Unexpected products from the formylation of N,N-dimethylanilines with 2-formamidopyridine in POCl3
Ying
Cheng
*a,
Peng
Jiao
a,
David J.
Williams
b and
Otto-Meth
Cohn
*c
aChemistry Department, Beijing Normal University, Beijing, 100875, China. E-mail: Yincheng@public2.east.net.cn
bChemistry Department, Imperial College, London, UK SW7 2AY
cChemistry Department, University of Sunderland, Sunderland, UK SR1 3SD. E-mail: Otto.meth-cohn@sunderland.ac.uk
First published on 11th December 2000
Abstract
2-Formamidopyridine in POCl3 solution reacts with N,N-dimethylaniline to give tris(4-dimethylaminophenyl)methane in 80% yield but with 4-X-N,N-dimethylanilines it gives 2-dimethylamino-5-X-phenyl[2-(N-methyl)formamido-5-X-phenyl](2-pyridylamino)methanes.
Introduction
Formanilide in the presence of POCl3 was first used as a formylating agent by Dimroth and Zoeppritz in 1902![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 who employed it to formylate resorcinol. However it failed to formylate N,N-dimethylaniline. Similar results were reported by Johnson and Lane,2 Pratt and Robinson,3 Froeschl and Bomberg,4 Nenitzescu and Isacescu
1 who employed it to formylate resorcinol. However it failed to formylate N,N-dimethylaniline. Similar results were reported by Johnson and Lane,2 Pratt and Robinson,3 Froeschl and Bomberg,4 Nenitzescu and Isacescu![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 and Oesterlin
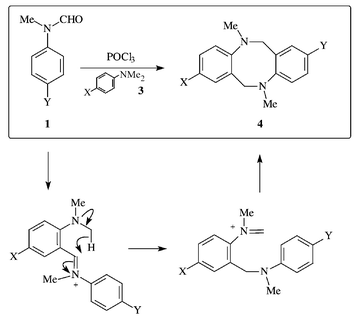
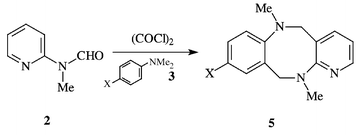
5 and Oesterlin![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 who were able to formylate related highly activated systems with this combination. The limitations of formanilide as the formylating amide led to the discovery by Vilsmeier and Haack of the excellent reagent, N-methylformanilide.7 From then on, the Vilsmeier’s reaction has been extensively studied and more recently, widely used in the preparation of heterocyclic compounds.8 In earlier work, we demonstrated that the Vilsmeier reagents derived from N-methylformanilides 1 and POCl3,9 or from 2-(N-methyl)formamidopyridine 2 and (COCl)2
6 who were able to formylate related highly activated systems with this combination. The limitations of formanilide as the formylating amide led to the discovery by Vilsmeier and Haack of the excellent reagent, N-methylformanilide.7 From then on, the Vilsmeier’s reaction has been extensively studied and more recently, widely used in the preparation of heterocyclic compounds.8 In earlier work, we demonstrated that the Vilsmeier reagents derived from N-methylformanilides 1 and POCl3,9 or from 2-(N-methyl)formamidopyridine 2 and (COCl)2![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 10 reacted with 4-substituted dimethylanilines 3 to afford N,N
10 reacted with 4-substituted dimethylanilines 3 to afford N,N![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′-dimethyl-5,6,11,12-tetrahydrodibenzo[b,
′-dimethyl-5,6,11,12-tetrahydrodibenzo[b,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f
f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ][1,5]diazocines 4 or N,N
][1,5]diazocines 4 or N,N![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ′-dimethyl-5,6,11,12-tetrahydrobenzo[
′-dimethyl-5,6,11,12-tetrahydrobenzo[![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f
f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ]pyrido[2,3-b][1,5]diazocines 5 respectively, by way of the ‘t-amino effect’
]pyrido[2,3-b][1,5]diazocines 5 respectively, by way of the ‘t-amino effect’![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 11 (Scheme 1). We considered that the N-unsubstituted [1,5]diazocines, which could be useful intermediates for the preparation of unsymmetrical Tröger’s bases (Tröger’s base is 5,11-methano-5,6,11,12-tetrahydrodibenzo[b,
11 (Scheme 1). We considered that the N-unsubstituted [1,5]diazocines, which could be useful intermediates for the preparation of unsymmetrical Tröger’s bases (Tröger’s base is 5,11-methano-5,6,11,12-tetrahydrodibenzo[b,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f
f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ][1,5]diazocine), might be accessible by the use of a formanilide as the Vilsmeier amide. Furthermore, 2-formamidopyridine should be a stronger formylator than formanilide itself, especially if N-protonated or acylated.
