Picolyl-armed 1,3-alternate calix[4]areneazacrown ethers
Jong
Seung Kim
*a,
Ok Jae
Shon
a,
Wonbo
Sim
a,
Sun
Kyu Kim
b,
Moon Hwan
Cho
b,
Jin-Gyu
Kim
c,
Il-Hwan
Suh
c and
Dong Won
Kim
d
aDepartment of Chemistry, Konyang University, Nonsan, 320-711, Korea
bDepartment of Chemistry, Kangwon National University, Chuncheon, 200-701, Korea
cDepartment of Physics, Chungnam National University, Taejon, 305-764, Korea
dDepartment of Chemistry, Chungbuk National University, Cheongju, 360-763, Korea
First published on 13th December 2000
Abstract
A series of novel 1,3-alternate calix[4]arene-azacrown ethers with 2-picolyl, 3-picolyl, and benzyl groups on the nitrogen atom were synthesized by reaction of 1,3-alternate calix[4]arene-azacrown ether and aryl halide in the presence of triethylamine as base. Based on two-phase extraction, bulk liquid membrane, 1H NMR, and solid-state studies on this ligand–metal complexation, 2-picolyl-armed calixazacrown ether showed the highest selectivity for silver ion due to electrostatic interaction through effective three-dimensional encapsulation assisted by the nitrogen atom of the 2-picolyl group.
Introduction
Those macrocyclic compounds, containing a nitrogen atom, called azacrown ethers are interesting because cation-ligating side arms such as carboxylic acid,1–4 chromogenic,1 and fluorogenic groups![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 on the nitrogen atom can strongly and selectively interact with various charged and neutral guest molecules by three-dimensional encapsulation. Molecular recognition by the nitrogen-functionalized arm group reportedly provides three-dimensional complexation with both transition metal cations and alkaline-earth metal cations, as well as their efficient transport across liquid membranes.6
5 on the nitrogen atom can strongly and selectively interact with various charged and neutral guest molecules by three-dimensional encapsulation. Molecular recognition by the nitrogen-functionalized arm group reportedly provides three-dimensional complexation with both transition metal cations and alkaline-earth metal cations, as well as their efficient transport across liquid membranes.6
Calix[4]arenes have been used as 3-D molecular building blocks for the synthesis of receptors with specific properties.7 They can exist in four different conformations: cone, partial cone, 1,2-alternate, and 1,3-alternate.8,9 Recently, calix[4]crown ethers in which conventional crown ethers are incorporated into a rigid calix[4]arene of the 1,3-alternate type have attracted intense interest as caesium-selective extractants.10 Caesium ion is encapsulated not only by the crown ether loop but also by the two aromatic rings (cation/π-interaction) when fixed in the 1,3-alternate conformation.11–13 It was also reported that this π-metal interaction between two aromatic rings in the 1,3-alternate conformer and silver ion plays an important role in the complexation by means ‘through the tubular cavity’.14 Previously, we reported that calix[4]azacrown ethers with N-hydroxynitrophenol as a proton-ionizable side arm showed exceptional potassium selectivity over other alkali metal ions in two-phase extraction and membrane-transport experiments.15 The phenolic oxygen of N-hydroxynitrophenol was observed to participate in metal-ion complexation by three-dimensional encapsulation under high-pH conditions.
To develop new types of calixarene receptors based on the above findings, we examined a series of picolyl-armed![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) † calixazacrown ethers to see if the pyridyl group can play an important role in metal-ion complexation by geometric encapsulation. With these complexation concepts in mind, we synthesized calixazacrown ethers 1–8 with azacrown rings of various sizes and with different picolyl groups (positions 2- and 3-) to investigate their complexation behaviors toward silver, alkali, alkaline earth and transition metal ions by means of two-phase extraction, bulk liquid membrane, solid-state and 1H NMR experiments.
