |
8.89 ± 0.02 |
2.09 ± 0.37 |
45.0 |
The weak catalysis exhibited by α-CD presumably results from a reduced interaction between the heteroaromatic rings of the substrates and the smaller cyclodextrin cavity. In comparison to either α-CD or β-CD, fewer binding studies have been performed with γ-CD.6 Therefore, at the present time, a more detailed analysis for the low activity of γ-CD towards C–N bond cleavage is unwarranted.
Effect of anomeric configuration.
The data listed in Table 9 clearly show that the CD-catalysed hydrolyses for both anomers of 2-deoxy-D-glucopyranosyl-4′-bromoisoquinolinium (7a and 8a) and isoquinolinium (7b and 8b) tetrafluoroborates display similar values for the parameter (1/KTS − 1/Kd).|| Consequently, the conformation of the carbohydrate ring is unimportant in these CD-catalysed reactions. The binding mode probably involves insertion of the aromatic portion of the guest molecule into the CD’s cavity while the sugar fragment remains in bulk solvent.
Effect of temperature on the β-CD catalysed reactions
The results listed in Table 9 clearly show that there is a significant effect of temperature on the calculated (1/KTS − 1/Kd) values [eqn. (7)], and that as the temperature decreases, the relative binding of the hydrolytic transition state to the ground state becomes tighter. In other words, the catalytic effect of the CD increases.
Since the magnitude of (1/KTS − 1/Kd) increases with decreasing temperatures, there must be a larger negative enthalpy of binding of the hydrolytic transition state to the hydrophobic cavity (1/KTS) compared to binding of the ground state to the CD’s cavity (1/Kd). This enthalpic difference is caused by a more favourable enthalpy of binding as the positive charge on the aromatic base diminishes as the reaction approaches the hydrolytic transition state. As a consequence it would be expected that these reactions should show significant product inhibition. That no such effects are seen results from the much greater concentration of cyclodextrin relative to that of the pyridinium compounds used in these experiments.
Conclusions
Spontaneous hydrolyses of 6a and 6b are accelerated in the presence of α-CD at pH values where the cyclodextrin remains neutral. However, at high pH values the addition of α-CD completely inhibits hydrolysis. The observed rate constants for the hydrolysis reactions in the presence of CD are consistent with the CD-catalysed reaction occurring from a 1∶1 complex which becomes catalytically inert when a single CD hydroxy group is ionised.
The spontaneous hydrolysis reactions of a series of 2-deoxy-D-glucopyranosylpyridinium tetrafluoroborates are accelerated by β-CD. Catalysis occurs because, during the catalysed reaction, the diminishing positive charge on the heteroaromatic ring increases the favourable binding enthalpy to the hydrophobic CD cavity.
Experimental
Materials and methods
All pH values were measured using a Radiometer pHM82 standard pH meter and a standard combination glass electrode standardised with Fisher certified buffers (pH = 4.0, 7.0, and 10.0). Quinoline was purchased from Aldrich and purified by recrystallisation of its hydrogen sulfate salt from HOAc–Et2O. Cyclodextrins (α-CD, β-CD, and γ-CD) were purchased from Sigma and these compounds were dried overnight in vacuo (0.25 mmHg) at 100 °C over P4O10 prior to use. Heptakis[6-O-(tert-butyldimethylsilyl)]-β-CD,15 2-(4-nitrophenoxy)tetrahydropyran and 2-(4-cyanophenoxy)tetrahydropyran![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 were synthesized according to published procedures. Chemical shifts (δH and δC) are in ppm downfield from signals for TMS. The residual signals from deuterated chloroform and external TMS-salts (D2O) were used as 1H NMR references; for 13C NMR spectra, natural abundance signals from CDCl3 and external TMS-salt (D2O) were used as references. Coupling constants (J
12 were synthesized according to published procedures. Chemical shifts (δH and δC) are in ppm downfield from signals for TMS. The residual signals from deuterated chloroform and external TMS-salts (D2O) were used as 1H NMR references; for 13C NMR spectra, natural abundance signals from CDCl3 and external TMS-salt (D2O) were used as references. Coupling constants (J![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) are given in Hz.
) are given in Hz.