][1,5]diazocine), might be accessible by the use of a formanilide as the Vilsmeier amide. Furthermore, 2-formamidopyridine should be a stronger formylator than formanilide itself, especially if N-protonated or acylated.
 | ||
| Scheme 1 | ||
Results and discussion
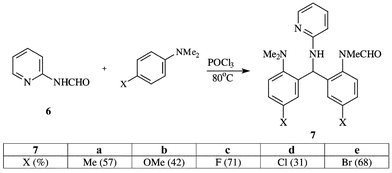
In fact, formanilides proved ineffective agents as indicated above. We therefore reacted 2-formamidopyridine with various para-substituted N,N-dimethylanilines in POCl3 solution. Surprisingly, the reaction took a totally different course leading to a product derived from two units of the t-aniline and one of the pyridine, less four protons, as indicated by mass spectral and CHN analytical data. The infrared and NMR spectra were insufficient to put the structure beyond doubt and the structure 7 (X = Cl) was established by X-ray crystallography for the product from 4-chloro-N,N-dimethylaniline, as shown in Fig. 1 Further examples are tabulated in Scheme 2, the yields being based upon the optimal use of 1∶2 mol of aniline to pyridine reactant respectively. Full details of conditions, yields and spectral data are recorded in Tables 1–3. Clearly, the 2-formamidopyridine is indeed a more powerful formylator than formanilide.| Yields (%) | |||||
|---|---|---|---|---|---|
| 3 X = | Ratio of 3∶6 | Time/h | Temp./°C | 7 | 8 |
| a Under N2. | |||||
| Me | 2∶1 | 8 | 80 | — | |
| Me | 2∶1 | 20 | 75 | — | |
| Me | 2∶1 | 17 | 94 | 25 | |
| Me | 1∶1 | 18 | 96 | 47![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
|
| Me | 1∶2 | 18 | 96 | 57 | |
| OMe | 2∶1 | 17 | 94 | 35 | |
| OMe | 2∶1 | 8 | 100 | 32 | |
| OMe | 1∶2 | 18 | 94 | 42 | |
| F | 1∶1 | 36 | 90 | 44 | |
| F | 1∶2 | 18 | 90 | 71 | |
| Cl | 2∶1 | 18 | 90 | 34 | |
| Cl | 1∶2 | 18 | 96 | 31 | |
| Br | 2∶1 | 19 | 90 | 17 | |
| Br | 1∶1 | 18 | 90 | 29 | |
| Br | 1∶2 | 18 | 94 | 48 | |
| Br | 1∶2 | 15 | 96 | 68 | |
| H | 2∶1 | 18 | 90 | 80 | |
| Compound | Mp/°C | CHN% |
|---|---|---|
| a Lit.15 mp 177–178 °C. | ||
| 7a | 152–153 | C, 74.51; H, 7.33; N, 14.29. C24H28N4O requires C, 74.20; H 7.26; N, 14.42 |
| 7b | 153–154 | C, 68.63; H, 6.96; N, 13.16. C24H28N4O3 requires C, 68.55; H, 6.71; N, 13.32 |
| 7c | 169–171 | C, 66.59; H, 5.46; N, 14.02. C22H22F2N4O requires C, 66.48; H, 5.83; N, 14.10 |
| 7d | 195–197 | C, 61.52; H, 5.14; N, 12.82. C22H22Cl2N4O requires C, 61.40; H, 5.38; N, 13.02 |
| 7e | 182–184 | C, 51.27; H, 4.41; N, 10.55. C22H22Br2N4O requires C, 50.99; H, 4.28; N, 10.81 |
| 8 | 177–179![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
|
| Cmpd. | IR (KBr) ν/cm−1 | 1H NMR (CDCl3) δ | 13C NMR (CDCl3) δ | MS m/z (%) |
|---|---|---|---|---|
| 7a | 3280, 1660, 1600, 1480 | 8.05 (1H, d, J 4.2, CHO), 6.87–7.41 (7H, m), 6.57(1H, dd, J 5.4 and 2.7), 6.38 (1H, d, J 8.1), 6.22 (1H, d, J 8.1), 5.04 (1H, d, J 8.1, NH), 3.02 (3H, br s, NCH3), 2.67 (1H, s, NCH), 2.54 (6H, s, 2NCH3), 2.33 (3H, s, ArCH3), 2.21 (3H, s, ArCH3) | 161.8, 157.7, 149.9, 147.5, 142.0, 137.7, 137.2, 136.4, 132.1, 128.7, 128.4, 128.2, 128.1, 128.0, 120.1, 111.9, 108.7, 48.3, 44.7, 32.3, 20.9, 20.6 | 134 (62), 222 (56), 235 (62), 294 (100), 388 (85, M+), 389 (24) |
