Cross-coupling reactions in Cinchona alkaloid chemistry: aryl-substituted and dimeric quinine, quinidine, as well as quincorine and quincoridine derivatives
Jens
Frackenpohl†
,
Wilfried M.
Braje‡
and
H. Martin R.
Hoffmann
*
Department of Organic Chemistry, Universität Hannover, Schneiderberg 1B, D-30167, Hannover, Germany. Fax: +(0) 511 762 3011; E-mail: hoffmann@mbox.oci.uni-hannover.de
First published on 11th December 2000
Abstract
Cross-coupling reactions of modified Cinchona alkaloids provide access to a wide variety of novel arylated and dimeric derivatives of quinine and quinidine containing a single and double 1,2-amino alcohol functionality. Sonogashira and Heck reactions allow functionalization of ethynyl and 11-iodovinyl precursors. The role of bystander functionality is investigated.
Introduction
In recent years, the study of transition-metal mediated reactions has facilitated progress in organic synthesis and vice versa.1 Metal-catalyzed cross-coupling reactions have been extensively employed, spanning the range of complex natural products to supramolecular chemistry and materials science. Among the many transition metals used, palladium![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2,3 complexes are probably most valuable. At the outset of our work it was not clear whether the basic bridgehead amine and other functionality of the alkaloids including the C9-hydroxy and its protection groups would help or hinder the planned carbon–carbon coupling reactions.
2,3 complexes are probably most valuable. At the outset of our work it was not clear whether the basic bridgehead amine and other functionality of the alkaloids including the C9-hydroxy and its protection groups would help or hinder the planned carbon–carbon coupling reactions.
The quest for novel ligands of neuronal receptors has been a further incentive, as the quinuclidine nucleus has been found to be a good mimic for the quaternary nitrogen in acetylcholine. Unlike acetylcholine, the quaternary nitrogen of which is necessarily charged, the unprotonated form of quinuclidines is able to cross the blood–brain barrier.4 Quinuclidine derivatives with aromatic substituents in the 3-position are able to block M1 (1 and 2), 5-HT3 (3) and NK1-receptors![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5,6 and to act as squalene synthase inhibitors (4) (Fig. 1).7 In contrast to de novo syntheses which provide the desired target molecules only in poor yields and as racemic mixtures, cross-coupling reactions of quinuclidine derivatives offer an efficient access to the desired lead structures.8
5,6 and to act as squalene synthase inhibitors (4) (Fig. 1).7 In contrast to de novo syntheses which provide the desired target molecules only in poor yields and as racemic mixtures, cross-coupling reactions of quinuclidine derivatives offer an efficient access to the desired lead structures.8
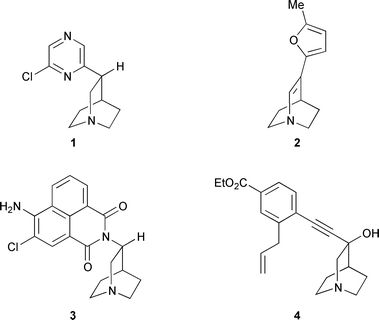 | ||
| Fig. 1 Quinuclidine lead structures. | ||
Dimeric alkaloids, e.g. vincristine and vinblastine, have been used as potent antitumor agents.9 Dimeric Cinchona alkaloids are decisive as ligands in the AD reaction. Thus, dimeric quinine- and quinidine-based phthalazine- and pyrimidine-bridged ligands have outperformed monomeric ligands in most reactions.10 Chloroquine is still one of the main antimalarial drugs, but its efficacy is being steadily eroded by the spread of resistant parasites. The development of alternative agents remains a major goal. Dimeric quinoline-based antimalaria agents such as piperaquine-like quinine derivative 5 (Fig. 2) are of current interest![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 11 since some of these compounds exhibit high activity against chloroquine-resistant parasites.12 Further examples of dimeric Cinchona alkaloid derivatives are chiral symmetrical pentamethinium cyanine dyes with hexahydroquininyl and -quinidinyl end groups (6).13
11 since some of these compounds exhibit high activity against chloroquine-resistant parasites.12 Further examples of dimeric Cinchona alkaloid derivatives are chiral symmetrical pentamethinium cyanine dyes with hexahydroquininyl and -quinidinyl end groups (6).13
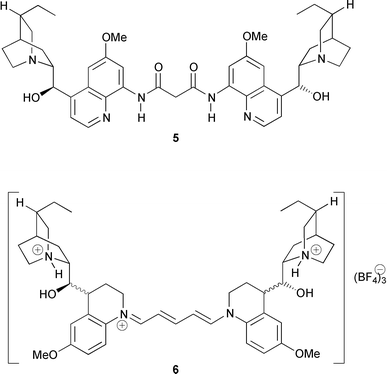 | ||
| Fig. 2 Dimerized Cinchona alkaloids. | ||
In the course of our work we have prepared a wide variety of arylated and dimeric derivatives from acetylenic and vinylic precursors. The acetylenic derivatives of the diyne and enediyne type described herein represent an entirely new class of Cinchona alkaloids.
Results and discussion
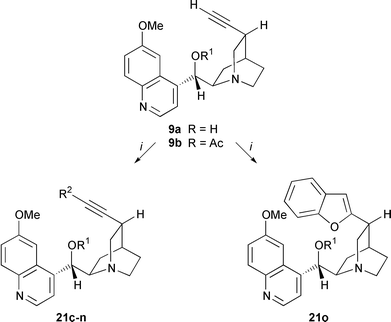
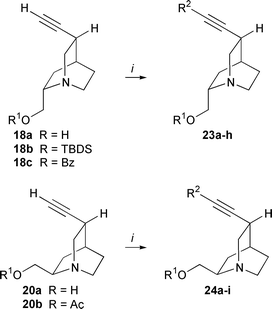
A useful route to arylalkynes and conjugated enynes is the Pd-catalyzed coupling of terminal alkynes with aryl or alkenyl halides as described by Sonogashira et al.14 Numerous applications have been reported, especially in the construction of complex unsaturated frameworks of enediyne antibiotics with (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-1,2-dichloroethylene.15 To explore the application of this cross-coupling reaction to Cinchona alkaloids we have used 10,11-didehydro-derivatives 9, 10, 18, 20 of quinine 8, quinidine 7, Quincorine® (QCI) 13 and Quincoridine® (QCD) 19, vinyl iodides 11, 12, 17 and vinylstannane 15 as precursors (Schemes 1 and 2). The series of terminal alkynes 9, 10, 18, 20 was prepared efficiently in two steps from the naturally occurring Cinchona alkaloids and from QCI (13) and QCD (19).16 Iodinated alkynes were easily transformed into corresponding (Z
)-1,2-dichloroethylene.15 To explore the application of this cross-coupling reaction to Cinchona alkaloids we have used 10,11-didehydro-derivatives 9, 10, 18, 20 of quinine 8, quinidine 7, Quincorine® (QCI) 13 and Quincoridine® (QCD) 19, vinyl iodides 11, 12, 17 and vinylstannane 15 as precursors (Schemes 1 and 2). The series of terminal alkynes 9, 10, 18, 20 was prepared efficiently in two steps from the naturally occurring Cinchona alkaloids and from QCI (13) and QCD (19).16 Iodinated alkynes were easily transformed into corresponding (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-vinyl iodides 11, 12, 17 by p-tolylsulfonyl hydrazide-mediated hydrogenation. (E
)-vinyl iodides 11, 12, 17 by p-tolylsulfonyl hydrazide-mediated hydrogenation. (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-Vinyl iodide 17 was obtained upon treatment of C10-aldehyde 16 with CrCl2 and CHI3.17 Vinylstannane 15 was prepared from ketone 14via hydrazide reduction.18
)-Vinyl iodide 17 was obtained upon treatment of C10-aldehyde 16 with CrCl2 and CHI3.17 Vinylstannane 15 was prepared from ketone 14via hydrazide reduction.18
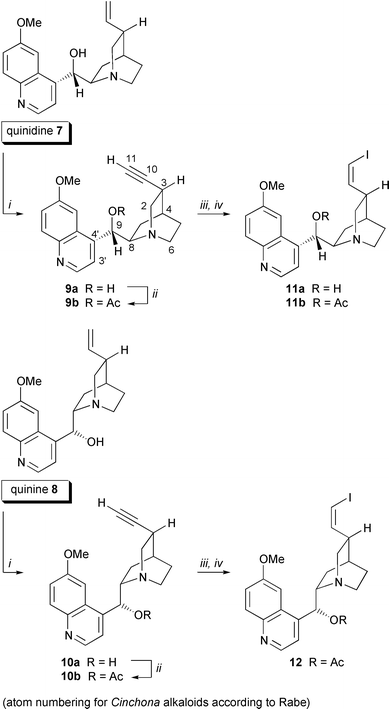 | ||
| Scheme 1 Synthesis of precursors for quinine and quinidine cross-coupling. Reagents and conditions: i, 1. Br2, CHCl3–CCl4, 0 °C, 2 h, 2. Et3N, CHCl3, rt, 2 h, 3. KOH, aliquat 336®, THF, 6–20 h, rt or 70 °C; ii, AcCl, Et3N, DCM, 0 °C→rt, 16 h; iii, I2, morpholine, toluene, 55 °C, 10 h, 91–97%; iv, TsNHNH2, NaOAc, THF, H2O, 55 °C, 4–6 h, 56–65%. | ||
![Synthesis of precursors for quincorine and quincoridine cross-coupling. Reagents and conditions: i, 1. Br2, CHCl3–CCl4, 0 °C, 2 h, 2. Et3N, CHCl3, rt, 2 h, 3. KOH, aliquat 336®, THF, 6–20 h, rt or 70 °C; ii, 1. 2,4,6-triisopropylbenzenesulfonyl hydrazide, Et2O, rt, 16 h, 2. n-BuLi, TMEDA, hexane, −78 °C→0 °C, 1 h; 3. Bu3SnCl, 0 °C→rt, 2 h; iii, 1. TBDSCl, Et3N, DMAP, DCM, rt, 14 h, 2. K3[Fe(CN)6], K2CO3, OsO4, tBuOH–H2O (1∶1), rt, 8 h; iv, NaIO4, SiO2, H2O, DCM, rt, 15 min; v, CrCl2, CHI3, THF, 0 °C→rt, 3 h.](/image/article/2001/P1/b004693k/b004693k-s2.gif) | ||
| Scheme 2 Synthesis of precursors for quincorine and quincoridine cross-coupling. Reagents and conditions: i, 1. Br2, CHCl3–CCl4, 0 °C, 2 h, 2. Et3N, CHCl3, rt, 2 h, 3. KOH, aliquat 336®, THF, 6–20 h, rt or 70 °C; ii, 1. 2,4,6-triisopropylbenzenesulfonyl hydrazide, Et2O, rt, 16 h, 2. n-BuLi, TMEDA, hexane, −78 °C→0 °C, 1 h; 3. Bu3SnCl, 0 °C→rt, 2 h; iii, 1. TBDSCl, Et3N, DMAP, DCM, rt, 14 h, 2. K3[Fe(CN)6], K2CO3, OsO4, tBuOH–H2O (1∶1), rt, 8 h; iv, NaIO4, SiO2, H2O, DCM, rt, 15 min; v, CrCl2, CHI3, THF, 0 °C→rt, 3 h. | ||
Usually, the Sonogashira coupling is carried out in the presence of catalytic amounts of a Pd(II)-complex and CuI in an amine as solvent. The use of cosolvents like THF or DMF has also been reported.19
In view of differences in reactivity of halides and alkynes we have optimized the (Ph3P)2PdCl2-mediated coupling of unprotected 10,11-didehydroquinidine 9a with iodo- and bromobenzene with respect to amine, solvent, temperature and reaction time. In accord with other optimization studies![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 20 reaction of the coupling precursors with (Ph3P)2PdCl2 (0.05 eq.) and CuI (0.1 eq.) in a mixture (1∶1) of Et3N and THF proved to be suitable (Scheme 3, Table 1). Due to strong basicity of the bridgehead nitrogen and the presence of a quinoline nitrogen, Sonogashira coupling of 9a is also feasible without addition of an external amine furnishing the desired internal alkyne in moderate yield (44%).