† calixazacrown ethers to see if the pyridyl group can play an important role in metal-ion complexation by geometric encapsulation. With these complexation concepts in mind, we synthesized calixazacrown ethers 1–8 with azacrown rings of various sizes and with different picolyl groups (positions 2- and 3-) to investigate their complexation behaviors toward silver, alkali, alkaline earth and transition metal ions by means of two-phase extraction, bulk liquid membrane, solid-state and 1H NMR experiments.
Results and discussion
The introduction of a pyridine moiety to the azacrown framework has been reported to produce a powerful binding site for metal cations.6 This led us to design a picolyl-armed calix[4]azacrown ether in which a picolyl-armed azacrown loop links to the 1,3-alternate calix[4]arene framework. The synthetic schemes for preparing the picolyl- and benzyl-armed calixazacrown ethers are shown in Scheme 1. Starting materials 1 and 2 in the 1,3-alternate conformation could be prepared in four steps from the calix[4]arene.15,16 The coupling reaction of 1 or 2 with 2-picolyl chloride, 3-picolyl chloride, or benzyl bromide in the presence of triethylamine in DMF gave the desired products 3–8 in poor to moderate (15–40%) yields. To the best of our knowledge, these are the first examples of calixazacrowns with a picolyl group as a pendent arm. All of the products were confirmed to be in the fixed 1,3-alternate conformation based on 1H and 13C NMR spectral assignments.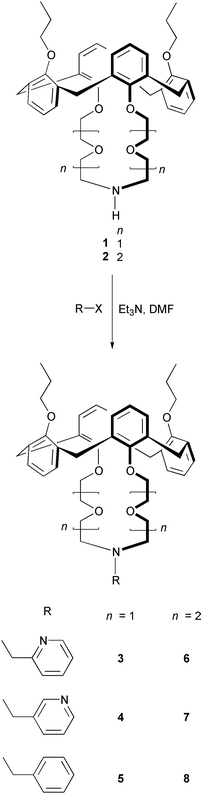 | ||
| Scheme 1 Synthetic scheme for compounds 3–8. | ||
The association constants (log Ka) of the synthesized ligands for alkali, alkaline earth and transition metal ions including silver cation were determined by two-phase picrate extraction, and are summarized in Table 1. The association constants of 1 and 2 could not be calculated, probably because the N–H hydrogens of 1 and 2 exchange with metal picrate at the interface between chloroform and the aqueous layer at pH 7, and protonated picric acid is then transported into the chloroform layer. The effect of the pyridine-functionalized arm on the stability constant was clearly demonstrated by comparison with the corresponding benzyl-substituted calixazacrown ether. In most cases, calixazacrown ethers coordinate more strongly with monovalent metal cations than with divalent cations. For the series with n = 1 (3–5), 3-picolyl-substituted calixazacrown ether (4) showed poor complexation ability toward all metal ions, even worse than that for its benzyl-substituted counterpart 5. This may be due to the fact that the nitrogen of the 3-picolyl group cannot complex with the target metal ion due to geometric constraints when the metal ion approaches the cavity of the calixazacrown ether. In addition, the introduction of a nitrogen atom to the benzene ring increases the electron density and the 3-picolyl substituent seems to act as an electron-withdrawing group, which reduces the stability constant. Interestingly, however, the 2-picolyl-substituted calixazacrown ether 3 has a higher binding constant than its 3-picolyl- (4) and benzyl-substituted (5) counterparts, indicating that the nitrogen of the 2-picolyl unit can participate in ligand–metal complexation by three-dimensional encapsulation. To examine the effect of size on ligand–metal-ion complexation, the azacrown loop was elongated by preparing crown ethers with n = 2. This gave relatively low binding constants compared with those for n = 1, implying that the azacrown loop of azacrown-7 is too large to wrap the target metal ions. Like compound 3, the 2-picolyl nitrogen of the calixazacrown ether 6 seems to participate in ligand–metal complexation. Among several metal ions, silver ion was selectively bound in the azacrown cavity of this particular host molecule.