Preparations
Mono[2-O-(ethoxycarbonylmethyl)]heptakis[6-O-(tert-butyldimethylsilyl)]-β-CD (3).
Silver oxide (0.57 g, 2.45 mmol) and ethyl 2-iodoacetate (0.16 cm3, 1.2 equiv.) were added in one portion to a solution of heptakis[6-O-(tert-butyldimethylsilyl)]-β-CD (2) (1.9 g, 0.98 mmol) in dry DMF (15 cm3) and the reaction mixture was stirred at RT for 20 h. After the precipitate had been removed by filtration through Celite, the solution was concentrated under reduced pressure to give a light yellow solid. The crude product was purified by flash column chromatography (ethyl acetate–ethanol–water 50∶2∶1) to give a colourless solid (0.39 g, 32% based on recovered starting material). Mp 180–183 °C (decomp.); νmax (KBr)/cm−1 1753 and 1727 (CO); δH(400 MHz; CDCl3) 0.00–0.10 (m, 42 H, CH3-Si), 0.80–0.95 (m, 63 H, (CH3)3-C), 1.25 (t, 3 H, J 7, CH3CH2), 3.23–3.29 (m, 1 H, H-2A), 3.50–4.00 (m, 39 H), 4.05–4.10 (m, 2 H, H-3A, H-4A), 4.12 (q, 2 H, J 7, CH2CH3), 4.39 (d, 1 H, J 10, OCHaHb), 4.51 (d, 1 H, OCHaHb), 5.03 (br s, 6 H, H-1S), 5.25 (d, 1 H, J1,2 = 3.5 Hz, H-1A); δC(100 MHz; CDCl3) −5.2, −5.1, −5.0, 14.2, 18.3, 18.5, 25.9, 26.0, 60.8, 61.7, 61.8, 69.6, 69.8, 72.5, 72.6, 72.7, 72.8, 73.0, 73.2, 73.4, 73.5, 73.6, 73.7, 74.0, 81.7, 81.9, 82.0, 82.1, 82.7, 101.1, 101.9, 102.2, 102.1, 102.2, 102.4, 103.0, 170.5 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O). Found: C 52.5; H 8.8. C88H174O37Si7 requires C 52.3; H 8.7%.
O). Found: C 52.5; H 8.8. C88H174O37Si7 requires C 52.3; H 8.7%.
Mono[2-O-(ethoxycarbonylmethyl)]-β-CD (4).
BF3·Et2O (0.11 cm3) was added to a solution of 3 (0.20 g, 0.01 mmol) in dry CHCl3 (5 cm3) and the solution was stirred at RT for 5 h. The precipitated cyclodextrin was filtered, washed with CHCl3 (10 cm3), and purified by precipitation from a water solution (1 cm3) using CH3CN (10 cm3). The resulting solid, which was collected and dried overnight at 110 °C under vacuum, gave an analytically pure sample of 4 (0.09 g, 75%). Mp 241–244 °C (decomp.); νmax (KBr)/cm−1 1748; δH(400 MHz; D2O) 1.25 (t, 3 H, J 7, CH3), 3.50–3.63 (m, 14 H, H-2A, H-2S, H-4A, H-4S), 3.76–3.95 (m, 21 H, H-5A, H-5S, H-6A, H-6S), 3.91 (br t, 6 H, J2,3 + J3,4 20.0, H-3S), 4.08 (t, 1 H, J2,3 + J3,4 19.0, H-3A), 4.22 (m, 2 H, CH2CH3), 4.40 (br s, 2 H, OCH2), 5.03 (br s, 6 H, H-1S), 5.25 (d, 1 H, J1,2 3.6, H-1A); δC(100 MHz; D2O) 16.2, 63.2, 65.1, 71.8, 74.4, 74.6, 74.7, 74.9, 75.4, 75.9, 83.7, 83.9, 84.7, 101.0, 101.7, 102.3, 102.1, 102.5, 102.7, 103.7, 174.8. Found: C 44.6; H 6.05. C46H76O37·H2O requires C 44.6; H 6.3%. m/z (MALDI-TOF![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) 1121.3 (M + H+).
) 1121.3 (M + H+).
Mono[2-O-(carboxymethyl)]-β-CD (5).