20 reaction of the coupling precursors with (Ph3P)2PdCl2 (0.05 eq.) and CuI (0.1 eq.) in a mixture (1∶1) of Et3N and THF proved to be suitable (Scheme 3, Table 1). Due to strong basicity of the bridgehead nitrogen and the presence of a quinoline nitrogen, Sonogashira coupling of 9a is also feasible without addition of an external amine furnishing the desired internal alkyne in moderate yield (44%).
| Entry | Alkyne | Ph-X (eq.) | Solvent, base![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
Time/h | Yield (%) |
|---|---|---|---|---|---|
| a Typically, reactions were carried out at rt. b Temperature: 60 °C. | |||||
| 1 | 21a | Ph-I (1.0) | THF, Et2NH | 6 | 60 |
| 2 | 21a | Ph-I (1.5) | THF, iPr2NH | 6 | 66 |
| 3 | 21a | Ph-I (1.5) | —, Et3N | 16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
51 |
| 4 | 21a | Ph-I (1.5) | THF, Et3N | 6 | 73 |
| 5 | 21a | Ph-I (1.5) | THF, Et3N | 14 | 80 |
| 6 | 21a | Ph-I (1.5) | Et2O, Et3N | 14 | 68 |
| 7 | 21a | Ph-I (1.5) | Dioxan, Et3N | 14 | 63 |
| 8 | 21a | Ph-I (1.5) | DMF, Et3N | 14 | 65 |
| 9 | 21a | Ph-I (1.5) | THF, — | 72 | 44 |
| 10 | 21a | Ph-Br (1.5) | THF, Et3N | 6 | 53 |
| 11 | 21a | Ph-Br (1.5) | THF, Et3N | 20 | 71 |
| 12 | 21b | Ph-I (1.5) | THF, Et3N | 14 | 94 |
| 13 | 22a | Ph-I (1.5) | THF, Et3N | 16 | 70 |
| 14 | 22b | Ph-I (1.5) | THF, Et3N | 16 | 86 |
 | ||
| Scheme 3 Optimized Sonogashira coupling of 10,11-didehydroquinidine 9a,b, and -quinine 10a,b. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), phenyl halide (1.0–1.5 eq.), THF, amine. | ||
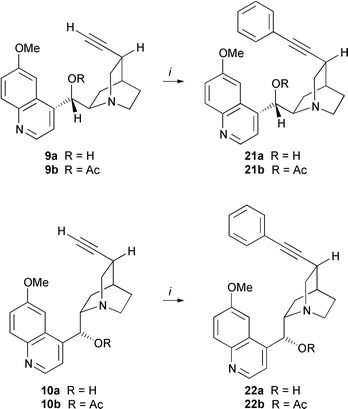
Although an unprotected 1,2-amino alcohol unit was present in terminal alkyne 9a the desired coupling product 21a was obtained in 80% yield using iodobenzene and in 71% yield using bromobenzene (Table 1, entries 5 and 11). Likewise, coupling of 10,11-didehydroquinine 10a with iodobenzene furnished internal alkyne 22a in 70% yield. Protection of the C9-hydroxy group by acetylation as in Cinchona alkaloid precursors 9b and 10b gave higher yields of up to 94% (Schemes 1 and 4, Tables 1 and 2). Reaction with less reactive bromobenzene required longer reaction times, but was also feasible under mild conditions (Table 1, entries 10, 11). Formation of symmetrical diyne side products by oxidative homocoupling was not observed in the optimized Sonogashira couplings of 10,11-didehydro-derivatives of quinine, quinidine, QCI and QCD. Moreover, ordinary, nondegassed reagent-grade solvents could be used without decrease in yield. Optimized Sonogashira coupling of 10,11-didehydroquinidine 9a,b with various substituted aryl and vinyl halides furnished the desired internal alkynes 21c–n with yields from 67 to 94%. When 2-iodophenol was used as halide benzofuran 21o was obtained exclusively by a tandem reaction involving cross-coupling and intramolecular cyclization. The corresponding coupling of 10,11-didehydroquinidine 9b with 2-iodoaniline furnished the substituted alkyne 21n.21 Indoles can only be obtained under forcing conditions.22
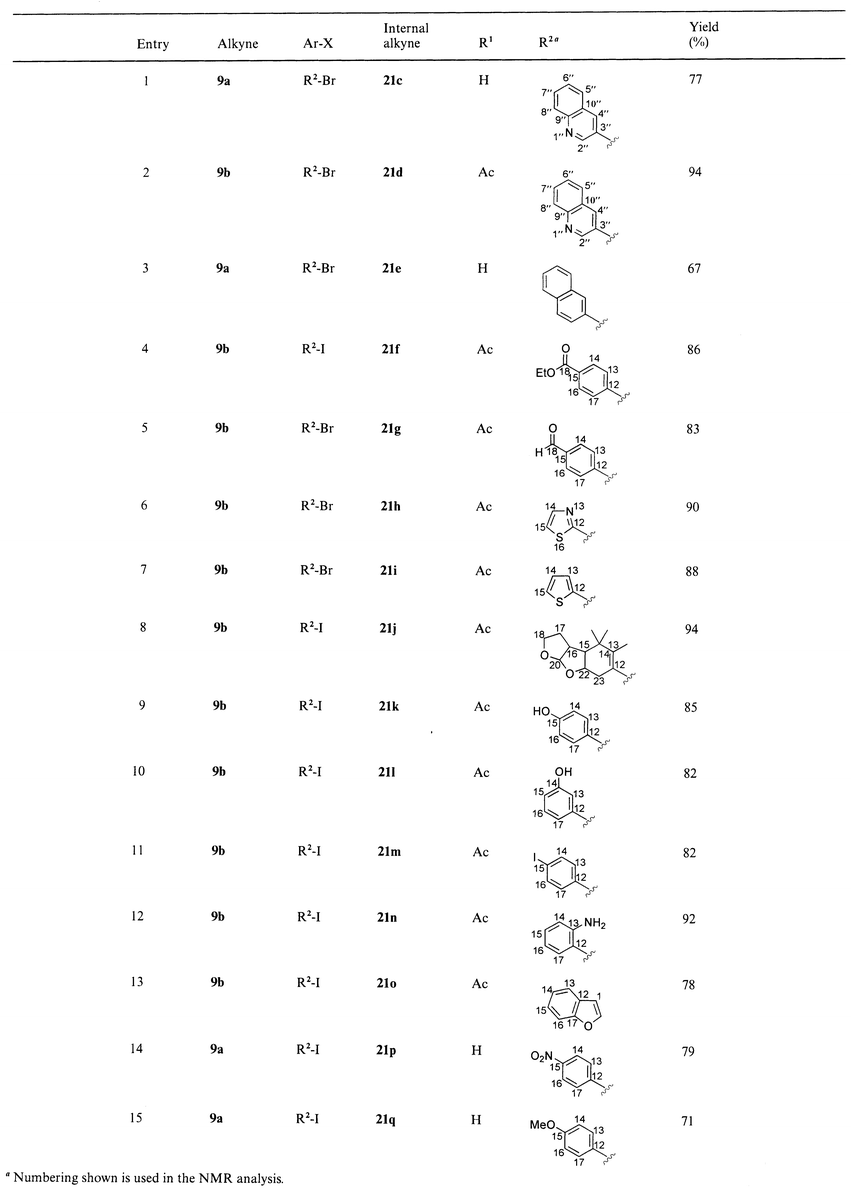
 | ||
| Scheme 4 Sonogashira coupling of 10,11-didehydroquinidine 9a,b with substituted aryl and vinyl halides. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), aryl or vinyl halide (1.0–1.5 eq.), THF, Et3N, 16 h. | ||
Pd-mediated cross-coupling was also applied to polar 1,2-amino alcohols didehydro-QCI and didehydro-QCD (Scheme 5). Unprotected alkynes 18a and 20a coupled under standard conditions to the desired internal alkynes in yields from 62 to 77% (Table 3, entries 1, 3, 4, 9, 10). Nevertheless, O-silylation and O-acylation of the 1,2-amino alcohol gave a considerable increase in yield (up to 92%, Table 3, entry 5). Moreover, no significant difference in reactivity between aryl iodides and aryl bromides was observed, although yields of coupling reactions with aryl bromides turned out to be slightly lower (Table 3, entries 6, 10, 12, 14, 15). Vinylic chlorides are inert for many coupling reactions.23 We were pleased to find that TBDS-protected 10,11-didehydroquincoridine 20b coupled smoothly when using diisopropylamine or piperidine instead of Et3N (Table 3, entries 16 and 17).
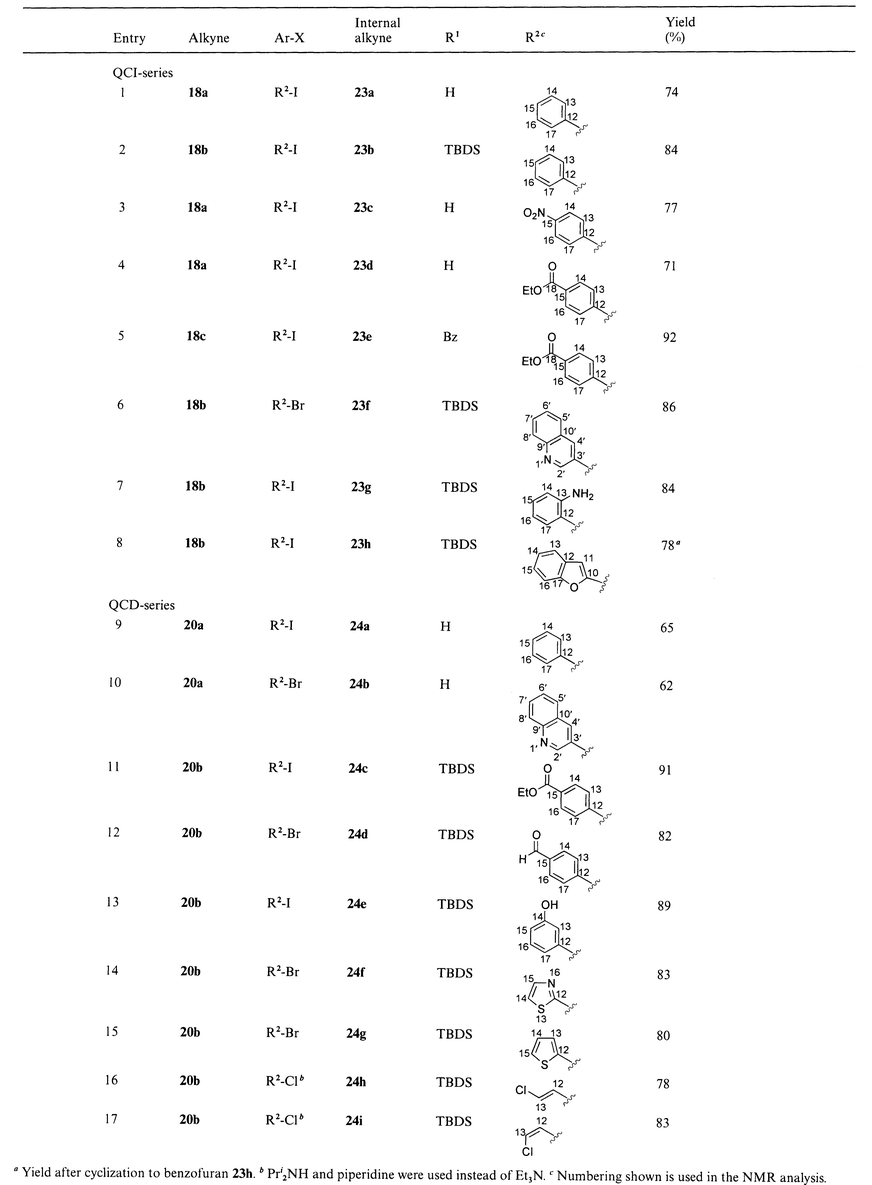
 | ||
| Scheme 5 Sonogashira coupling of 10,11-didehydroquincorine 18a,c and 10,11-didehydroquincoridine 20a,b with substituted aryl and vinyl halides. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), aryl or vinyl halide (1.0–1.5 eq.), THF, Et3N, 16 h. | ||
Quinidine- and quinine-based vinyl iodides 11a,b and 12 coupled with terminal alkynes (Scheme 6) giving (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enynes 25a–c and 26 in fair yield (78–86%). Likewise, vinylstannane 15 was transformed into α-amino enyne 27.
)-enynes 25a–c and 26 in fair yield (78–86%). Likewise, vinylstannane 15 was transformed into α-amino enyne 27.