| 7b | 3380, 3260, 1680, 1600, 1500, 1480 | 8.0 (1H, d, J 4.8 CHO), 7.47 (1H, t, J 8.4), 7.17 (1H, d, J 8.7), 6.70–7.08 (5H, m), 6.63 (1H, t, J 5.7), 6.41 (1H, t, J 6.8), 6.34 (1H, d, J 8.5), 5.90 (1H, br s, NH), 3.76 (3H, s, OCH3), 3.71 (3H, s, OCH3), 3.06 (3H, br s, NCH3), 2.64 (1H, s, NCH), 2.50 (6H, s, 2NCH3) | 163.2, 162.6, 159.7, 156.5, 145.8, 142.1, 139.2, 137.7, 137.1, 133.1, 130.3, 122.5, 113.9, 113.3, 113.2, 112.8, 112.7, 107.2, 55.7, 51.1, 45.8, 33.4, 30.8 | 254 (58), 267 (70), 268 (54), 326 (100), 420 (51, M+), 421 (17) |
| 7c | 3300, 1680, 1620, 1500, 1480 | 8.01 (1H, d, J 4.7, CHO), 7.42 (1H, t, J 7.2), 6.94–7.27 (5H, m), 6.79 (1H, dd, J 9.3 and 2.9), 6.64 (1H, t, J 5.5), 6.50 (1H, d, J 6.4), 6.31 (1H, d, J 8.3), 5.27 (1H, d, J 6.3, NH), 3.07 (3H, s, NCH3), 2.65 (1H, s, NCH), 2.54 (6H, s, 2NCH3) | 164.2, 157.8, 156.8, 148.7, 147.9, 138.4/138.3, 138.0, 131.0/130.9, 122.8/122.7, 115.6, 115.4, 115.3, 115.1, 114.8, 114.5, 113.9, 113.5, 107.0, 50.5, 45.5, 33.1 | 124 (56), 136 (54), 138 (56), 230 (100), 243 (58), 302 (68), 396 (50, M+), 397 (13) |
| 7d | 3280, 1660, 1600, 1480 | 8.05 (1H, d, J 5.4, CHO), 7.52 (1H, d, J 2.7), 6.98–7.44 (6H, m), 6.62 (1H, dd, J 8.1 and 5.4), 6.52 (1H, d, J 8.1), 6.32 (1H, d, J 8.1), 4.92 (1H, d, J 5.4, NH), 3.03 (3H, s, NCH3), 2.67 (1H, s, NCH), 2.58 (6H, s, 2NCH3) | 162.1, 157.4, 151.6, 147.9, 144.3, 139.4, 138.9, 137.2, 133.1, 131.0, 128.4, 128.3, 128.2, 127.7, 127.5, 122.7, 113.0, 109.3, 48.7, 44.6, 32.7 | 154 (61), 246 (100), 334 (64), 428 (53)/430 (34)/432 (6) (M+) |
| 7e | 3300, 1680, 1610, 1490 | 8.03 (1H, d, J 4.8, CHO), 6.95–7.67 (7H, m), 6.65 (1H, t, J 5.6), 6.49 (1H, d, J 6.5), 6.25 (1H, d, J 6.0), 5.35 (1H, br s, NH), 3.03 (3H, s, NCH3), 2.67 (1H, s, NCH), 2.57 (6H, s, 2NCH3) | 117 (99), 290 (100), 292 (88), 405 (54), 407 (46), 424 (77), 516 (27)/518 (52)/520 (25) (M+) | |
| 8 | 1620, 1530 | 7.03 (6H, d, J 8.4), 6.91 (6H, d, J 7.7), 5.37 (1H, s, CH), 2.98 (18H, s, 6NCH3) | 252 (65), 253 (100), 254 (20), 372 (44), 373 (96, M+), 374 (26) |
![the X-ray structure of 7 (X = Cl).12 The principal intermolecular interaction is an N–H ⋯ O hydrogen bond between N(14) in one molecule and O(24) in the next [N ⋯ O, H ⋯ O distances 2.97, 2.27 Å, N–H ⋯ O angle 134°]. This interaction is supplemented by a weaker C–H ⋯ π interaction between C(10)–H in one molecule and the C(15) pyridyl ring of another [H ⋯ π distance 2.82 Å, C–H ⋯ π angle 151°].](/image/article/2001/P1/b006148o/b006148o-f1.gif) | ||
Fig. 1 the X-ray structure of 7 (X = Cl).12 The principal intermolecular interaction is an N–H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O hydrogen bond between N(14) in one molecule and O(24) in the next [N O hydrogen bond between N(14) in one molecule and O(24) in the next [N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O, H O, H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O distances 2.97, 2.27 Å, N–H O distances 2.97, 2.27 Å, N–H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O angle 134°]. This interaction is supplemented by a weaker C–H O angle 134°]. This interaction is supplemented by a weaker C–H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) π interaction between C(10)–H in one molecule and the C(15) pyridyl ring of another [H π interaction between C(10)–H in one molecule and the C(15) pyridyl ring of another [H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) π distance 2.82 Å, C–H π distance 2.82 Å, C–H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⋯ ⋯![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) π angle 151°]. π angle 151°].
| ||
 | ||
| Scheme 2 | ||