Compound 4 (100 mg, 0.082 mmol) was dissolved in NaOH (0.1 M, 10 cm3), and the reaction mixture was stirred at 50–60 °C for 4 h. After cooling to room temperature, Amberlite resin IR-120 (H+-form) was added to the reaction mixture and the resultant solution was stirred at RT until it became weakly acidic. After the resin was removed by filtration, the solution was concentrated under vacuum to give a white solid (0.088 g, 91%). This material was further purified by dissolving in water (1 cm3) followed by the addition of CH3CN (10 cm3) to give a colourless precipitate (0.083 g, 85% after drying under vacuum for 24 h). Mp 206–208 °C (decomp.); νmax (KBr)/cm−1 1750; δH(400 MHz; D2O) 3.49–3.62 (m, 14 H, H-2A, H-2S, H-4A, H-4S), 3.78–3.88 (m, 21 H, H-5A, H-5S, H-6A, H-6S, H-6′A, H-6′S), 3.91 (dd, 6 H, J2,3 = J3,4 10, H-3S), 4.05 (dd, 1 H, J2,3 = J3,4 10 Hz, H-3A), 4.31 (br s, 2 H, OCH2), 5.02 (br s, 6 H, H-1S), 5.24 (d, 1 H, J1,2 3.5, H-1A); δC(100 MHz; D2O) 60.6, 61.9, 62.0, 74.4, 74.6, 74.7, 74.9, 75.0, 83.8, 83.9, 84.3, 101.3, 101.5, 102.0, 102.1, 102.5, 103.0, 103.2, 174.4. Found: C 43.6; H 6.1. C44H72O37·H2O requires C 43.6; H 6.2%.
All kinetic runs were monitored using a Cary 3E UV–Vis spectrophotometer equipped with the Cary six-cell Peltier constant-temperature accessory. The hydrolysis reactions of 6a and 6b were followed under basic conditions at 402 and 275.5 nm, respectively, while the corresponding reactions at pH values of 3.5 and 5.0 were monitored at 349 and 238 nm, respectively. Reactions were initiated by the injection of an aqueous stock solution of the required substrate and cyclodextrin into a pre-equilibrated buffer solution (1.00 cm3, μ = 0.2 KCl). The temperature dependence studies of the spontaneous hydrolysis reactions of 2-deoxy-α-D-arabino-hexopyranosylquinolinium tetrafluoroborate (7b) and of 2-deoxy-α-D-arabino-hexopyranosylisoquinolinium tetrafluoroborate (7c) were performed using buffer concentrations of 0.01 M and an ionic strength of 1.0 M (NaClO4), while the pH–rate profile of 7c used an ionic strength of 2.0 (NaClO4). The hydrolysis reactions of 7a, 7b, 7c, 7d, 8a, and 8b were followed at 346, 338, 320, 261, 345, and 235 nm, respectively. Reactions were initiated by the injection of an aqueous stock solution of the required substrate and cyclodextrin into a pre-equilibrated buffer solution (1.00 cm3, μ = 0.5 KCl). All pseudo-first-order rate constants were calculated by non-linear least-squares fitting of the observed absorbance versus time data to a standard single exponential rate equation.
Acknowledgements
The authors gratefully acknowledge the Natural Sciences and Engineering Research Council of Canada and Simon Fraser University for financial support of this work. The authors would like to thank Dr T. E. Kitos for editorial assistance with this manuscript and Mr G. C. Winters for acquiring the mass spectrometric data.
References
- A. Villiers, C. R. Hebd. Seances Acad. Sci., 1891, 112, 536 Search PubMed.
- F. Schardinger, Z. Unters. Nahr.-Genussm. Gebrauchsgegenstaende, 1903, 6, 865 Search PubMed.
-
M. Bender
and M. Komiyama
, Cyclodextrin Chemistry, Springer Verlag, New York, 1978.
Search PubMed.
-
J. Szejtli
, Cyclodextrins and Their Inclusion Complexes, Akademiai Kiado, Budapest, 1982;
Search PubMed;
J. Szejtli
, Cyclodextrin Technology, Kluwer Academic Publishers, Budapest, 1988.
Search PubMed.
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875 CrossRef CAS; I. Tabushi, Tetrahedron, 1984, 40, 269 CrossRef CAS; R. Breslow and C. Schmuck, J. Am. Chem. Soc., 1996, 118, 6601 CrossRef CAS.