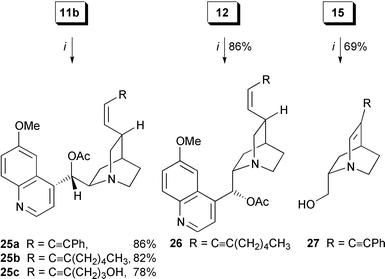 | ||
Scheme 6 Sonogashira coupling of (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-vinyl iodides 11b, 12 and vinylstannane 15 with alkylated alkynes. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), aryl or halide (1.0 eq.), THF, Et3N, 16 h. )-vinyl iodides 11b, 12 and vinylstannane 15 with alkylated alkynes. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), aryl or halide (1.0 eq.), THF, Et3N, 16 h.
| ||
Homocoupling of 10,11-didehydro-quinidine and -quinine furnished the novel class of dimeric Cinchona alkaloids which are linked via the C10–C11-side chain. This is in contrast to AD-ligands of the second generation which incorporate heteroaromatic spacers (phthalazine or pyrimidine) between C9-oxygen atoms. A variety of synthetic methods is available for the homocoupling.24 Although being suitable for the synthesis of porphyrin oligomers, the classic approach to diynes, the Glaser coupling, afforded the desired quinidine dimerics 28a,b only in poor yield (<15%). Modified Sonogashira coupling was more suitable for the synthesis of symmetrical diynes 28 and 29.25 10,11-Didehydroquinidine 9a,b and 10,11-didehydroquinine 10a,b underwent self-coupling in the presence of (Ph3P)2PdCl2, CuI, I2 (0.5 eq.) and Et3N to give the corresponding diynes 28a,b and 29a,b exclusively (yields from 71 to 95%). The resulting products exhibit strongly enhanced basicity compared with parent quinine and quinidine (Scheme 7).
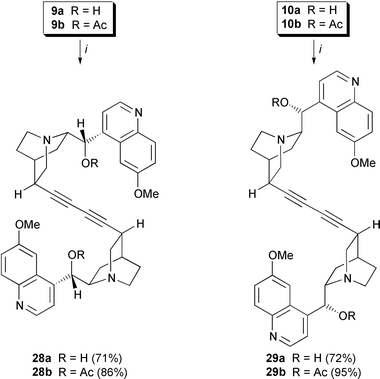 | ||
| Scheme 7 Pd-mediated dimerization of 10,11-didehydroquinine and 10,11-didehydroquinidine. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), I2 (0.5 eq.), THF, Et3N, 16 h. | ||
Homocoupling of simple 10,11-didehydroquincorine 18a and 10,11-didehydroquincoridines 20a,b afforded the corresponding diynes (64–85%) without by-products. Moreover, further spacers could be introduced giving enediyne 32 and quincoridine-based diyne 33 with a benzene spacer. Interestingly, enediyne 32 underwent a Bergman cycloaromatization at moderately elevated temperature (60–70 °C) in CHCl3 (86% yield), although the enediyne moiety was not incorporated into a strained medium sized ring (Scheme 8).
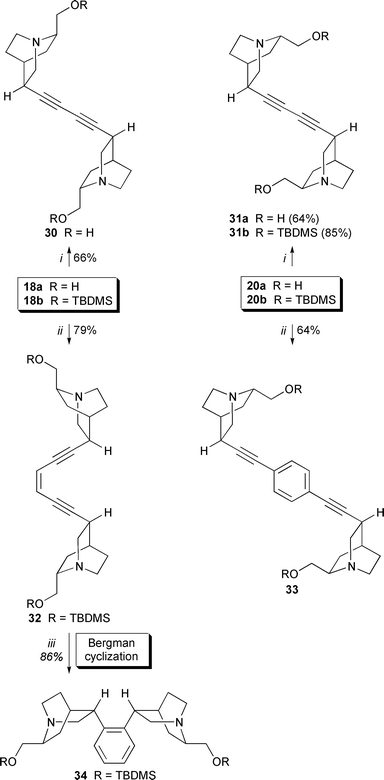 | ||
Scheme 8 Pd-mediated dimerization of 10,11-didehydroquincorine and 10,11-didehydroquincoridine. Reagents and conditions: i, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), I2 (0.5 eq.), THF, Et3N, 16 h; ii, (Ph3P)2PdCl2 (0.05 eq.), CuI (0.1 eq.), 1,4-diiodobenzene or (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-1,2-dichloroethene (0.5 eq.), THF, Et3N, 16 h; iii, CHCl3, 60–70 °C, 4 h. )-1,2-dichloroethene (0.5 eq.), THF, Et3N, 16 h; iii, CHCl3, 60–70 °C, 4 h.
| ||
Over the years the Heck reaction has emerged as a powerful method for the formation of carbon–carbon bonds. Hallmarks of Heck reactions are excellent functional group tolerance and predictable regio- and stereochemistry. A telling indication of the utility of intramolecular Heck reactions is their recent use as the key step in the synthesis of many complex natural products, especially alkaloids.26 Various protocols have been introduced, and tetraalkylammonium salts in particular have been highly successful in enhancing reactivity and selectivity of inter- and intramolecular Heck-type reactions. We therefore used the optimized phase-transfer protocol developed by Jeffery (Pd(OAc)2, K2CO3, n-Bu4NI, DMF![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ).27 Under these conditions quinidine-(Z
).27 Under these conditions quinidine-(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-vinyl iodides 11a,b and quincoridine-based (E
)-vinyl iodides 11a,b and quincoridine-based (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-vinyl iodide could be coupled stereoselectively with α,β-unsaturated carbonyl compounds to give conjugated alkenes 35a–e and 36 at room temperature. O-Protection of the 1,2-amino alcohol unit was not essential. Heck coupling of vinyl iodide 11a with methyl acrylate furnished dienoic ester 35b (84%) (Scheme 9).
)-vinyl iodide could be coupled stereoselectively with α,β-unsaturated carbonyl compounds to give conjugated alkenes 35a–e and 36 at room temperature. O-Protection of the 1,2-amino alcohol unit was not essential. Heck coupling of vinyl iodide 11a with methyl acrylate furnished dienoic ester 35b (84%) (Scheme 9).
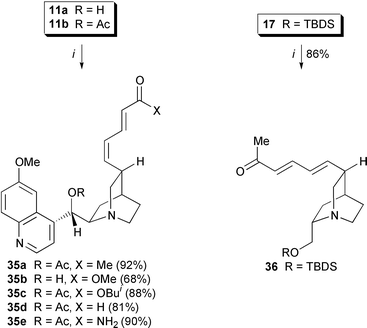 | ||
Scheme 9 Heck reactions of (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )- and (Z )- and (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-vinyl iodides. Reagents and conditions: i, Pd(OAc)2 (0.05 eq.), K2CO3 (2.5 eq.), TBAI (1.0 eq.), α,β-unsatd. compound (4.0 eq.), DMF, RT, 12 h. )-vinyl iodides. Reagents and conditions: i, Pd(OAc)2 (0.05 eq.), K2CO3 (2.5 eq.), TBAI (1.0 eq.), α,β-unsatd. compound (4.0 eq.), DMF, RT, 12 h.
| ||
Conclusion
Cinchona alkaloid chemistry continues to contribute to medicinal chemistry, supramolecular chemistry![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 28 and asymmetric synthesis. As we have shown 10,11-didehydro Cinchona alkaloids represent a significantly new class of semi-natural Cinchona alkaloids
28 and asymmetric synthesis. As we have shown 10,11-didehydro Cinchona alkaloids represent a significantly new class of semi-natural Cinchona alkaloids![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 16 showing enhanced basicity and polarity. We have found that the C10–C11 triple bond facilitates crystallization including that of simple didehydro-QCI (18a) and didehydro-QCD (20a). These alkynes show also little twisting and considerable eclipsing of the ethano bridge of the azabicyclic cage (torsion angles Φ = 5–10° from X-ray crystal studies).16 The additional functionality of the Cinchona alkaloid is tolerated by palladium-mediated cross-coupling without protection.
16 showing enhanced basicity and polarity. We have found that the C10–C11 triple bond facilitates crystallization including that of simple didehydro-QCI (18a) and didehydro-QCD (20a). These alkynes show also little twisting and considerable eclipsing of the ethano bridge of the azabicyclic cage (torsion angles Φ = 5–10° from X-ray crystal studies).16 The additional functionality of the Cinchona alkaloid is tolerated by palladium-mediated cross-coupling without protection.
Experimental
General
Infrared spectra were recorded on a Perkin-Elmer 1710 infrared spectrometer. 1H NMR and 13C NMR spectra were recorded on Bruker AVS 400 and Bruker AVM 500 spectrometers in deuterated chloroform unless otherwise stated, with tetramethylsilane as internal standard. Mass spectra were recorded on a Finnigan MAT 312 (70 eV) or a VG Autospec spectrometer. Microanalyses were performed in the Department of Organic Chemistry of the University of Hannover. Preparative column chromatography was performed on J. T. Baker silica gel (particle size 30–60 μm). Analytical TLC was carried out on aluminium-backed 0.2 mm silica gel 60 F254 plates (E. Merck). Ethyl acetate (EA) and methyl tert-butyl ether (MTBE) were distilled before use. Methanol was dried over calcium hydride and citric acid and distilled over magnesium before use. Silver triflate was purchased from Aldrich, whereas silver benzoate was freshly prepared before use. Coupling precursors 9–12, 18 and 20 were prepared as described previously.16(1S,2S,4S![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-2-Hydroxymethyl-5-tributylstannanyl-1-azabicyclo[2.2.2]oct-5-ene 15
)-2-Hydroxymethyl-5-tributylstannanyl-1-azabicyclo[2.2.2]oct-5-ene 15
Ketone 14![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 18 (1.0 eq.) and 2,4,6-triisopropylbenzenesulfonyl hydrazide (1.05 eq.) were dissolved in Et2O at rt. The resulting yellow reaction mixture was stirred for 16 h at rt, sat. aq. NaHCO3 was added and the aqueous layer was extracted with DCM. The collected organic layer was dried over MgSO4, filtered and the solvent removed in vacuo. The residue was purified by column chromatography (EtOAc–MeOH 10∶1) and redissolved (300 mg, 0.45 mmol, 1 eq.) in a 1∶1 mixture of TMEDA (2 ml) and cyclohexane (2 ml). After stirring for 10 min at rt, the homogeneous solution was cooled to −78 °C and treated with n-BuLi (0.84 ml, 1.34 mmol, 3 eq.) and Bu3SnCl (0.24 ml, 0.89 mmol, 2 eq.). The reaction mixture was stirred for 2 h at 0 °C, treated with sat. aq. NaHCO3 and extracted with CH2Cl2. The collected organic layer was dried (MgSO4), filtered and concentrated under reduced pressure. The resulting crude product was purified by column chromatography (EtOAc–MeOH 6∶1) to afford vinylstannane 15 (80%, 152 mg, 0.36 mmol); νmax(CHCl3)/cm−1 3304, 2960, 2929, 2872, 1564, 1463, 1419, 1379, 1362, 1342, 1292, 1230, 1150, 1082, 1050, 1007, 939 and 878; δH(400 MHz; CDCl3) 6.93 (s, 1 H, H-6), 3.94–3.90 (m, 1 H, H-9), 3.81–3.74 (m, 1 H, H-9), 3.40–3.32 (m, 1 H, H-2), 2.90–2.76 (m, 2 H, H-7, H-7), 1.90–1.81 (m, 1 H, H-4), 1.62–1.54 (m, 2 H, H-3, H-8), 1.48–1.42 (m, 2 H, H-8, H-3), 1.32–1.16 (m, 9 H, SnBu3) and 0.88–0.82 (m, 18 H, SnBu3); δC(100 MHz; CDCl3) 135.93 (CH, C-6), 121.95 (C, C-5), 62.61 (CH, C-2), 61.07 (CH2, C-9), 43.16 (CH2, C-7), 34.32 (CH, C-4), 29.37 (CH2, SnBu3), 28.15 (CH2, SnBu3), 27.12 (CH2, SnBu3), 26.93 (CH2, SnBu3), 26.19 (CH2, C-8), 24.94 (CH3, SnBu3), 24.55 (CH3, SnBu3), 23.86 (CH3, SnBu3), 23.09 (CH2, C-3), −9.02 (CH2, SnBu3), −9.13 (CH2, SnBu3) and −9.22 (CH2, SnBu3); m/z (MAT, 70 °C) (EI) 429.2054 (M+, C20H39N1O1120Sn requires 429.2054), 313 (M+ − 2 Bu, 15%), 292 (7), 269 (100), 239 (10), 213 (20), 195 (1), 177 (27), 155 (23) and 121 (12).