| Log Ka | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligand | Na+ | K+ | Rb+ | Cs+ | NH4+ | Ag+ | Sr2+ | Br2+ | Pb2+ |
| a Average association constant was determined at 25 °C by three indpendent experiments at pH 7. | |||||||||
| 3 | 10.68 | 10.38 | 10.30 | 10.03 | 10.54 | 11.44 | 9.71 | 9.75 | 10.11 |
| 4 | 9.93 | 9.58 | 9.62 | 8.95 | 9.69 | 9.73 | 9.26 | 9.19 | 9.43 |
| 5 | 10.47 | 10.13 | 10.20 | 9.84 | 10.20 | 10.35 | 9.57 | 9.65 | 9.83 |
| 6 | 9.60 | 9.24 | 9.34 | 8.98 | 9.32 | 10.88 | 8.88 | 9.03 | 10.12 |
| 7 | 9.19 | 8.74 | 8.99 | 8.52 | 8.87 | 8.93 | 8.43 | 8.70 | 8.85 |
| 8 | 9.56 | 9.30 | 9.35 | 9.03 | 9.50 | 9.47 | 8.96 | 9.02 | 9.26 |
A liquid-membrane experiment was also performed to measure transport rates of metal ions from an aqueous source phase into an aqueous receiving phase through an organic bulk membrane. The measured flux values are listed in Table 2. Attachment of side arms (3–8) gave faster transport than without side arms (1 and 2). Picolyl-armed calixazacrown ethers, especially compound 3, gave a faster transport rate than did the benzyl-armed one 5, consistent with the results of the two-phase extraction experiments. In addition, calixazacrown-7 with a 2-picolyl unit (6) gave the highest flux value. In this case, we assume that, when n = 2, the rate of decomplexation is faster than that for n = 1. High selectivity in the case of the 2-picolyl series in this membrane-transport experiment is another important clue to help elucidate the three-dimensional encapsulating behavior of the pyridine unit. A competitive ion-transport experiment, which is the most applicable in an industrial setting, was also carried out. In a two-component system, silver ion was again selectively transported, as shown in Table 3. Compound 3 with a 2-picolyl arm on the nitrogen atom gave the highest transport rate while compounds 1 and 2, which do not have pendent side arms, gave slow transport. Substituting a benzyl unit for the 2-picolyl group (compound 5) gave slow transport because the benzyl group cannot act as an additional binding site. Considering these transport values, 3 showed good selectivity for silver ion over other metal ions. This compound could be useful for separating noble metal ions in industrial applications which require the selective separation of silver ion from other transition and heavy-metal ions. In this stage of host–metal ion interaction, we assume three important possibilities in determining the selectivity: (I) electrostatic interactions between the silver ion and heteroatoms based on their size correspondence, (II) extra-coordination by the picolyl group attached to the nitrogen of the azacrown moiety, and (III) π–metal interactions between the metal ion and two rotated aromatic nuclei of the 1,3-alternate calixarene.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a
| Flux value (×10−8 mol m−2 s−1) | |||||||
|---|---|---|---|---|---|---|---|
| Mn+ | 1 | 2 | 3 | 5 | 6 | 7 | 8 |
| a Transport conditions: source phase (aqueous solution of metal nitrate, 0.1 M, 0.8 mL); membrane phase (1,2-dichloroethane saturated with water, 3 mL), [carrier] = 1.0 mM; receiving phase (0.1 M HNO3, 5.0 mL). | |||||||
| Ag+ | 9.58 | 24.29 | 155.51 | 34.25 | 476.66 | 125.15 | 104.65 |