The formation of these surprising products involves an oxidation of one of the methyl groups of an N,N-dimethylaniline moiety, a process that we propose is initiated by a ‘t-amino effect’ interaction. A possible pathway is illustrated in Scheme 3. In fact, the reaction appears to require aerial oxidation for optimal yields. Nevertheless it does proceed under nitrogen. As noted in earlier work![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 10 it is probable that iminium intermediates behave as dehydrogenating agents and hence the need for an excess of the pyridine amide. Addition of an oxidant, e.g. copper(II) acetate, does not improve yields.
10 it is probable that iminium intermediates behave as dehydrogenating agents and hence the need for an excess of the pyridine amide. Addition of an oxidant, e.g. copper(II) acetate, does not improve yields.
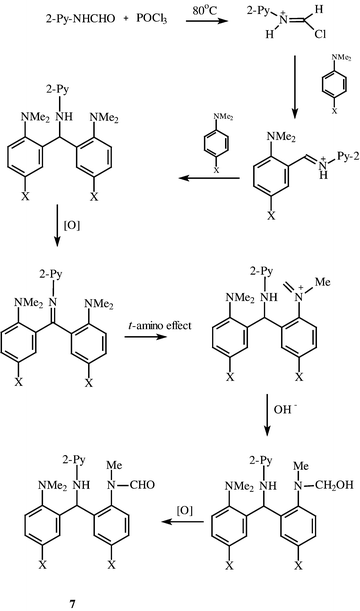 | ||
| Scheme 3 | ||
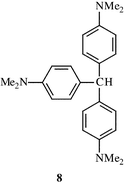
When N,N-dimethylaniline itself was treated with 2-formamidopyridine in POCl3 solution the reaction took a different course yielding tris(4-dimethylaminophenyl)methane 8 in high yield (80%). This well known compound, the precursor to ‘Crystal Violet’ (the tritylium salt thereof), has in fact been formed by, for example, the interaction of 4-dimethylaminobenzaldehyde with N,N-dimethylaniline and an acid![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 or by treatment of a 4,4′-bis(dimethylamino)benzhydryl
13 or by treatment of a 4,4′-bis(dimethylamino)benzhydryl![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) † derivative with N,N-dimethylaniline under acid catalysis.14 Similar benzaldehyde (i.e. an iminium salt derivative) and benzhydryl analogues can be easily formed in our case to account for this efficient reaction. It is of interest that while classical Vilsmeier reagents with N,N-dimethylaniline yield solely 4-formyldimethylaniline, this reagent proceeds further, due to the greater formylating ability of the derived iminium ion (cf. Scheme 3). When the iminium intermediate of the formylation of N,N-dimethylaniline with DMF–POCl3 is reacted with further N,N-dimethylaniline, the same triarylmethane is formed.15
† derivative with N,N-dimethylaniline under acid catalysis.14 Similar benzaldehyde (i.e. an iminium salt derivative) and benzhydryl analogues can be easily formed in our case to account for this efficient reaction. It is of interest that while classical Vilsmeier reagents with N,N-dimethylaniline yield solely 4-formyldimethylaniline, this reagent proceeds further, due to the greater formylating ability of the derived iminium ion (cf. Scheme 3). When the iminium intermediate of the formylation of N,N-dimethylaniline with DMF–POCl3 is reacted with further N,N-dimethylaniline, the same triarylmethane is formed.15
Experimental
Melting points are uncorrected. 1H NMR and 13C NMR spectra in CDCl3 were obtained on Varian Unity 200 and 300 spectrometers. IR spectra were recorded using a Perkin-Elmer 782 spectrometer and mass spectra were recorded on a KYKY-ZHT-5 instrument. Elemental analyses were performed on a GMBH Vario EL instrument. Light petroleum refers to bp 60–80 °C.2-Dimethylamino-5-X-phenyl[2-N-(methyl)formamido-5-X-phenyl](2-pyridylamino)methane 7
Acknowledgements
This work was supported by the National Natural Science Foundation of China.References
- O. Dimroth and R. Zoeppritz, Chem. Ber., 1902, 35, 994 Search PubMed.