18 (1.0 eq.) and 2,4,6-triisopropylbenzenesulfonyl hydrazide (1.05 eq.) were dissolved in Et2O at rt. The resulting yellow reaction mixture was stirred for 16 h at rt, sat. aq. NaHCO3 was added and the aqueous layer was extracted with DCM. The collected organic layer was dried over MgSO4, filtered and the solvent removed in vacuo. The residue was purified by column chromatography (EtOAc–MeOH 10∶1) and redissolved (300 mg, 0.45 mmol, 1 eq.) in a 1∶1 mixture of TMEDA (2 ml) and cyclohexane (2 ml). After stirring for 10 min at rt, the homogeneous solution was cooled to −78 °C and treated with n-BuLi (0.84 ml, 1.34 mmol, 3 eq.) and Bu3SnCl (0.24 ml, 0.89 mmol, 2 eq.). The reaction mixture was stirred for 2 h at 0 °C, treated with sat. aq. NaHCO3 and extracted with CH2Cl2. The collected organic layer was dried (MgSO4), filtered and concentrated under reduced pressure. The resulting crude product was purified by column chromatography (EtOAc–MeOH 6∶1) to afford vinylstannane 15 (80%, 152 mg, 0.36 mmol); νmax(CHCl3)/cm−1 3304, 2960, 2929, 2872, 1564, 1463, 1419, 1379, 1362, 1342, 1292, 1230, 1150, 1082, 1050, 1007, 939 and 878; δH(400 MHz; CDCl3) 6.93 (s, 1 H, H-6), 3.94–3.90 (m, 1 H, H-9), 3.81–3.74 (m, 1 H, H-9), 3.40–3.32 (m, 1 H, H-2), 2.90–2.76 (m, 2 H, H-7, H-7), 1.90–1.81 (m, 1 H, H-4), 1.62–1.54 (m, 2 H, H-3, H-8), 1.48–1.42 (m, 2 H, H-8, H-3), 1.32–1.16 (m, 9 H, SnBu3) and 0.88–0.82 (m, 18 H, SnBu3); δC(100 MHz; CDCl3) 135.93 (CH, C-6), 121.95 (C, C-5), 62.61 (CH, C-2), 61.07 (CH2, C-9), 43.16 (CH2, C-7), 34.32 (CH, C-4), 29.37 (CH2, SnBu3), 28.15 (CH2, SnBu3), 27.12 (CH2, SnBu3), 26.93 (CH2, SnBu3), 26.19 (CH2, C-8), 24.94 (CH3, SnBu3), 24.55 (CH3, SnBu3), 23.86 (CH3, SnBu3), 23.09 (CH2, C-3), −9.02 (CH2, SnBu3), −9.13 (CH2, SnBu3) and −9.22 (CH2, SnBu3); m/z (MAT, 70 °C) (EI) 429.2054 (M+, C20H39N1O1120Sn requires 429.2054), 313 (M+ − 2 Bu, 15%), 292 (7), 269 (100), 239 (10), 213 (20), 195 (1), 177 (27), 155 (23) and 121 (12).
(1S,2S,4S,5R)-2-[(tert-Butyl)(dimethyl)silyloxymethyl]-5-[(E![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-2-iodovinyl]-1-azabicyclo[2.2.2]octane 17
)-2-iodovinyl]-1-azabicyclo[2.2.2]octane 17
CrCl2 (612 mg, 4.98 mmol, 8 eq.) was dissolved in absolute THF, cooled (0 °C) and treated with CHI3 (490 mg, 1.24 mmol, 2 eq.). After 10 min TBDMS-protected C10-aldehyde 16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 29 (176 mg, 0.62 mmol, 1 eq.) was added at 0 °C and the resulting reaction mixture was stirred for 3 h at rt. After filtration over Celite® sat. aq. NaHCO3, sat. aq. NaCl and H2O were added and the aqueous layer was extracted with CHCl3. The combined organic layer was dried (MgSO4), filtered and concentrated in vacuo. Purification of the crude product by column chromatography (EtOAc–MeOH 20∶1) furnished the desired vinyl iodide 17 (66%, 167 mg, 0.41 mmol, E∶Z ratio: 81∶19); νmax(CHCl3)/cm−1 2956, 2932, 2900, 2860, 1672, 1604, 1460, 1408, 1388, 1256, 1228, 1172, 1132, 1092, 1064, 1004 and 948; δH(400 MHz; CDCl3) 6.61–6.53 (m, 1 H, H-10), 6.41–6.37 (d, 1 H, J 14.5, H-11), 3.85–3.72 (m, 2 H, H-9, H-9), 3.62–3.54 (m, 1 H, H-2), 3.43–3.34 (m, 1 H, H-6), 3.32–3.24 (m, 1 H, H-6), 3.18–3.10 (m, 1 H, H-7), 3.08–2.97 (m, 1 H, H-7), 2.49–2.45 (m, 1 H, H-5), 2.28–2.22 (m, 1 H, H-4), 2.17–2.11 (m, 1 H, H-3), 2.10–1.93 (m, 1 H, H-8), 1.92–1.81 (m, 1 H, H-8), 1.80–1.71 (m, 1 H, H-3), 0.88 (s, 9 H, SiC(CH3)3) and 0.13–0.08 (m, 6 H, SiCH3); δC(100 MHz; CDCl3) 144.42 (CH, C-10), 79.65 (CH, C-11), 62.29 (CH2, C-9), 58.79 (CH, C-2), 53.26 (CH2, C-6), 43.81 (CH2, C-7), 31.49 (CH, C-5), 27.85 (CH, C-4), 25.97 (CH3, SiC(C
29 (176 mg, 0.62 mmol, 1 eq.) was added at 0 °C and the resulting reaction mixture was stirred for 3 h at rt. After filtration over Celite® sat. aq. NaHCO3, sat. aq. NaCl and H2O were added and the aqueous layer was extracted with CHCl3. The combined organic layer was dried (MgSO4), filtered and concentrated in vacuo. Purification of the crude product by column chromatography (EtOAc–MeOH 20∶1) furnished the desired vinyl iodide 17 (66%, 167 mg, 0.41 mmol, E∶Z ratio: 81∶19); νmax(CHCl3)/cm−1 2956, 2932, 2900, 2860, 1672, 1604, 1460, 1408, 1388, 1256, 1228, 1172, 1132, 1092, 1064, 1004 and 948; δH(400 MHz; CDCl3) 6.61–6.53 (m, 1 H, H-10), 6.41–6.37 (d, 1 H, J 14.5, H-11), 3.85–3.72 (m, 2 H, H-9, H-9), 3.62–3.54 (m, 1 H, H-2), 3.43–3.34 (m, 1 H, H-6), 3.32–3.24 (m, 1 H, H-6), 3.18–3.10 (m, 1 H, H-7), 3.08–2.97 (m, 1 H, H-7), 2.49–2.45 (m, 1 H, H-5), 2.28–2.22 (m, 1 H, H-4), 2.17–2.11 (m, 1 H, H-3), 2.10–1.93 (m, 1 H, H-8), 1.92–1.81 (m, 1 H, H-8), 1.80–1.71 (m, 1 H, H-3), 0.88 (s, 9 H, SiC(CH3)3) and 0.13–0.08 (m, 6 H, SiCH3); δC(100 MHz; CDCl3) 144.42 (CH, C-10), 79.65 (CH, C-11), 62.29 (CH2, C-9), 58.79 (CH, C-2), 53.26 (CH2, C-6), 43.81 (CH2, C-7), 31.49 (CH, C-5), 27.85 (CH, C-4), 25.97 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 23.91 (CH2, C-8), 22.19 (CH2, C-3), 18.22 (C, SiC(CH3)3), −5.14 (CH3, SiC
H3)3), 23.91 (CH2, C-8), 22.19 (CH2, C-3), 18.22 (C, SiC(CH3)3), −5.14 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.37 (CH3, SiC
H3) and −5.37 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 408 (M+ + H, 100%), 394 (10), 282 (9), 221 (8), 207 (12), 147 (23) and 133 (19).
H3); m/z (FAB) (EI) 408 (M+ + H, 100%), 394 (10), 282 (9), 221 (8), 207 (12), 147 (23) and 133 (19).
General procedure for the Sonogashira coupling of aryl and vinyl halides with terminal alkynes
(Ph3P)2PdCl2 (0.05 eq.) and CuI (0.1 eq.) were dissolved in Et3N and absolute THF (1∶1 mixture, 10 ml mmol−1 alkaloid). The reaction mixture was stirred for 15 min at rt under argon and a solution of the corresponding aryl or vinyl halide (1.5 eq.) in absolute THF was added. After stirring for 45 min at rt a solution of the terminal alkyne in absolute THF was added dropwise within 15 min. After having been stirred for 14–20 h at rt, the resulting orange–brown reaction mixture was treated with sat. aq. NaHCO3 and sat. aq. NaCl. The aqueous layer was extracted several times with CH2Cl2 (up to 8×) and the combined organic layer was dried (MgSO4) and concentrated under reduced pressure. The resulting crude product was purified by column chromatography (EtOAc–MeOH) to yield the desired internal alkyne.![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-11-Phenyl-10,11-didehydro-6′-methoxycinchonan-9-ol 21a.
10,11-Didehydroquinidine 9a (644 mg, 2.00 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (70 mg, 0.10 mmol, 0.05 eq.), CuI (95 mg, 0.20 mmol, 0.1 eq.) and phenyl iodide (0.33 ml, 3.00 mmol, 1.5 eq.) to afford internal alkyne 21a (80%, 637 mg, 1.60 mmol); νmax(CHCl3)/cm−1 3064, 2944, 2876, 2836, 2224, 1620, 1592, 1508, 1472, 1432, 1388, 1364, 1320, 1256, 1228, 1172, 1092, 1032, 996 and 828; δH(400 MHz; CDCl3) 8.55 (d, 1 H, J 4.6, H-2′), 7.93 (d, 1 H, J 9.1, H-8′), 7.47–7.42 (m, 3 H, H-3′, 2 Ar-H), 7.33–7.28 (m, 3 H, Ar-H), 7.27 (d, 1 H, J 2.6, H-5′), 7.24 (dd, 1 H, J 9.2 and 2.6, H-7′), 5.67 (d, 1 H, J 5.4, H-9), 3.80 (s, 3 H, H-11′), 3.54–3.49 (ddd, 1 H, J 13.6, 7.0 and 2.0, H-2endo), 3.20–3.14 (m, 1 H, H-8), 3.11–3.05 (dd, 1 H, J 13.3 and 8.5, H-2exo), 2.91–2.83 (m, 1 H, H-6), 2.75–2.65 (m, 2 H, H-6, H-3), 2.43–2.37 (m, 1 H, H-7), 2.09–2.05 (m, 1 H, H-4), 1.57–1.49 (m, 2 H, H-7, H-5) and 1.46–1.38 (m, 1 H, H-5); δC(100 MHz; CDCl3) 157.61 (C, C-6′), 147.62 (C, C-10′), 147.45 (CH, C-2′), 144.02 (C, C-4′), 131.63 (CH, Ar-H), 131.28 (CH, C-8′), 128.28 (CH, Ar-H), 127.77 (CH, Ar-H), 126.81 (C, C-9′), 123.69 (C, Ar-C), 121.52 (CH, C-7′), 118.74 (CH, C-3′), 101.39 (CH, C-5′), 92.68 (C, C-11), 81.70 (C, C-10), 71.41 (CH, C-9), 60.04 (CH, C-8), 55.64 (CH3, C-11′), 50.61 (CH2, C-2), 49.54 (CH2, C-6), 28.81 (CH, C-3), 28.12 (CH, C-4), 25.03 (CH2, C-7) and 22.94 (CH2, C-5); m/z (MAT, 190 °C) (EI) 398.1999 (M+, C26H26N2O2 requires 398.1994), 381 (3%), 369 (4), 341 (5), 326 (3), 312 (3), 283 (11), 262 (3), 240 (2), 226 (3), 210 (100), 200 (4), 189 (61), 172 (9), 155 (11), 128 (152), 115 (15), 91 (7) and 77 (9).
)-11-Phenyl-10,11-didehydro-6′-methoxycinchonan-9-ol 21a.
10,11-Didehydroquinidine 9a (644 mg, 2.00 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (70 mg, 0.10 mmol, 0.05 eq.), CuI (95 mg, 0.20 mmol, 0.1 eq.) and phenyl iodide (0.33 ml, 3.00 mmol, 1.5 eq.) to afford internal alkyne 21a (80%, 637 mg, 1.60 mmol); νmax(CHCl3)/cm−1 3064, 2944, 2876, 2836, 2224, 1620, 1592, 1508, 1472, 1432, 1388, 1364, 1320, 1256, 1228, 1172, 1092, 1032, 996 and 828; δH(400 MHz; CDCl3) 8.55 (d, 1 H, J 4.6, H-2′), 7.93 (d, 1 H, J 9.1, H-8′), 7.47–7.42 (m, 3 H, H-3′, 2 Ar-H), 7.33–7.28 (m, 3 H, Ar-H), 7.27 (d, 1 H, J 2.6, H-5′), 7.24 (dd, 1 H, J 9.2 and 2.6, H-7′), 5.67 (d, 1 H, J 5.4, H-9), 3.80 (s, 3 H, H-11′), 3.54–3.49 (ddd, 1 H, J 13.6, 7.0 and 2.0, H-2endo), 3.20–3.14 (m, 1 H, H-8), 3.11–3.05 (dd, 1 H, J 13.3 and 8.5, H-2exo), 2.91–2.83 (m, 1 H, H-6), 2.75–2.65 (m, 2 H, H-6, H-3), 2.43–2.37 (m, 1 H, H-7), 2.09–2.05 (m, 1 H, H-4), 1.57–1.49 (m, 2 H, H-7, H-5) and 1.46–1.38 (m, 1 H, H-5); δC(100 MHz; CDCl3) 157.61 (C, C-6′), 147.62 (C, C-10′), 147.45 (CH, C-2′), 144.02 (C, C-4′), 131.63 (CH, Ar-H), 131.28 (CH, C-8′), 128.28 (CH, Ar-H), 127.77 (CH, Ar-H), 126.81 (C, C-9′), 123.69 (C, Ar-C), 121.52 (CH, C-7′), 118.74 (CH, C-3′), 101.39 (CH, C-5′), 92.68 (C, C-11), 81.70 (C, C-10), 71.41 (CH, C-9), 60.04 (CH, C-8), 55.64 (CH3, C-11′), 50.61 (CH2, C-2), 49.54 (CH2, C-6), 28.81 (CH, C-3), 28.12 (CH, C-4), 25.03 (CH2, C-7) and 22.94 (CH2, C-5); m/z (MAT, 190 °C) (EI) 398.1999 (M+, C26H26N2O2 requires 398.1994), 381 (3%), 369 (4), 341 (5), 326 (3), 312 (3), 283 (11), 262 (3), 240 (2), 226 (3), 210 (100), 200 (4), 189 (61), 172 (9), 155 (11), 128 (152), 115 (15), 91 (7) and 77 (9).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-11-(3″-Quinolyl)-10,11-didehydro-6′-methoxycinchonan-9-ol 21c.