| Cd2+ | 0 | 0 | 2.15 | 0 | 4.59 | 0 | 0 |
| Co2+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cu2+ | 2.71 | 0 | 25.27 | 0 | 89.63 | 0 | 0 |
| Fe3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ni2+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pb2+ | 0 | 1.17 | 6.47 | 0 | 134.25 | 0 | 0.65 |
| Zn2+ | 1.59 | 0.10 | 0.60 | 0.20 | 0.30 | 0.22 | 0.22 |
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a
Flux value (×10−8 mol m−2 s−1)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
||||
|---|---|---|---|---|
| Mn+ | 1 | 2 | 3 | 5 |
| a Transport conditions: source phase (aqueous solution of metal nitrate, 0.8 mL, 0.1 M); membrane phase (ClCH2CH2Cl, 3.0 mL), [carrier] = 1.0 mM; i.d. of glass vial = 18 mm, stirred by 13 mm Teflon-coated magnetic stirring bar driven by a Hurst Synchronous motor (120 rpm); receiving phase (0.1 M HNO3, 5.0 mL). b The average value of three independent determinations at 25 °C. The experimental values deviate from the reported values by an average of 10%. | ||||
| Ag+/Cd2+ | 11.71/0 | 45.99/0 | 156.39/0 | 38.63/0 |
| Ag+/Co2+ | 9.42/0 | 58.51/0 | 146.89/0 | 45.48/0 |
| Ag+/Cu2+ | 9.66/0 | 73.13/0 | 162.32/19.94 | 34.43/0 |
| Ag+/Fe3+ | 0/0 | 37.31/0 | 44.15/0 | 8.97/0 |
| Ag+/Ni2+ | 10.75/0 | 54.54/0 | 144.66/0 | 36.07/0 |
| Ag+/Pb2+ | 8.69/0 | 56.04/0.42 | 160.47/0.70 | 39.94/0.41 |
| Ag+/Zn2+ | 9.88/0.27 | 53.42/0.18 | 156.39/0.28 | 43.47/0.17 |
To obtain a better understanding of concept (III) above, it is important to elucidate the X-ray crystal structure. Therefore, we attempted to make crystals of 3·Ag+ suitable for X-ray study, but failed. Instead, we obtained a crystal of 5·K+, in which a benzyl group was attached to the nitrogen atom. The crystal structure and corresponding data are shown in Fig. 1 and Table 4, respectively. Five carbon atoms on the calixazacrown (C16, C17, C35, C36, and C42) and an oxygen atom (O10) on the perchlorate were disordered into two positions. This conformation enables four oxygen atoms and a nitrogen atom on the lower azacrown loop to be directed toward the central metal ion K+. The distances between K+ and (O3–O6) in the downward crown ring are 2.77, 2.82, 2.83, and 2.76 Å, respectively. In this crystal structure, we can also clearly observe cation–π interaction between the potassium ion and the para- and meta-carbons of the two rotated benzene rings. It has been reported that the sum of the half-thickness of the benzene π-electron (1.70 Å) and the potassium-ion radius (1.33 Å) is 3.03 Å.14 In this experiment, the distances for K+–C11 (para-carbon) and K+–C28 (para-carbon) were 3.388 and 3.307 Å, respectively. Thus, the distances between the metal ion and the para-carbons of the benzene rings are close enough for cation–π interaction. meta-Carbons (C10 and C27) also seem to interact with potassium cation because of the short distances between the metal ion and the carbon atoms (3.429 for K+–C10 and 3.444 Å for K+–C27). To the best of our knowledge, this is the first example of an X-ray crystal structure showing that a calixazacrown ether encapsulates a potassium cation. From this crystal structure, we can assume that the silver cation could also be encapsulated through electrostatic interaction via cation–π interaction.