-
K. A. Connors
, in Comprehensive Supramolecular Chemistry: Cyclodextrins, eds. J. Szejtli and T. Osa, Pergamon Press, New York, 1996, vol. 3, ch. 6.
Search PubMed.
-
T. Osa
and I. Suzuki
, in Comprehensive Supramolecular Chemistry: Cyclodextrins, eds. J. Szejtli and T. Osa, Pergamon Press, New York, 1996, vol. 3, ch. 11;
Search PubMed; A. J. Kirby, Angew. Chem., Int. Ed. Engl., 1994, 33, 551 Search PubMed; R. Breslow, Pure Appl. Chem., 1994, 66, 1573 CrossRef; R. Breslow, Acc. Chem. Res., 1995, 28, 146 CAS; Y. Murakami, J. I. Kikuchi, Y. Hisaeda and O. Hayashida, Chem. Rev., 1996, 96, 721 CrossRef CAS; R. Breslow and S. D. Dong, Chem. Rev., 1998, 98, 1997 CrossRef CAS.
-
L. Jicsinszky
, É. Fenyvesi
and H. Hashimoto
, in Comprehensive Supramolecular Chemistry: Cyclodextrins, eds. J. Szejtli and T. Osa, Pergamon Press, New York, 1996, vol. 3, ch. 4.
Search PubMed.
- B. Siegel, A. Pinter and R. Breslow, J. Am. Chem. Soc., 1977, 99, 2309 CrossRef CAS.
- O. S. Tee, A. A. Fedortchenko and P. L. Soo, J. Chem. Soc., Perkin Trans. 2, 1998, 123 RSC.
- E. H. Cordes, Prog. Phys. Org. Chem., 1967, 4, 1 Search PubMed.
- T. H. Fife and L. K. Jao, J. Am. Chem. Soc., 1968, 90, 4081 CrossRef CAS.
- T. H. Fife and L. H. Brod, J. Am. Chem. Soc., 1970, 92, 1681 CrossRef CAS.
- B. L. Knier and W. P. Jencks, J. Am. Chem. Soc., 1980, 102, 6789 CrossRef CAS.
- M. J. Pregel and E. Buncel, Can. J. Chem., 1991, 69, 130 CAS.
- A. W. Coleman, P. Zhang, H. Parrot-Lopez, C.-C. Ling, M. Miocque and L. Mascrier, Tetrahedron Lett., 1991, 32, 3997 CrossRef CAS.
- F. M. Menger and M. A. Dulany, Tetrahedron Lett., 1985, 26, 267 CrossRef CAS.
- A. Ueno and R. Breslow, Tetrahedron Lett., 1982, 23, 3451 CrossRef CAS.
- K. Bock and C. Peterson, J. Chem. Soc., Perkin Trans. 2, 1974, 293 RSC.
-
D. T. H. Chou
and A. J. Bennet
, unpublished results.
.
- R. Breslow, G. Trainer and A. Ueno, J. Am. Chem. Soc., 1983, 105, 2739 CrossRef CAS; F. M. Menger and M. Ladika, J. Am. Chem. Soc., 1987, 109, 3145 CrossRef CAS.
-
M. H. Kleinman
and C. Bohne
, in Molecular and Supramolecular Photochemistry, eds. V. Ramamurthy and K. S. Schanze, Marcel Dekker, New York, 1997, vol. 1, p. 391.
Search PubMed.
- W. Saenger, Angew. Chem., Int. Ed. Engl., 1980, 19, 344 CrossRef; G. Wenz, Angew. Chem., Int. Ed. Engl., 1994, 33, 803 CrossRef; S. Li and W. C. Purdy, Chem. Rev., 1992, 92, 1457 CrossRef CAS.
- O. S. Tee, Adv. Phys. Org. Chem., 1994, 29, 1 Search PubMed.
- A. Buvári and L. Barcza, J. Chem. Soc., Perkin Trans. 2, 1988, 543 RSC.
- X. Huang, C. Surry, T. Hiebert and A. J. Bennet, J. Am. Chem. Soc., 1995, 117, 10614 CrossRef CAS; J. Zhu and A. J. Bennet, J. Am. Chem. Soc., 1998, 120, 3887 CrossRef CAS.