10,11-Didehydroquinidine 9a (644 mg, 2.00 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (70 mg, 0.10 mmol, 0.05 eq.), CuI (95 mg, 0.20 mmol, 0.1 eq.) and 3-bromoquinoline (0.41 ml, 3.00 mmol, 1.5 eq.) to afford internal alkyne 21c (77%, 691 mg, 1.54 mmol); νmax(CHCl3)/cm−1 3340, 3068, 2948, 2876, 2220, 1620, 1592, 1508, 1488, 1472, 1432, 1388, 1360, 1340, 1320, 1256, 1240, 1172, 1092, 1032, 908 and 828; δH(400 MHz; CDCl3) 8.69 (d, 1 H, J 1.9, H-2″), 8.55 (d, 1 H, J 4.6, H-2′), 8.12 (d, 1 H, J 1.9, H-4″), 8.06 (d, 1 H, J 8.5, H-8″), 7.97 (d, 1 H, J 9.3, H-8′), 7.69 (d, 1 H, J 8.0, H-5″), 7.66 (ddd, 1 H, J 8.4, 6.9 and 1.5, H-7″), 7.57 (d, 1 H, J 4.5, H-3′), 7.51–7.48 (ddd, 1 H, J 8.0, 6.9 and 1.1, H-6″), 7.34 (d, 1 H, J 2.6, H-5′), 7.27 (dd, 1 H, J 9.2, 2.6, H-7′), 5.68 (d, 1 H, J 5.4, H-9), 3.80 (s, 3 H, H-11′), 3.61–3.54 (m, 1 H, H-2endo), 3.25–3.19 (m, 1 H, H-8), 3.14–3.07 (dd, 1 H, J 13.4 and 10.4, H-2exo), 2.94–2.85 (m, 1 H, H-6), 2.76–2.69 (m, 1 H, H-6), 2.46–2.40 (m, 1 H, H-3), 2.14–2.09 (m, 1 H, H-4), 1.65–1.47 (m, 3 H, H-7, H-7, H-5) and 1.35–1.26 (m, 1 H, H-5); δC(100 MHz; CDCl3) 157.58 (C, C-6′), 152.30 (CH, C-6″), 147.99 (C, C-10′), 147.50 (CH, C-2′), 146.30 (C, C-10″), 144.17 (C, C-4′), 138.15 (CH, C-2″), 131.37 (CH, C-8′), 129.85 (CH, C-8″), 128.99 (CH, C-7″), 127.43 (CH, C-5″), 127.27 (C, C-9″), 127.23 (CH, C-4″), 126.96 (C, C-9′), 121.43 (CH, C-7′), 118.97 (CH, C-3′), 117.90 (C, C-3″), 101.70 (CH, C-5′), 96.57 (C, C-11), 78.80 (C, C-10), 71.71 (CH, C-9), 60.08 (CH, C-8), 55.59 (CH3, C-11′), 50.44 (CH2, C-2), 49.54 (CH2, C-6), 29.09 (CH, C-3), 28.19 (CH, C-4), 25.07 (CH2, C-7) and 23.31 (CH2, C-5); m/z (MAT, 180 °C) (EI) 449.2108 (M+, C29H27N3O2 requires 449.2103), 434 (7%), 421 (8), 392 (7), 378 (7), 363 (7), 283 (18), 261 (100), 249 (8), 233 (26), 220 (15), 206 (19), 189 (45), 179 (16), 167 (14), 158 (11), 128 (10), 117 (10), 91 (17) and 82 (10).
)-11-(3″-Quinolyl)-10,11-didehydro-6′-methoxycinchonan-9-ol 21c.
10,11-Didehydroquinidine 9a (644 mg, 2.00 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (70 mg, 0.10 mmol, 0.05 eq.), CuI (95 mg, 0.20 mmol, 0.1 eq.) and 3-bromoquinoline (0.41 ml, 3.00 mmol, 1.5 eq.) to afford internal alkyne 21c (77%, 691 mg, 1.54 mmol); νmax(CHCl3)/cm−1 3340, 3068, 2948, 2876, 2220, 1620, 1592, 1508, 1488, 1472, 1432, 1388, 1360, 1340, 1320, 1256, 1240, 1172, 1092, 1032, 908 and 828; δH(400 MHz; CDCl3) 8.69 (d, 1 H, J 1.9, H-2″), 8.55 (d, 1 H, J 4.6, H-2′), 8.12 (d, 1 H, J 1.9, H-4″), 8.06 (d, 1 H, J 8.5, H-8″), 7.97 (d, 1 H, J 9.3, H-8′), 7.69 (d, 1 H, J 8.0, H-5″), 7.66 (ddd, 1 H, J 8.4, 6.9 and 1.5, H-7″), 7.57 (d, 1 H, J 4.5, H-3′), 7.51–7.48 (ddd, 1 H, J 8.0, 6.9 and 1.1, H-6″), 7.34 (d, 1 H, J 2.6, H-5′), 7.27 (dd, 1 H, J 9.2, 2.6, H-7′), 5.68 (d, 1 H, J 5.4, H-9), 3.80 (s, 3 H, H-11′), 3.61–3.54 (m, 1 H, H-2endo), 3.25–3.19 (m, 1 H, H-8), 3.14–3.07 (dd, 1 H, J 13.4 and 10.4, H-2exo), 2.94–2.85 (m, 1 H, H-6), 2.76–2.69 (m, 1 H, H-6), 2.46–2.40 (m, 1 H, H-3), 2.14–2.09 (m, 1 H, H-4), 1.65–1.47 (m, 3 H, H-7, H-7, H-5) and 1.35–1.26 (m, 1 H, H-5); δC(100 MHz; CDCl3) 157.58 (C, C-6′), 152.30 (CH, C-6″), 147.99 (C, C-10′), 147.50 (CH, C-2′), 146.30 (C, C-10″), 144.17 (C, C-4′), 138.15 (CH, C-2″), 131.37 (CH, C-8′), 129.85 (CH, C-8″), 128.99 (CH, C-7″), 127.43 (CH, C-5″), 127.27 (C, C-9″), 127.23 (CH, C-4″), 126.96 (C, C-9′), 121.43 (CH, C-7′), 118.97 (CH, C-3′), 117.90 (C, C-3″), 101.70 (CH, C-5′), 96.57 (C, C-11), 78.80 (C, C-10), 71.71 (CH, C-9), 60.08 (CH, C-8), 55.59 (CH3, C-11′), 50.44 (CH2, C-2), 49.54 (CH2, C-6), 29.09 (CH, C-3), 28.19 (CH, C-4), 25.07 (CH2, C-7) and 23.31 (CH2, C-5); m/z (MAT, 180 °C) (EI) 449.2108 (M+, C29H27N3O2 requires 449.2103), 434 (7%), 421 (8), 392 (7), 378 (7), 363 (7), 283 (18), 261 (100), 249 (8), 233 (26), 220 (15), 206 (19), 189 (45), 179 (16), 167 (14), 158 (11), 128 (10), 117 (10), 91 (17) and 82 (10).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(3″-quinolyl)-10,11-didehydro-6′-methoxycinchonan 21d.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-bromoquinoline (0.07 ml, 0.54 mmol, 1.5 eq.) to afford quininyl-substituted alkyne 21d (94%, 165 mg, 0.34 mmol); νmax(CHCl3)/cm−1 2952, 2876, 2224, 1744, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1320, 1300, 1264, 1232, 1136, 1092, 1032 and 908; δH(400 MHz; CDCl3) 9.03 (d, 1 H, J 2.0, H-2″), 8.81 (d, 1 H, J 4.4, H-2′), 8.37 (d, 1 H, J 1.9, H-4″), 8.16 (d, 1 H, J 8.4, H-8″), 8.08 (d, 1 H, J 9.2, H-8′), 7.85 (d, 1 H, J 8.1, H-5″), 7.83 (ddd, 1 H, J 8.4, 7.0 and 1.5, H-7″), 7.63–7.56 (ddd, 1 H, J 8.1, 7.0 and 1.1, H-6″), 7.55 (d, 1 H, J 2.6, H-5′), 7.45 (d, 1 H, J 4.6, H-3′), 7.41 (dd, 1 H, J 9.2 and 2.7, H-7′), 6.78 (d, 1 H, J 7.2, H-9), 3.89 (s, 3 H, H-11′), 3.47–3.41 (m, 1 H, H-8), 3.29–3.23 (m, 1 H, H-2endo), 3.19–3.12 (dd, 1 H, J 13.8 and 8.5, H-2exo), 2.92–2.74 (m, 2 H, H-6, H-6), 2.33–2.27 (m, 1 H, H-3), 2.21–2.17 (m, 1 H, H-4), 2.16 (s, 3 H, H-13), 1.76–1.55 (m, 3 H, H-7, H-7, H-5) and 1.36–1.25 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.91 (C, C-12), 157.99 (C, C-6′), 152.43 (CH, C-6″), 147.49 (CH, C-2′), 146.64 (C, C-10″), 144.71 (C, C-10′), 143.56 (C, C-4′), 138.29 (CH, C-2″), 131.77 (CH, C-8′), 129.90 (CH, C-8″), 129.34 (CH, C-7″), 127.52 (CH, C-5″), 127.36 (C, C-9″), 127.28 (CH, C-4″), 127.03 (C, C-9′), 121.91 (CH, C-7′), 118.87 (CH, C-3′), 117.82 (C, C-3″), 101.43 (CH, C-5′), 96.38 (C, C-11), 79.18 (C, C-10), 73.45 (CH, C-9), 58.94 (CH, C-8), 55.52 (CH3, C-11′), 50.39 (CH2, C-2), 49.49 (CH2, C-6), 29.09 (CH, C-3), 27.81 (CH, C-4), 25.05 (CH2, C-7), 24.42 (CH2, C-5) and 21.14 (CH3, C-13); m/z (MAT, 180 °C) (EI) 491.2197 (M+, C31H29N3O3 requires 491.2195), 476 (9%), 448 (11), 432 (26), 421 (8), 402 (8), 392 (9), 375 (9), 325 (22), 284 (8), 261 (100), 245 (10), 233 (34), 220 (14), 204 (22), 189 (37), 179 (20), 166 (17), 154 (13), 128 (11), 117 (8), 94 (11) and 82 (10).