| Chemical formula | C49H57ClKNO10 |
| Formula weight | 894.51 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| μ/mm−1 | 0.234 |
| R and Rw | 0.0863 and 0.2096 |
| Unit-cell dimensions | |
| a/Å | 16.4923(15) |
| b/Å | 13.9235(11) |
| c/Å | 19.913(5) |
| β/° | 92.856(14) |
| V/Å3 | 4567.0(13) |
| T/K | 288 |
| Z | 4 |
| Measured reflections | 8234 |
| No. of independent reflections | 7981 |
| R int | 0.0240 |
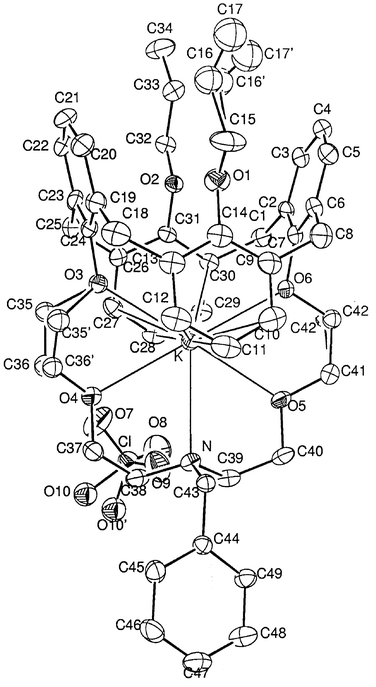 | ||
| Fig. 1 X-Ray crystal structure of 5·K+. | ||
To better understand metal-ion complexation, we performed 1H NMR spectroscopic studies in CDCl3 solution for complex 3·Ag+. These 1H NMR studies based on the chemical shifts of selected proton signals of the calixazacrown 3 can provide metal-ion selectivity, the extent of pyridyl unit participation, and the possibility of cation–π interaction in metal-ion complexation. The metal-ion-induced chemical-shift changes are listed in Table 5. The shifted values for the induced proton signal were highly dependent upon the nature of the guest ion. Silver ion showed the greatest changes in chemical shift (ppm), reflecting silver-ion selectivity as observed in the liquid membrane and two-phase extraction experiments. In addition, metal-induced changes in chemical shifts of Hb, Hc, and Hd were observed upon silver-ion complexation, whereas compound 5, which has a benzyl group, showed no chemical-shift changes for the aromatic hydrogen atoms, providing an important clue for the three-dimensional participation of the pyridyl group in metal-ion complexation. Two sets of triplet peaks for Hg and Hg′ of 3 were shifted downfield upon silver-ion complexation by Δ = 0.22 and Δ = 0.18 ppm, respectively. Two doublet peaks for Hf and Hf′ were also observed to shift downfield, by Δ = 0.28 and Δ = 0.08 ppm, respectively, implying that the para- and meta-carbons of two pairs of the downward-benzene rings participate in the cation–π interactions. In contrast, only a small change was observed in the chemical shifts of the corresponding Hg′ and Hf′ of 3. This 1H NMR investigation clearly demonstrated that the silver ion can be encapsulated by the cavity made by the distally located azacrown ring of the calix[4]arene as well as by the 2-picolyl arm attached to the nitrogen atom of the calixazacrown ether.