- T. B. Johnson and F. W. Lane, J. Am. Chem. Soc., 1921, 43, 348 CrossRef CAS.
- D. D. Pratt and R. Robinson, J. Chem. Soc., 1924, 125, 188 Search PubMed.
- J. Froeschl and P. Bomberg, Monatsh. Chem., 1927, 48, 571.
- C. D. Nenitzescu and D. Isacescu, Bull. Soc. Chim. Rom., 1929, 11, 135 Search PubMed.
- D. M. Oesterlin, Angew. Chem., 1932, 45, 536.
- A. Vilsmeier and A. Haack, Chem. Ber., 1927, 60, 119 Search PubMed.
- O. Meth-Cohn, Heterocycles, 1993, 35, 539 Search PubMed.
- Y. Cheng, O. Meth-Cohn and D. L. Taylor, J. Chem. Soc., Perkin Trans. 1, 1998, 1257 RSC.
- Y. Cheng, Q.-X. Liu and O. Meth-Cohn, Tetrahedron Lett., 2000, 41, 3475 CrossRef CAS.
- O. Meth-Cohn, Adv. Heterocycl. Chem., 1996, 65, 1 CAS.
-
Crystal data for
7 (X = Cl): C22H22N4OCl2, M = 429.3, monoclinic, space group P21/c (no. 14), a = 9.667(3), b = 14.060(2), c = 17.366(3) Å, β = 105.90(2)°, V = 2270.0(8) Å3, Z = 4, Dc = 1.256 g cm−3, μ(Cu-Kα) = 27.3 cm−1, F(000) = 896, T = 293 K; colourless blocks, 0.33 × 0.30 × 0.17 mm, Siemens P4/PC diffractometer, ω-scans, 3555 independent reflections. The structure was solved by direct methods and the non-hydrogen atoms were refined anisotropically using full matrix least-squares based on F
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 to give R1 = 0.058, wR2 = 0.151 for 2771 independent observed absorption corrected reflections [|Fo| > 4σ(|Fo|), 2θ ≤ 124°] and 270 parameters. CCDC reference number 207/497. See http://www.rsc.org/suppdata/p1/b0/b006148o/ for crystallographic files in .cif format..
2 to give R1 = 0.058, wR2 = 0.151 for 2771 independent observed absorption corrected reflections [|Fo| > 4σ(|Fo|), 2θ ≤ 124°] and 270 parameters. CCDC reference number 207/497. See http://www.rsc.org/suppdata/p1/b0/b006148o/ for crystallographic files in .cif format.. - Z.-H. Zhang, F. Yang, T.-S. Li and C.-G. Fu, Synth. Commun., 1997, 27, 3823 CAS.
- H. J. Schneider, T. Schiestel and P. Zimmermann, J. Am. Chem. Soc., 1992, 114, 7698 CrossRef CAS.
- M. Tsujimoto , H. Akahori , K. Hasegawa and M. Asano , PCT Int. Appl. WO 8303840, 1983 (Chem. Abstr., 1984, 100, 69872m). Search PubMed.
Footnote |
| † The IUPAC name for benzhydryl is diphenylmethyl. |
| This journal is © The Royal Society of Chemistry 2001 |