)-9-Acetoxy-11-(3″-quinolyl)-10,11-didehydro-6′-methoxycinchonan 21d.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-bromoquinoline (0.07 ml, 0.54 mmol, 1.5 eq.) to afford quininyl-substituted alkyne 21d (94%, 165 mg, 0.34 mmol); νmax(CHCl3)/cm−1 2952, 2876, 2224, 1744, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1320, 1300, 1264, 1232, 1136, 1092, 1032 and 908; δH(400 MHz; CDCl3) 9.03 (d, 1 H, J 2.0, H-2″), 8.81 (d, 1 H, J 4.4, H-2′), 8.37 (d, 1 H, J 1.9, H-4″), 8.16 (d, 1 H, J 8.4, H-8″), 8.08 (d, 1 H, J 9.2, H-8′), 7.85 (d, 1 H, J 8.1, H-5″), 7.83 (ddd, 1 H, J 8.4, 7.0 and 1.5, H-7″), 7.63–7.56 (ddd, 1 H, J 8.1, 7.0 and 1.1, H-6″), 7.55 (d, 1 H, J 2.6, H-5′), 7.45 (d, 1 H, J 4.6, H-3′), 7.41 (dd, 1 H, J 9.2 and 2.7, H-7′), 6.78 (d, 1 H, J 7.2, H-9), 3.89 (s, 3 H, H-11′), 3.47–3.41 (m, 1 H, H-8), 3.29–3.23 (m, 1 H, H-2endo), 3.19–3.12 (dd, 1 H, J 13.8 and 8.5, H-2exo), 2.92–2.74 (m, 2 H, H-6, H-6), 2.33–2.27 (m, 1 H, H-3), 2.21–2.17 (m, 1 H, H-4), 2.16 (s, 3 H, H-13), 1.76–1.55 (m, 3 H, H-7, H-7, H-5) and 1.36–1.25 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.91 (C, C-12), 157.99 (C, C-6′), 152.43 (CH, C-6″), 147.49 (CH, C-2′), 146.64 (C, C-10″), 144.71 (C, C-10′), 143.56 (C, C-4′), 138.29 (CH, C-2″), 131.77 (CH, C-8′), 129.90 (CH, C-8″), 129.34 (CH, C-7″), 127.52 (CH, C-5″), 127.36 (C, C-9″), 127.28 (CH, C-4″), 127.03 (C, C-9′), 121.91 (CH, C-7′), 118.87 (CH, C-3′), 117.82 (C, C-3″), 101.43 (CH, C-5′), 96.38 (C, C-11), 79.18 (C, C-10), 73.45 (CH, C-9), 58.94 (CH, C-8), 55.52 (CH3, C-11′), 50.39 (CH2, C-2), 49.49 (CH2, C-6), 29.09 (CH, C-3), 27.81 (CH, C-4), 25.05 (CH2, C-7), 24.42 (CH2, C-5) and 21.14 (CH3, C-13); m/z (MAT, 180 °C) (EI) 491.2197 (M+, C31H29N3O3 requires 491.2195), 476 (9%), 448 (11), 432 (26), 421 (8), 402 (8), 392 (9), 375 (9), 325 (22), 284 (8), 261 (100), 245 (10), 233 (34), 220 (14), 204 (22), 189 (37), 179 (20), 166 (17), 154 (13), 128 (11), 117 (8), 94 (11) and 82 (10).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-10,11-Didehydro-11-(2-naphthyl)-6′-methoxycinchonan-9-ol 21e.
10,11-Didehydroquinidine 9a (130 mg, 0.40 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (14 mg, 0.02 mmol, 0.05 eq.), CuI (8 mg, 0.04 mmol, 0.1 eq.) and 3-bromonaphthalene (125 mg, 0.61 mmol, 1.5 eq.) to afford naphthyl-substituted alkyne 21e (67%, 121 mg, 0.27 mmol); νmax(CHCl3)/cm−1 3412, 3084, 2948, 2876, 1620, 1592, 1508, 1472, 1432, 1388, 1364, 1320, 1240, 1188, 1104, 1032, 844 and 832; δH(400 MHz; CDCl3) 8.46 (d, 1 H, J 4.6, H-2′), 7.90–7.82 (m, 3 H, H-8′, 2 naphthyl-H), 7.41 (d, 1 H, J 4.5, H-3′), 7.33–7.20 (m, 5 H, H-5′, H-7′, 3 naphthyl-H), 6.80–6.59 (m, 2 H, naphthyl-H), 5.76 (s, 1 H, H-9), 3.84 (s, 3 H, H-11′), 3.68–3.63 (m, 1 H, H-8), 3.11–3.02 (m, 1 H, H-2), 2.98–2.81 (m, 2 H, H-2, H-6), 2.73–2.59 (m, 1 H, H-6), 2.38–2.30 (m, 1 H, H-3), 1.98–1.92 (m, 1 H, H-4), 1.56–1.39 (m, 3 H, H-7, H-7, H-5) and 1.31–1.14 (m, 1 H, H-5); δC(100 MHz; CDCl3) 157.82 (C, C-6′), 147.69 (C, C-10′), 147.37 (CH, C-2′), 143.81 (C, C-4′), 133.44 (C, C-13), 133.28 (C, C-18), 131.17 (CH, C-8′), 130.21 (CH, C-12), 130.09 (C, C-21), 128.69–126.88 (CH, C-14, C-15, C-16, C-17, C-19, C-20), 126.54 (C, C-9′), 121.69 (CH, C-7′), 118.98 (CH, C-3′), 101.26 (CH, C-5′), 96.42 (C, C-11), 80.09 (C, C-10), 69.98 (CH, C-9), 59.82 (CH, C-8), 55.87 (CH3, C-11′), 49.29 (CH2, C-2), 46.63 (CH2, C-6), 29.98 (CH, C-3), 27.88 (CH, C-4), 24.34 (CH2, C-7) and 22.58 (CH2, C-5); m/z (MAT, 50 °C) (EI) 448.2151 (M+, C30H28N2O2 requires 448.2151), 442 (6%), 426 (4), 411 (3), 399 (3), 373 (3), 355 (2), 321 (4), 284 (5), 267 (12), 254 (8), 239 (7), 202 (5), 189 (31), 173 (18), 160 (22), 129 (7), 116 (10), 99 (12) and 83 (100).
)-10,11-Didehydro-11-(2-naphthyl)-6′-methoxycinchonan-9-ol 21e.
10,11-Didehydroquinidine 9a (130 mg, 0.40 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (14 mg, 0.02 mmol, 0.05 eq.), CuI (8 mg, 0.04 mmol, 0.1 eq.) and 3-bromonaphthalene (125 mg, 0.61 mmol, 1.5 eq.) to afford naphthyl-substituted alkyne 21e (67%, 121 mg, 0.27 mmol); νmax(CHCl3)/cm−1 3412, 3084, 2948, 2876, 1620, 1592, 1508, 1472, 1432, 1388, 1364, 1320, 1240, 1188, 1104, 1032, 844 and 832; δH(400 MHz; CDCl3) 8.46 (d, 1 H, J 4.6, H-2′), 7.90–7.82 (m, 3 H, H-8′, 2 naphthyl-H), 7.41 (d, 1 H, J 4.5, H-3′), 7.33–7.20 (m, 5 H, H-5′, H-7′, 3 naphthyl-H), 6.80–6.59 (m, 2 H, naphthyl-H), 5.76 (s, 1 H, H-9), 3.84 (s, 3 H, H-11′), 3.68–3.63 (m, 1 H, H-8), 3.11–3.02 (m, 1 H, H-2), 2.98–2.81 (m, 2 H, H-2, H-6), 2.73–2.59 (m, 1 H, H-6), 2.38–2.30 (m, 1 H, H-3), 1.98–1.92 (m, 1 H, H-4), 1.56–1.39 (m, 3 H, H-7, H-7, H-5) and 1.31–1.14 (m, 1 H, H-5); δC(100 MHz; CDCl3) 157.82 (C, C-6′), 147.69 (C, C-10′), 147.37 (CH, C-2′), 143.81 (C, C-4′), 133.44 (C, C-13), 133.28 (C, C-18), 131.17 (CH, C-8′), 130.21 (CH, C-12), 130.09 (C, C-21), 128.69–126.88 (CH, C-14, C-15, C-16, C-17, C-19, C-20), 126.54 (C, C-9′), 121.69 (CH, C-7′), 118.98 (CH, C-3′), 101.26 (CH, C-5′), 96.42 (C, C-11), 80.09 (C, C-10), 69.98 (CH, C-9), 59.82 (CH, C-8), 55.87 (CH3, C-11′), 49.29 (CH2, C-2), 46.63 (CH2, C-6), 29.98 (CH, C-3), 27.88 (CH, C-4), 24.34 (CH2, C-7) and 22.58 (CH2, C-5); m/z (MAT, 50 °C) (EI) 448.2151 (M+, C30H28N2O2 requires 448.2151), 442 (6%), 426 (4), 411 (3), 399 (3), 373 (3), 355 (2), 321 (4), 284 (5), 267 (12), 254 (8), 239 (7), 202 (5), 189 (31), 173 (18), 160 (22), 129 (7), 116 (10), 99 (12) and 83 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-10,11-didehydro-11-(4-ethoxycarbonylphenyl)-6′-methoxycinchonan 21f.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 4-iodobenzoate (127 mg, 0.53 mmol, 1.5 eq.) to afford ethoxycarbonylphenyl-substituted alkyne 21f (86%, 157 mg, 0.31 mmol); νmax(CHCl3)/cm−1 3076, 2944, 2876, 2220, 1740, 1712, 1620, 1604, 1508, 1472, 1432, 1368, 1276, 1228, 1176, 1108, 1068, 1028, 856 and 824; δH(400 MHz; CDCl3) 8.77 (d, 1 H, J 4.5, H-2′), 8.07 (d, 2 H, J 8.4, H-14, H-16), 8.06 (d, 1 H, J 9.2, H-8′), 7.61 (d, 2 H, J 8.6, H-13, H-17), 7.52 (d, 1 H, J 2.6, H-5′), 7.42 (d, 1 H, J 4.6, H-3′), 7.40 (dd, 1 H, J 9.2 and 2.6, H-7′), 6.73 (d, 1 H, J 7.0, H-9), 4.44 (q, 2 H, J 7.1, H3C-H2C-O), 3.89 (s, 3 H, H-11′), 3.43–3.36 (m, 1 H, H-8), 3.24–3.18 (m, 1 H, H-2), 3.14–3.08 (dd, 1 H, J 13.8 and 10.1, H-2exo), 2.91–2.83 (m, 1 H, H-6), 2.81–2.72 (m, 1 H, H-6), 2.29–2.22 (m, 1 H, H-3), 2.14 (s, 3 H, H-20), 2.15–2.10 (m, 1 H, H-4), 1.71–1.55 (m, 3 H, H-7, H-7, H-5), 1.45–1.41 (t, 3 H, J 7.1, H3C-H2C-O) and 1.32–1.28 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.90 (C, C-19), 166.14 (C, C-18), 157.97 (C, C-6′), 148.19 (C, C-15), 147.39 (CH, C-2′), 144.62 (C, C-10′), 143.59 (C, C-4′), 131.75 (CH, C-8′), 131.56 (2 CH, C-14, C-16), 129.52 (2 CH, C-13, C-17), 129.50 (C, C-12), 128.38 (C, C-9′), 121.96 (CH, C-7′), 118.76 (CH, C-3′), 101.35 (CH, C-5′), 96.09 (C, C-11), 81.33 (C, C-10), 73.57 (CH, C-9), 61.13 (CH2, H3C-H2C-O), 58.97 (CH, C-8), 55.52 (CH3, C-11′), 50.42 (CH2, C-2), 49.50 (CH2, C-6), 29.05 (CH, C-3), 27.76 (CH, C-4), 25.08 (CH2, C-7), 24.38 (CH2, C-5), 21.09 (CH3, C-20) and 14.32 (CH3, H3C-H2C-O); m/z (MAT, 180 °C) (EI) 512.2335 (M+, C31H32N2O5 requires 512.2331), 497 (5%), 467 (10), 453 (30), 439 (6), 414 (9), 379 (4), 365 (18), 340 (4), 325 (22), 305 (8), 283 (100), 254 (19), 231 (34), 226 (8), 209 (9), 189 (44), 171 (18), 155 (18), 136 (14), 115 (10), 91 (7) and 77 (9).