Induced chemical shift (ppm)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal | a | b | c | d | e | f | f′ | g | g′ | h | i |
| a Induced chemical shift (ppm) = (new chemical shift value of the complex) − (chemical shift of the parent ligand); the (+) and (−) imply down-field- and up-field-shifted signs on metal-ion complexation, respectively. | |||||||||||
| Na+ | −0.01 | +0.01 | 0 | −0.01 | +0.03 | +0.01 | 0 | +0.01 | 0.01 | 0 | +0.03 |
| K+ | 0 | +0.04 | −0.03 | −0.33 | −0.02 | +0.15 | +0.05 | +0.08 | +0.06 | +0.17 | +0.17 |
| Rb+ | 0 | 0 | −0.03 | −0.33 | −0.05 | +0.15 | +0.06 | +0.1 | +0.06 | +0.15 | +0.15 |
| Cs+ | −0.01 | +0.01 | 0 | −0.02 | +0.01 | +0.01 | +0.02 | −0.01 | +0.02 | 0 | 0 |
| NH4+ | −0.01 | +0.54 | −0.01 | −0.32 | −0.02 | +0.15 | +0.06 | +0.05 | +0.07 | +0.01 | +0.10 |
| Ag+ | +0.03 | +0.24 | +0.15 | +0.1 | +0.38 | +0.28 | +0.08 | +0.22 | +0.18 | +0.10 | +0.12 |
In conclusion, calix[4]arene azacrown ethers with picolyl and benzyl groups on the nitrogen atom were synthesized in poor to moderate yield in a 1,3-alternate conformation. 1H NMR, two-phase extraction, and bulk liquid membrane studies of complexation showed high silver-ion selectivity over other metal ions. This silver selectivity is due to (i) electrostatic interaction between the metal ion and the polyether cavity composed of oxygen atoms and nitrogen as electron donors, (ii) π–metal interaction between the metal ion and two aromatic rings of the 1,3-alternate calixarene, and (iii) additional coordination of the 2-picolyl group through three-dimensional encapsulation. The design, development, and elucidation of other molecules with a fluorogenic side arm according to the above concepts are in progress.
Experimental
Unless specified otherwise, reagent-grade reactants and solvents were obtained from chemical suppliers and used as received. Dry solvents were prepared as follows: tetrahydrofuran (THF) was freshly distilled from sodium metal ribbon or chunks; dimethylformamide (DMF) was dried over 4 Å molecular sieves. Compounds 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15 and 2
15 and 2![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15 were prepared as described in the literature.
15 were prepared as described in the literature.
Synthesis
1H NMR spectroscopy of complexes
Samples of metal picrate complexes were prepared for 1H NMR analysis as follows. A mixture of 3 (20 mg) and excess of metal picrate (at least 5 mole equivalents) in CDCl3 (3 mL) was stirred for 1 h. After filtration of the excess of metal picrate, the 1H NMR (400 MHz) spectrum of the filtrate was obtained.Two-phase extraction
The picrate concentration in the organic layer was analyzed with a UV-visible spectrometer. Metal picrate was prepared by reaction of picric acid and metal carbonate.17 To obtain an association constant (Ka) and a stoichiometry coefficient between the extractant and metal picrate, an aqueous solution (2.0 mL) containing 0.20 mM metal picrate and chloroform solutions of the same volume according to the extractant concentration (0.1 mM) were mixed and equilibrated by shaking for 30 min at 25 °C. Concentrations of picrate anion extracted from the aqueous phase into the organic layer were determined by UV spectrophotometry (λmax = 373 nm).Transport of alkali metal ions in a bulk liquid membrane system
Liquid membrane transport experiments were carried out using a bulk liquid membrane apparatus consisting of a bulk, stirred organic phase that separated the aqueous source and receiving phases.18 The two aqueous phases were separated by a 1,2-dichloroethane phase that constituted the membrane. The details of the transport conditions for single and competitive experiments are summarized in the footnotes of Tables 2 and 3, respectively. Each experiment was independently repeated three times in a room temperature of 25 ± 1 °C. 3.0 mL of the aqueous receiving phase was collected and the flux values (moles transported s−1 m−2) were determined using a Perkin-Elmer 2380 atomic absorption spectrophotometer. Blank experiments in which no organic ligand was present were performed to determine membrane leakage.Determination of the X-ray crystal structure
A crystal of approximately 0.264 × 0.264 × 0.264 mm was obtained by the slow evaporation of solvent from a solution of 5 and potassium perchlorate in methanol. The crystal was mounted and aligned on an Enraf-Nonius CAD-4 diffractometer![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19 and accurate monoclinic cell parameters were refined from setting angles of 25 reflections. A total of 7981 independent reflections were collected in the range 2 < θ < 25° with graphite-monochromated Mo-Kα radiation, using the ω/2θ scan technique at room temperature. The data were collected for L-p, decay and empirical absorption corrections, and 3298 reflections were assumed as observed by applying the condition Fo ≥ 4σ(Fo) and space group P21/c with Z = 4. The structure was solved by direct methods and was refined by a program for the refinement of crystal structures.20,21 All hydrogen atoms were placed in their calculated positions and were allowed to ride on their parent carbon atoms.