- E. A. Lewis and L. D. Hansen, J. Chem. Soc., Perkin Trans. 2, 1973, 2081 RSC.
Footnotes |
| † Complete tables of observed rate constants for the hydrolyses of 6a, 6b, 7a–d, 8a and 8bversus cyclodextrin concentrations are available as supplementary data. For direct electronic access see http://www.rsc.org/suppdata/p2/b0/b006568o/ |
| ‡ When seven OCH2CO2H groups are substituted onto the secondary β-CD face, the 13C NMR (100 MHz; D2O) chemical shift for C2 is 7.2 ppm downfield from the corresponding resonance in β-CD itself.20 |
| § Eqn. (2) corresponds to the standard Michaelis–Menten expression when khyd = 0. |
| ¶ Average and standard deviation for the 10 middle values given in Table 5, ref. 6. |
| || The extrapolated [(kcat/kuncat) − 1]/Kd value for 7b at 90 °C is 22.2 M−1. |
|
| This journal is © The Royal Society of Chemistry 2001 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) †
†![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 was soon followed by their preparation and isolation by Schardinger in the early 1900s.2 Since that time, continued scientific interest has led to the exploration and development of a variety of novel chemical applications for these cyclic oligosaccharides.3,4 The ability of these compounds to form inclusion complexes with hydrophobic guest molecules
1 was soon followed by their preparation and isolation by Schardinger in the early 1900s.2 Since that time, continued scientific interest has led to the exploration and development of a variety of novel chemical applications for these cyclic oligosaccharides.3,4 The ability of these compounds to form inclusion complexes with hydrophobic guest molecules![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4–6 highlights the potential for use of cyclodextrins and their derivatives as artificial enzyme systems.7 In view of their possible role as enzyme models, a vast array of derivatised cyclodextrins have been synthesized, thus expanding the cyclodextrin functionality in order to increase their catalytic potential.8
4–6 highlights the potential for use of cyclodextrins and their derivatives as artificial enzyme systems.7 In view of their possible role as enzyme models, a vast array of derivatised cyclodextrins have been synthesized, thus expanding the cyclodextrin functionality in order to increase their catalytic potential.8
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9,10 or basic reaction conditions, undoubtedly because very few of these hydrolysis reactions have significant non-catalysed reaction rates. In general, acetal hydrolysis involves specific-acid catalysis, i.e., rapid, reversible protonation followed by rate-limiting unimolecular C–O bond cleavage.11 However, acetals that contain activated leaving groups can hydrolyse via a dissociative, uncatalysed mechanism. For example, 2-(4-aryloxy)tetrahydropyrans hydrolyse via both general-acid catalysed and spontaneous uncatalysed reaction pathways.12,13 Acetals that contain positively charged leaving groups can also hydrolyse without acid-catalysis.14
9,10 or basic reaction conditions, undoubtedly because very few of these hydrolysis reactions have significant non-catalysed reaction rates. In general, acetal hydrolysis involves specific-acid catalysis, i.e., rapid, reversible protonation followed by rate-limiting unimolecular C–O bond cleavage.11 However, acetals that contain activated leaving groups can hydrolyse via a dissociative, uncatalysed mechanism. For example, 2-(4-aryloxy)tetrahydropyrans hydrolyse via both general-acid catalysed and spontaneous uncatalysed reaction pathways.12,13 Acetals that contain positively charged leaving groups can also hydrolyse without acid-catalysis.14![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡
‡



![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat is the maximal rate constant in the presence of CD, khyd is the rate constant in the absence of CD, and K
′cat is the maximal rate constant in the presence of CD, khyd is the rate constant in the absence of CD, and K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d is the apparent dissociation constant of the 1∶1 complex.9
′d is the apparent dissociation constant of the 1∶1 complex.9![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) §
§
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat, khyd, and K
′cat, khyd, and K![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d are listed in Tables 1–4. The derived values of khyd (Tables 1 and 2) were fitted to eqn. (1) to give estimates for the acid-catalysed (kH = 1.59 ± 0.05 M−1 s−1) and the spontaneous (kuncat = 1.115 ± 0.005 × 10−3 s−1) hydrolysis rate constants for 6a at 50 °C. These calculated values for kuncat and kH are very similar to the value of 1.175 × 10−3 s−1 (μ = 0.5, KCl) for kuncat and 2.17 M−1 s−1 (μ = 0.5, KCl) for kH measured at 50 °C by Fife and Brod.13