)-9-Acetoxy-10,11-didehydro-11-(4-ethoxycarbonylphenyl)-6′-methoxycinchonan 21f.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 4-iodobenzoate (127 mg, 0.53 mmol, 1.5 eq.) to afford ethoxycarbonylphenyl-substituted alkyne 21f (86%, 157 mg, 0.31 mmol); νmax(CHCl3)/cm−1 3076, 2944, 2876, 2220, 1740, 1712, 1620, 1604, 1508, 1472, 1432, 1368, 1276, 1228, 1176, 1108, 1068, 1028, 856 and 824; δH(400 MHz; CDCl3) 8.77 (d, 1 H, J 4.5, H-2′), 8.07 (d, 2 H, J 8.4, H-14, H-16), 8.06 (d, 1 H, J 9.2, H-8′), 7.61 (d, 2 H, J 8.6, H-13, H-17), 7.52 (d, 1 H, J 2.6, H-5′), 7.42 (d, 1 H, J 4.6, H-3′), 7.40 (dd, 1 H, J 9.2 and 2.6, H-7′), 6.73 (d, 1 H, J 7.0, H-9), 4.44 (q, 2 H, J 7.1, H3C-H2C-O), 3.89 (s, 3 H, H-11′), 3.43–3.36 (m, 1 H, H-8), 3.24–3.18 (m, 1 H, H-2), 3.14–3.08 (dd, 1 H, J 13.8 and 10.1, H-2exo), 2.91–2.83 (m, 1 H, H-6), 2.81–2.72 (m, 1 H, H-6), 2.29–2.22 (m, 1 H, H-3), 2.14 (s, 3 H, H-20), 2.15–2.10 (m, 1 H, H-4), 1.71–1.55 (m, 3 H, H-7, H-7, H-5), 1.45–1.41 (t, 3 H, J 7.1, H3C-H2C-O) and 1.32–1.28 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.90 (C, C-19), 166.14 (C, C-18), 157.97 (C, C-6′), 148.19 (C, C-15), 147.39 (CH, C-2′), 144.62 (C, C-10′), 143.59 (C, C-4′), 131.75 (CH, C-8′), 131.56 (2 CH, C-14, C-16), 129.52 (2 CH, C-13, C-17), 129.50 (C, C-12), 128.38 (C, C-9′), 121.96 (CH, C-7′), 118.76 (CH, C-3′), 101.35 (CH, C-5′), 96.09 (C, C-11), 81.33 (C, C-10), 73.57 (CH, C-9), 61.13 (CH2, H3C-H2C-O), 58.97 (CH, C-8), 55.52 (CH3, C-11′), 50.42 (CH2, C-2), 49.50 (CH2, C-6), 29.05 (CH, C-3), 27.76 (CH, C-4), 25.08 (CH2, C-7), 24.38 (CH2, C-5), 21.09 (CH3, C-20) and 14.32 (CH3, H3C-H2C-O); m/z (MAT, 180 °C) (EI) 512.2335 (M+, C31H32N2O5 requires 512.2331), 497 (5%), 467 (10), 453 (30), 439 (6), 414 (9), 379 (4), 365 (18), 340 (4), 325 (22), 305 (8), 283 (100), 254 (19), 231 (34), 226 (8), 209 (9), 189 (44), 171 (18), 155 (18), 136 (14), 115 (10), 91 (7) and 77 (9).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-10,11-didehydro-11-(4-formylphenyl)-6′-methoxycinchonan 21g.
10,11-Didehydroquinidine 9b (195 mg, 0.54 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (19 mg, 0.03 mmol, 0.05 eq.), CuI (10 mg, 0.05 mmol, 0.1 eq.) and 4-bromobenzaldehyde (149 mg, 0.80 mmol, 1.5 eq.) to afford p-benzaldehyde-substituted alkyne 21g (83%, 208 mg, 0.44 mmol); νmax(CHCl3)/cm−1 2948, 2876, 2836, 2220, 1744, 1700, 1620, 1600, 1508, 1472, 1456, 1432, 1372, 1304, 1232, 1164, 1092, 1068, 1032 and 832; δH(400 MHz; CDCl3) 10.05 (s, 1 H, H-18), 8.79 (d, 1 H, J 4.4, H-2′), 8.07 (d, 1 H, J 9.2, H-8′), 7.91 (d, 2 H, J 8.4, H-14, H-16), 7.71 (d, 2 H, J 8.4, H-13, H-17), 7.53 (d, 1 H, J 2.7, H-5′), 7.43 (d, 1 H, J 4.6, H-3′), 7.41 (dd, 1 H, J 9.4 and 2.7, H-7′), 6.73 (d, 1 H, J 7.3, H-9), 3.90 (s, 3 H, H-11′), 3.46–3.39 (m, 1 H, H-8), 3.24–3.19 (ddd, 1 H, J 14.0, 6.8 and 2.0, H-2endo), 3.15–3.09 (dd, 1 H, J 14.0 and 10.4, H-2exo), 2.91–2.72 (m, 3 H, H-6, H-6, H-3), 2.27–2.19 (m, 1 H, H-4), 2.15 (s, 3 H, H-20), 1.74–1.58 (m, 3 H, H-7, H-7, H-5) and 1.31–1.27 (m, 1 H, H-5); δC(100 MHz; CDCl3) 191.42 (CH, C-18), 169.92 (C, C-19), 157.94 (C, C-6′), 147.48 (CH, C-2′), 144.80 (C, C-10′), 143.49 (C, C-4′), 135.21 (C, C-15), 132.23 (2 CH, C-14, C-16), 131.83 (CH, C-8′), 130.12 (C, C-12), 129.59 (2 CH, C-13, C-17), 127.02 (C, C-9′), 121.86 (CH, C-7′), 118.89 (CH, C-3′), 101.44 (CH, C-5′), 97.44 (C, C-11), 81.28 (C, C-10), 73.49 (CH, C-9), 58.97 (CH, C-8), 55.49 (CH3, C-11′), 50.30 (CH2, C-2), 49.46 (CH2, C-6), 29.11 (CH, C-3), 27.75 (CH, C-4), 25.06 (CH2, C-7), 24.55 (CH2, C-5) and 21.08 (CH3, C-20); m/z (MAT, 190 °C) (EI) 468.2050 (M+, C29H28N2O4 requires 468.2049), 453 (4%), 440 (2), 425 (8), 410 (26), 381 (3), 370 (4), 355 (2), 326 (26), 307 (2), 296 (4), 283 (3), 253 (7), 238 (100), 231 (21), 210 (9), 188 (44), 172 (14), 155 (19), 128 (11), 115 (14), 91 (7) and 77 (10).
)-9-Acetoxy-10,11-didehydro-11-(4-formylphenyl)-6′-methoxycinchonan 21g.
10,11-Didehydroquinidine 9b (195 mg, 0.54 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (19 mg, 0.03 mmol, 0.05 eq.), CuI (10 mg, 0.05 mmol, 0.1 eq.) and 4-bromobenzaldehyde (149 mg, 0.80 mmol, 1.5 eq.) to afford p-benzaldehyde-substituted alkyne 21g (83%, 208 mg, 0.44 mmol); νmax(CHCl3)/cm−1 2948, 2876, 2836, 2220, 1744, 1700, 1620, 1600, 1508, 1472, 1456, 1432, 1372, 1304, 1232, 1164, 1092, 1068, 1032 and 832; δH(400 MHz; CDCl3) 10.05 (s, 1 H, H-18), 8.79 (d, 1 H, J 4.4, H-2′), 8.07 (d, 1 H, J 9.2, H-8′), 7.91 (d, 2 H, J 8.4, H-14, H-16), 7.71 (d, 2 H, J 8.4, H-13, H-17), 7.53 (d, 1 H, J 2.7, H-5′), 7.43 (d, 1 H, J 4.6, H-3′), 7.41 (dd, 1 H, J 9.4 and 2.7, H-7′), 6.73 (d, 1 H, J 7.3, H-9), 3.90 (s, 3 H, H-11′), 3.46–3.39 (m, 1 H, H-8), 3.24–3.19 (ddd, 1 H, J 14.0, 6.8 and 2.0, H-2endo), 3.15–3.09 (dd, 1 H, J 14.0 and 10.4, H-2exo), 2.91–2.72 (m, 3 H, H-6, H-6, H-3), 2.27–2.19 (m, 1 H, H-4), 2.15 (s, 3 H, H-20), 1.74–1.58 (m, 3 H, H-7, H-7, H-5) and 1.31–1.27 (m, 1 H, H-5); δC(100 MHz; CDCl3) 191.42 (CH, C-18), 169.92 (C, C-19), 157.94 (C, C-6′), 147.48 (CH, C-2′), 144.80 (C, C-10′), 143.49 (C, C-4′), 135.21 (C, C-15), 132.23 (2 CH, C-14, C-16), 131.83 (CH, C-8′), 130.12 (C, C-12), 129.59 (2 CH, C-13, C-17), 127.02 (C, C-9′), 121.86 (CH, C-7′), 118.89 (CH, C-3′), 101.44 (CH, C-5′), 97.44 (C, C-11), 81.28 (C, C-10), 73.49 (CH, C-9), 58.97 (CH, C-8), 55.49 (CH3, C-11′), 50.30 (CH2, C-2), 49.46 (CH2, C-6), 29.11 (CH, C-3), 27.75 (CH, C-4), 25.06 (CH2, C-7), 24.55 (CH2, C-5) and 21.08 (CH3, C-20); m/z (MAT, 190 °C) (EI) 468.2050 (M+, C29H28N2O4 requires 468.2049), 453 (4%), 440 (2), 425 (8), 410 (26), 381 (3), 370 (4), 355 (2), 326 (26), 307 (2), 296 (4), 283 (3), 253 (7), 238 (100), 231 (21), 210 (9), 188 (44), 172 (14), 155 (19), 128 (11), 115 (14), 91 (7) and 77 (10).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-10,11-didehydro-11-(thiazolin-2-yl)-6′-methoxycinchonan 21h.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiazole (48 μl, 0.54 mmol, 1.5 eq.) to yield thiazolinyl-substituted alkyne 21h (90%, 144 mg, 0.32 mmol); νmax(CHCl3)/cm−1 2948, 2876, 2220, 1744, 1620, 1592, 1508, 1476, 1456, 1432, 1372, 1320, 1304, 1232, 1136, 1088, 1056, 1032 and 844; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.08 (d, 1 H, J 9.0, H-8′), 7.86 (d, 1 H, J 3.3, H-13), 7.44–7.40 (m, 3 H, H-5′, H-3′, H-7′), 7.38 (d, 1 H, J 3.3, H-14), 6.63 (d, 1 H, J 6.1, H-9), 3.98 (s, 3 H, H-11′), 3.38–3.27 (m, 2 H, H-8, H-2), 3.21–3.15 (dd, 1 H, J 14.0 and 10.2, H-2exo), 2.90–2.73 (m, 2 H, H-6, H-6), 2.33–2.26 (m, 1 H, H-3), 2.22–2.18 (m, 1 H, H-4), 2.20 (s, 3 H, H-16), 1.64–1.56 (m, 3 H, H-7, H-7, H-5) and 1.37–1.28 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.85 (C, C-15), 157.99 (C, C-6′), 149.09 (C, C-12), 147.48 (CH, C-2′), 144.67 (C, C-10′), 143.69 (C, C-4′), 143.29 (CH, C-13), 131.85 (CH, C-8′), 126.76 (C, C-9′), 121.92 (CH, C-7′), 120.14 (CH, C-14), 118.32 (CH, C-3′), 101.25 (CH, C-5′), 98.31 (C, C-11), 75.07 (C, C-10), 73.91 (CH, C-9), 58.91 (CH, C-8), 55.66 (CH3, C-11′), 49.92 (CH2, C-2), 49.58 (CH2, C-6), 29.11 (CH, C-3), 27.66 (CH, C-4), 24.92 (CH2, C-7), 23.77 (CH2, C-5) and 21.18 (CH3, C-16); m/z (MAT, 190 °C) (EI) 447.1602 (M+, C25H25N3O3S requires 447.1606), 432 (2%), 404 (9), 388 (49), 372 (2), 348 (8), 325 (12), 297 (4), 265 (4), 253 (4), 231 (10), 217 (100), 202 (6), 188 (37), 172 (11), 162 (11), 132 (15), 117 (7), 104 (9) and 77 (9).