19 and accurate monoclinic cell parameters were refined from setting angles of 25 reflections. A total of 7981 independent reflections were collected in the range 2 < θ < 25° with graphite-monochromated Mo-Kα radiation, using the ω/2θ scan technique at room temperature. The data were collected for L-p, decay and empirical absorption corrections, and 3298 reflections were assumed as observed by applying the condition Fo ≥ 4σ(Fo) and space group P21/c with Z = 4. The structure was solved by direct methods and was refined by a program for the refinement of crystal structures.20,21 All hydrogen atoms were placed in their calculated positions and were allowed to ride on their parent carbon atoms.
CCDC reference number 207/499. See http://www.rsc.org/suppdata/p1/b0/b006801m/ for crystallographic files in .cif format.
Acknowledgements
This research was supported by a Grant from the Korea Research Foundation (KRF-2000-015-DP0246).References
- M. Takagi and K. Ueno, Top. Curr. Chem., 1984, 121, 39 Search PubMed.
- R. M. Sink, D. C. Buster and A. D. Sherry, Inorg. Chem., 1990, 29, 3645 CrossRef CAS.
- R. Delgado and J. J. R. Frasto da Silva, Talanta, 1982, 29, 815 CrossRef CAS.
- A. Reisen, M. Zehnder and T. A. Kaden, J. Chem. Soc., Chem. Commun., 1985, 1336 RSC.
- H.-G. Löhr and F. Vötle, Acc. Chem. Res., 1985, 18, 65 CrossRef.
- (a) H. Tsukube, K. Yamashita, T. Iwachido and M. Zenki, Tetrahedron Lett., 1989, 30, 3983 CrossRef CAS; (b) K. Matsumoto, H. Minatogawa, M. Munakawa, M. Toda and H. Tsukube, Tetrahedron Lett., 1990, 31, 3923 CrossRef CAS; (c) A. V. Bordunov, P. C. Hellier, J. S. Bradshaw, N. K. Dalley, X. Kou, X. X. Zhang and R. M. Izatt, J. Org. Chem., 1995, 60, 6097 CrossRef CAS; (d) H. Tsukube, K. Yamashita, T. Iwachido and M. Zenki, J. Org. Chem., 1991, 56, 268 CrossRef CAS; (e) H. Tsukube, T. Inoue and K. Hori, J. Org. Chem., 1994, 59, 8047 CrossRef CAS.
- (a) C. D. Gutsche , Calixarenes, Royal Society of Chemistry, Cambridge, 1989; Search PubMed; (b) C. D. Gutsche , in Synthesis of Macrocycles: Design of Selective Complexing Agents, ed. R. M. Izatt and J. J. Christensen, Wiley, New York, 1987, p. 93; Search PubMed; (c) Calixarenes: A Versatile Class of Macrocyclic Compounds, ed. J. Vicens and V. Bömer, Kluwer, Dordrecht, 1991; Search PubMed; (d) V. Böhmer and M. A. McKervey, Chem. Unserer Zeit, 1991, 195 Search PubMed; (e) C. D. Gutsche , Calixarenes, Monographs in Supramolecular Chemistry, ed. J. F. Stoddart, Royal Society of Chemistry, Cambridge, 1989; Search PubMed; (f) C. D. Gutsche , in Inclusion Compounds, ed. J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Oxford University Press, New York, 1991, vol. 4, pp. 27–63; Search PubMed; (g) J.-D. Van Loon, W. Verboom and D. N. Reinhoudt, Org. Prep. Proced. Int., 1992, 24, 437 Search PubMed; (h) R. Ungaro and A. Pochini , in Frontiers in Supramolecular Organic Chemistry and Photochemistry, ed. H.-J. Schneider, VCH, Weinheim, Germany, 1991, pp. 57–81; Search PubMed; (i) G. D. Andreetti , F. Ugozzoli , R. Ungaro and A. Pochini , in Inclusion Compounds, ed. J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Oxford University Press, New York, 1991, vol. 4, pp. 64–125. Search PubMed.