′d are listed in Tables 1–4. The derived values of khyd (Tables 1 and 2) were fitted to eqn. (1) to give estimates for the acid-catalysed (kH = 1.59 ± 0.05 M−1 s−1) and the spontaneous (kuncat = 1.115 ± 0.005 × 10−3 s−1) hydrolysis rate constants for 6a at 50 °C. These calculated values for kuncat and kH are very similar to the value of 1.175 × 10−3 s−1 (μ = 0.5, KCl) for kuncat and 2.17 M−1 s−1 (μ = 0.5, KCl) for kH measured at 50 °C by Fife and Brod.13![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM
′d/mM![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1
′cat/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM
′d/mM![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1
′cat/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM
′d/mM![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1
′cat/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM
′d/mM![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1
′cat/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat) for the CD-catalysed hydrolysis of 6a (Tables 1 and 2). Consequently, the derived values for k
′cat) for the CD-catalysed hydrolysis of 6a (Tables 1 and 2). Consequently, the derived values for k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat (Tables 1 and 2) were fitted to eqn. (3), where kcat is the maximal rate constant for the neutral cyclodextrin-catalysed hydrolysis of 6a, kcat− is the rate constant for the hydrolysis of the CD–acetal complex when the cyclodextrin is fully ionised, and Ka is the acid dissociation constant for the CD.
′cat (Tables 1 and 2) were fitted to eqn. (3), where kcat is the maximal rate constant for the neutral cyclodextrin-catalysed hydrolysis of 6a, kcat− is the rate constant for the hydrolysis of the CD–acetal complex when the cyclodextrin is fully ionised, and Ka is the acid dissociation constant for the CD.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d) were fitted to a standard pH-titration model [eqn. (4)], where Kd and Kd− are the dissociation constants for the 1∶1 inclusion complexes of 6a with, respectively, the neutral form of CD and the monoanionic form of CD.
′d) were fitted to a standard pH-titration model [eqn. (4)], where Kd and Kd− are the dissociation constants for the 1∶1 inclusion complexes of 6a with, respectively, the neutral form of CD and the monoanionic form of CD.

![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values versus pH for hydrolysis of 6a in the presence of α-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3).
′cat values versus pH for hydrolysis of 6a in the presence of α-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3).

![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values versus pH for hydrolysis of 6a in the presence of β-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3).
′cat values versus pH for hydrolysis of 6a in the presence of β-CD, T = 50 °C. The line shown is the best non-linear least-squares fit to eqn. (3).
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat decreases at high pH values (Tables 5 and 6). Unfortunately, the measured rate constant data for the hydrolysis of 6b become ill-conditioned at high pH values, presumably as a result of the onset of base-promoted hydrolysis of the cyano (CN) group.
′cat decreases at high pH values (Tables 5 and 6). Unfortunately, the measured rate constant data for the hydrolysis of 6b become ill-conditioned at high pH values, presumably as a result of the onset of base-promoted hydrolysis of the cyano (CN) group.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM
′d/mM![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1
′cat/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′d/mM
′d/mM![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat/s−1
′cat/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b
b![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b
b![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c
c![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c
c![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d
d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d
d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e
e![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e
e![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e
e![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6
6![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ¶ and β-CD;25 and (2) binding of cations to the hydrophobic cavity of α-CD and β-CD is generally very weak.5,6 Thus, the CD-catalysed reaction cannot proceed via the “head last” binding mode to give the tetrahydropyranosyl oxacarbenium ion inside the cavity since this would yield much higher values for KTS. In addition, it would be expected that at high pH values this reaction mode would become more favourable as a hydroxy group on the CD rim becomes ionised. Hence, it appears that even at high pHs it is the aromatic ring that is bound in the cyclodextrin cavity, and complete inhibition of the acetal hydrolysis reaction occurs due to the energetic impediment to forming an aryloxide ion in close proximity to a CD-based alkoxide ion. This very unfavourable situation would result in the generation of a strong electrostatic repulsion.