- A. Casnati, A. Pochini, R. Ungaro, F. Ugozzoli, F. Arnaud, S. Fanni, M.-J. Schwing, R. J. M. Egberink, F. de Jong and D. N. Reinhoudt, J. Am. Chem. Soc., 1995, 117, 2767 CrossRef CAS.
- J. S. Kim, I. H. Suh, J. K. Kim and M. H. Cho, J. Chem. Soc., Perkin Trans. 1, 1998, 2307 RSC.
- (a) C. Bressot, J. F. Dozol, J. Vicens, Z. Asfari, R. Ungaro and A. Casnati, Sep. Sci. Technol., 1997, 32, 175 Search PubMed; (b) Z. Asfari, C. Bressot, J. Vicens, C. Hill, J. F. Dozol, H. Rouquette, S. Eymard, V. Lamare and B. Yournois, Anal. Chem., 1995, 67, 3133 CrossRef CAS.
- (a) J. S. Kim, A. Ohki, R. Ueki, T. Ishizuka, T. Shimotashiro and S. Maeda, Talanta, 1999, 48, 705 CrossRef CAS; (b) J. S. Kim, A. Ohki, M. H. Cho, J. K. Kim, D. Y. Ra, N. S. Cho, R. A. Bartsch, K. W. Lee and W. Z. Oh, Bull. Korean Chem. Soc., 1997, 18, 1014 Search PubMed.
- J. S. Kim, W. K. Lee, I. H. Suh, J. G. Kim, J. Y. Yoon and J. H. Lee, J. Org. Chem., 2000, 65, 7215 CrossRef CAS.
- (a) J. S. Kim, J. H. Pang, I. Y. Yu, W. K. Lee, I. H. Suh, J. K. Kim, M. H. Cho, E. T. Kim and D. Y. Ra, J. Chem. Soc., Perkin Trans. 2, 1999, 837 RSC; (b) W. H. Kruizinga and R. M. Kellogg, J. Am. Chem. Soc., 1981, 103, 5183 CrossRef CAS.
- A. Ikeda and S. Shinkai, J. Am. Chem. Soc., 1994, 116, 3102 CrossRef CAS.
- J. S. Kim, O. J. Shon, J. W. Ko, M. H. Cho, I. Y. Yu and J. Vicens, J. Org. Chem., 2000, 65, 2386 CrossRef CAS.
- J. S. Kim, W. K. Lee, K. H. No, Z. Asfari and J. Vicens, Tetrahedron Lett., 2000, 41, 3345 CrossRef CAS.
- R. A. Bartsch, I. W. Yang, E. G. Jeon, W. Walkowiak and W. A. Charewicz, J. Coord. Chem., 1992, 27, 75 Search PubMed.
- (a) G. Gokel , in Crown Ethers and Cryptands, ed. J. F. Stoddart, Royal Society of Chemistry, London, 1991, p. 81; Search PubMed; (b) S. S. Lee, J. H. Jung, S. H. Yu and M. H. Cho, Thermochim. Acta, 1995, 259, 133 CrossRef CAS.
- Enraf-Nonius Structure Determination Package, Enraf-Nonius, Delft, The Netherlands, 1994. Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 1990, 46, 467 CrossRef.
- G. M. Sheldrick , SHELX-97—Program for Crystal Structure Analysis, University of Göttingen, Germany, 1997. Search PubMed.
Footnote |
| † Picolyl = pyridylmethyl. |
| This journal is © The Royal Society of Chemistry 2001 |