¶ and β-CD;25 and (2) binding of cations to the hydrophobic cavity of α-CD and β-CD is generally very weak.5,6 Thus, the CD-catalysed reaction cannot proceed via the “head last” binding mode to give the tetrahydropyranosyl oxacarbenium ion inside the cavity since this would yield much higher values for KTS. In addition, it would be expected that at high pH values this reaction mode would become more favourable as a hydroxy group on the CD rim becomes ionised. Hence, it appears that even at high pHs it is the aromatic ring that is bound in the cyclodextrin cavity, and complete inhibition of the acetal hydrolysis reaction occurs due to the energetic impediment to forming an aryloxide ion in close proximity to a CD-based alkoxide ion. This very unfavourable situation would result in the generation of a strong electrostatic repulsion.



![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values for hydrolysis of 2-(4-nitrophenoxy)tetrahydropyran 6a. Although 6a binds approximately 2-fold more tightly to β-CD than it does to β-CD-acid, binding of the hydrolytic TS to β-CD and β-CD-acid displays a similar 2-fold difference. Therefore, cancellation of these two binding perturbations results in the observed k
′cat values for hydrolysis of 2-(4-nitrophenoxy)tetrahydropyran 6a. Although 6a binds approximately 2-fold more tightly to β-CD than it does to β-CD-acid, binding of the hydrolytic TS to β-CD and β-CD-acid displays a similar 2-fold difference. Therefore, cancellation of these two binding perturbations results in the observed k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat values being essentially unchanged. This minor perturbation in k
′cat values being essentially unchanged. This minor perturbation in k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′cat arises from an almost exact cancellation of the changes in binding of 6a and its hydrolytic TS to both β-CD and β-CD-acid (Table 7).
′cat arises from an almost exact cancellation of the changes in binding of 6a and its hydrolytic TS to both β-CD and β-CD-acid (Table 7).
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = 27.9 ± 0.2 kcal mol−1, ΔS
‡ = 27.9 ± 0.2 kcal mol−1, ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = +14.6 ± 0.7 cal mol−1 K−1, and ΔH
‡ = +14.6 ± 0.7 cal mol−1 K−1, and ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = 31.8 ± 0.5 kcal mol−1, ΔS
‡ = 31.8 ± 0.5 kcal mol−1, ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ = +14.6 ± 1.5 cal mol−1 K−1, respectively. Therefore, the difference in the pH-independent hydrolytic rate constants for 7c and 7b originates from a variation in their individual activation enthalpies (27.9 ± 0.2 and 31.8 ± 0.5 kcal mol−1, respectively). In all likelihood this difference arises from a greater release of ground state strain energy for 7c that is caused by a severe steric interaction between the sugar and the quinoline moiety.
‡ = +14.6 ± 1.5 cal mol−1 K−1, respectively. Therefore, the difference in the pH-independent hydrolytic rate constants for 7c and 7b originates from a variation in their individual activation enthalpies (27.9 ± 0.2 and 31.8 ± 0.5 kcal mol−1, respectively). In all likelihood this difference arises from a greater release of ground state strain energy for 7c that is caused by a severe steric interaction between the sugar and the quinoline moiety.



![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b
b![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c
c![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 were synthesized according to published procedures. Chemical shifts (δH and δC) are in ppm downfield from signals for TMS. The residual signals from deuterated chloroform and external TMS-salts (D2O) were used as 1H NMR references; for 13C NMR spectra, natural abundance signals from CDCl3 and external TMS-salt (D2O) were used as references. Coupling constants (J
12 were synthesized according to published procedures. Chemical shifts (δH and δC) are in ppm downfield from signals for TMS. The residual signals from deuterated chloroform and external TMS-salts (D2O) were used as 1H NMR references; for 13C NMR spectra, natural abundance signals from CDCl3 and external TMS-salt (D2O) were used as references. Coupling constants (J![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) are given in Hz.
) are given in Hz.
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O). Found: C 52.5; H 8.8. C88H174O37Si7 requires C 52.3; H 8.7%.
O). Found: C 52.5; H 8.8. C88H174O37Si7 requires C 52.3; H 8.7%.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) 1121.3 (M + H+).
) 1121.3 (M + H+).