)-9-Acetoxy-10,11-didehydro-11-(thiazolin-2-yl)-6′-methoxycinchonan 21h.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiazole (48 μl, 0.54 mmol, 1.5 eq.) to yield thiazolinyl-substituted alkyne 21h (90%, 144 mg, 0.32 mmol); νmax(CHCl3)/cm−1 2948, 2876, 2220, 1744, 1620, 1592, 1508, 1476, 1456, 1432, 1372, 1320, 1304, 1232, 1136, 1088, 1056, 1032 and 844; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.08 (d, 1 H, J 9.0, H-8′), 7.86 (d, 1 H, J 3.3, H-13), 7.44–7.40 (m, 3 H, H-5′, H-3′, H-7′), 7.38 (d, 1 H, J 3.3, H-14), 6.63 (d, 1 H, J 6.1, H-9), 3.98 (s, 3 H, H-11′), 3.38–3.27 (m, 2 H, H-8, H-2), 3.21–3.15 (dd, 1 H, J 14.0 and 10.2, H-2exo), 2.90–2.73 (m, 2 H, H-6, H-6), 2.33–2.26 (m, 1 H, H-3), 2.22–2.18 (m, 1 H, H-4), 2.20 (s, 3 H, H-16), 1.64–1.56 (m, 3 H, H-7, H-7, H-5) and 1.37–1.28 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.85 (C, C-15), 157.99 (C, C-6′), 149.09 (C, C-12), 147.48 (CH, C-2′), 144.67 (C, C-10′), 143.69 (C, C-4′), 143.29 (CH, C-13), 131.85 (CH, C-8′), 126.76 (C, C-9′), 121.92 (CH, C-7′), 120.14 (CH, C-14), 118.32 (CH, C-3′), 101.25 (CH, C-5′), 98.31 (C, C-11), 75.07 (C, C-10), 73.91 (CH, C-9), 58.91 (CH, C-8), 55.66 (CH3, C-11′), 49.92 (CH2, C-2), 49.58 (CH2, C-6), 29.11 (CH, C-3), 27.66 (CH, C-4), 24.92 (CH2, C-7), 23.77 (CH2, C-5) and 21.18 (CH3, C-16); m/z (MAT, 190 °C) (EI) 447.1602 (M+, C25H25N3O3S requires 447.1606), 432 (2%), 404 (9), 388 (49), 372 (2), 348 (8), 325 (12), 297 (4), 265 (4), 253 (4), 231 (10), 217 (100), 202 (6), 188 (37), 172 (11), 162 (11), 132 (15), 117 (7), 104 (9) and 77 (9).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-10,11-didehydro-11-(2-thienyl)-6′-methoxycinchonan 21i.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiophene (52 μl, 0.53 mmol, 1.5 eq.) to yield thiophenyl-substituted alkyne 21i (88%, 140 mg, 0.31 mmol); νmax(CHCl3)/cm−1 2956, 2876, 2224, 1744, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1300, 1236, 1136, 1092, 1028, 988, 844 and 828; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.07 (d, 1 H, J 9.0, H-8′), 7.51 (d, 1 H, J 2.6, H-5′), 7.43–7.39 (m, 2 H, H-3′, H-7′), 7.29–7.26 (m, 2 H, H-13, H-15), 7.04 (dd, 1 H, J 5.3 and 3.7, H-14), 6.71 (d, 1 H, J 6.4, H-9), 3.95 (s, 3 H, H-11′), 3.42–3.33 (m, 1 H, H-8), 3.26–3.20 (m, 1 H, H-2), 3.07–2.99 (m, 1 H, H-2), 2.92–2.74 (m, 2 H, H-6, H-6), 2.32–2.24 (m, 1 H, H-3), 2.16–2.11 (m, 1 H, H-4), 2.19 (s, 3 H, H-17), 1.67–1.54 (m, 3 H, H-7, H-7, H-5) and 1.37–1.28 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.88 (C, C-16), 158.01 (C, C-6′), 147.42 (CH, C-2′), 144.38 (C, C-10′), 143.73 (C, C-4′), 131.77 (CH, C-8′), 131.43 (CH, C-15), 128.59 (C, C-12), 126.96 (C, C-9′), 126.29 (CH, C-13), 122.01 (CH, C-7′), 120.14 (CH, C-14), 118.53 (CH, C-3′), 101.29 (CH, C-5′), 96.71 (C, C-11), 74.87 (C, C-10), 73.79 (CH, C-9), 59.01 (CH, C-8), 55.57 (CH3, C-11′), 50.49 (CH2, C-2), 49.59 (CH2, C-6), 29.24 (CH, C-3), 27.83 (CH, C-4), 25.11 (CH2, C-7), 24.13 (CH2, C-5) and 21.14 (CH3, C-17); m/z (MAT, 140 °C) (EI) 446.1186 (M+, C26H26N2O3S requires 446.1188), 366 (10%), 324 (7), 306 (4), 278 (7), 262 (4), 231 (2), 216 (6), 201 (3), 183 (5), 167 (11), 149 (19), 136 (10), 115 (50), 101 (17), 86 (100) and 72 (37).
)-9-Acetoxy-10,11-didehydro-11-(2-thienyl)-6′-methoxycinchonan 21i.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiophene (52 μl, 0.53 mmol, 1.5 eq.) to yield thiophenyl-substituted alkyne 21i (88%, 140 mg, 0.31 mmol); νmax(CHCl3)/cm−1 2956, 2876, 2224, 1744, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1300, 1236, 1136, 1092, 1028, 988, 844 and 828; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.07 (d, 1 H, J 9.0, H-8′), 7.51 (d, 1 H, J 2.6, H-5′), 7.43–7.39 (m, 2 H, H-3′, H-7′), 7.29–7.26 (m, 2 H, H-13, H-15), 7.04 (dd, 1 H, J 5.3 and 3.7, H-14), 6.71 (d, 1 H, J 6.4, H-9), 3.95 (s, 3 H, H-11′), 3.42–3.33 (m, 1 H, H-8), 3.26–3.20 (m, 1 H, H-2), 3.07–2.99 (m, 1 H, H-2), 2.92–2.74 (m, 2 H, H-6, H-6), 2.32–2.24 (m, 1 H, H-3), 2.16–2.11 (m, 1 H, H-4), 2.19 (s, 3 H, H-17), 1.67–1.54 (m, 3 H, H-7, H-7, H-5) and 1.37–1.28 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.88 (C, C-16), 158.01 (C, C-6′), 147.42 (CH, C-2′), 144.38 (C, C-10′), 143.73 (C, C-4′), 131.77 (CH, C-8′), 131.43 (CH, C-15), 128.59 (C, C-12), 126.96 (C, C-9′), 126.29 (CH, C-13), 122.01 (CH, C-7′), 120.14 (CH, C-14), 118.53 (CH, C-3′), 101.29 (CH, C-5′), 96.71 (C, C-11), 74.87 (C, C-10), 73.79 (CH, C-9), 59.01 (CH, C-8), 55.57 (CH3, C-11′), 50.49 (CH2, C-2), 49.59 (CH2, C-6), 29.24 (CH, C-3), 27.83 (CH, C-4), 25.11 (CH2, C-7), 24.13 (CH2, C-5) and 21.14 (CH3, C-17); m/z (MAT, 140 °C) (EI) 446.1186 (M+, C26H26N2O3S requires 446.1188), 366 (10%), 324 (7), 306 (4), 278 (7), 262 (4), 231 (2), 216 (6), 201 (3), 183 (5), 167 (11), 149 (19), 136 (10), 115 (50), 101 (17), 86 (100) and 72 (37).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(6,6,7-trimethyl-2,3,3a,3b,4,7,7a,8a-octahydrofuro[2,3-b]benzofuryl)-10,11-didehydro-6′-methoxycinchonan 21j.
10,11-Didehydroquinidine 9b (73 mg, 0.20 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (8 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and the tricyclic vinyl iodide (100 mg, 0.30 mmol, 1.5 eq.) to yield substituted alkyne 21j (94%, 107 mg, 0.19 mmol); νmax(CHCl3)/cm−1 2960, 2940, 2884, 1744, 1620, 1592, 1508, 1472, 1456, 1368, 1304, 1228, 1136, 1108, 1084, 1040, 996, 940 and 852; δH(400 MHz; CDCl3) 8.82 (d, 1 H, J 4.6, H-2′), 8.13 (d, 1 H, J 9.2, H-8′), 7.49 (d, 1 H, J 2.6, H-5′), 7.44–7.40 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.39 (d, 1 H, J 4.6, H-3′), 6.69 (d, 1 H, J 6.3, H-9), 5.73 (d, 1 H, J 4.8, H-20), 4.18–4.13 (m, 1 H, H-22), 4.02 (s, 3 H, H-11′), 3.85–3.78 (m, 1 H, H-18), 3.69–3.61 (m, 1 H, H-18), 3.32–3.21 (m, 2 H, H-8, H-2), 3.00–2.93 (m, 1 H, H-2), 2.90–2.74 (m, 2 H, H-6, H-6), 2.62–2.54 (m, 1 H, H-3), 2.22 (s, 3 H, H-25), 2.15–2.02 (m, 3 H, H-4, H-15, H-16), 1.94–1.87 (m, 1 H, H-7), 1.85–1.67 (m, 2 H, H-7, H-5), 1.62–1.53 (m, 4 H, H-17, H-17, H-23, H-23), 1.32–1.28 (m, 1 H, H-5), 1.13 (s, 3 H, C-13-Me), 1.04 (s, 3 H, C-14-Me) and 1.03 (s, 3 H, C-14-Me); δC(100 MHz; CDCl3) 169.76 (C, C-24), 158.21 (C, C-6′), 147.89 (CH, C-2′), 147.40 (C, C-10′), 144.59 (C, C-4′), 132.17 (C, C-13), 131.71 (CH, C-8′), 126.89 (C, C-9′), 122.09 (CH, C-7′), 118.53 (CH, C-3′), 112.11 (C, C-12), 109.66 (CH, C-20), 101.45 (CH, C-5′), 98.02 (C, C-11), 82.05 (C, C-10), 77.25 (CH, C-22), 71.74 (CH, C-9), 70.06 (CH2, C-18), 58.39 (CH, C-8), 58.14 (CH2, C-2), 53.60 (CH3, C-11′), 49.26 (CH2, C-6), 44.89 (CH, C-15), 39.33 (C, C-14), 37.89 (CH, C-16), 28.27 (CH, C-3), 27.28 (CH, C-4), 26.53 (CH2, C-7), 25.81 (CH2, C-23), 24.19 (CH2, C-17), 23.27 (CH2, C-5), 21.16 (CH3, C-25), 16.37 (CH3, C-13-Me), 15.59 (CH3, C-14-Me) and 15.57 (CH3, C-14-Me); m/z (MAT, 220 °C) (EI) 570.3127 (M+, C35H42N2O5: requires 570.3134), 556 (9%), 528 (6), 512 (31), 340 (100), 325 (12), 312 (17), 298 (13), 285 (11), 251 (7), 231 (61), 211 (9), 189 (59), 172 (28), 160 (17), 136 (15), 121 (70), 105 (11) and 91 (28.95).
)-9-Acetoxy-11-(6,6,7-trimethyl-2,3,3a,3b,4,7,7a,8a-octahydrofuro[2,3-b]benzofuryl)-10,11-didehydro-6′-methoxycinchonan 21j.
10,11-Didehydroquinidine 9b (73 mg, 0.20 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (8 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and the tricyclic vinyl iodide (100 mg, 0.30 mmol, 1.5 eq.) to yield substituted alkyne 21j (94%, 107 mg, 0.19 mmol); νmax(CHCl3)/cm−1 2960, 2940, 2884, 1744, 1620, 1592, 1508, 1472, 1456, 1368, 1304, 1228, 1136, 1108, 1084, 1040, 996, 940 and 852; δH(400 MHz; CDCl3) 8.82 (d, 1 H, J 4.6, H-2′), 8.13 (d, 1 H, J 9.2, H-8′), 7.49 (d, 1 H, J 2.6, H-5′), 7.44–7.40 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.39 (d, 1 H, J 4.6, H-3′), 6.69 (d, 1 H, J 6.3, H-9), 5.73 (d, 1 H, J 4.8, H-20), 4.18–4.13 (m, 1 H, H-22), 4.02 (s, 3 H, H-11′), 3.85–3.78 (m, 1 H, H-18), 3.69–3.61 (m, 1 H, H-18), 3.32–3.21 (m, 2 H, H-8, H-2), 3.00–2.93 (m, 1 H, H-2), 2.90–2.74 (m, 2 H, H-6, H-6), 2.62–2.54 (m, 1 H, H-3), 2.22 (s, 3 H, H-25), 2.15–2.02 (m, 3 H, H-4, H-15, H-16), 1.94–1.87 (m, 1 H, H-7), 1.85–1.67 (m, 2 H, H-7, H-5), 1.62–1.53 (m, 4 H, H-17, H-17, H-23, H-23), 1.32–1.28 (m, 1 H, H-5), 1.13 (s, 3 H, C-13-Me), 1.04 (s, 3 H, C-14-Me) and 1.03 (s, 3 H, C-14-Me); δC(100 MHz; CDCl3) 169.76 (C, C-24), 158.21 (C, C-6′), 147.89 (CH, C-2′), 147.40 (C, C-10′), 144.59 (C, C-4′), 132.17 (C, C-13), 131.71 (CH, C-8′), 126.89 (C, C-9′), 122.09 (CH, C-7′), 118.53 (CH, C-3′), 112.11 (C, C-12), 109.66 (CH, C-20), 101.45 (CH, C-5′), 98.02 (C, C-11), 82.05 (C, C-10), 77.25 (CH, C-22), 71.74 (CH, C-9), 70.06 (CH2, C-18), 58.39 (CH, C-8), 58.14 (CH2, C-2), 53.60 (CH3, C-11′), 49.26 (CH2, C-6), 44.89 (CH, C-15), 39.33 (C, C-14), 37.89 (CH, C-16), 28.27 (CH, C-3), 27.28 (CH, C-4), 26.53 (CH2, C-7), 25.81 (CH2, C-23), 24.19 (CH2, C-17), 23.27 (CH2, C-5), 21.16 (CH3, C-25), 16.37 (CH3, C-13-Me), 15.59 (CH3, C-14-Me) and 15.57 (CH3, C-14-Me); m/z (MAT, 220 °C) (EI) 570.3127 (M+, C35H42N2O5: requires 570.3134), 556 (9%), 528 (6), 512 (31), 340 (100), 325 (12), 312 (17), 298 (13), 285 (11), 251 (7), 231 (61), 211 (9), 189 (59), 172 (28), 160 (17), 136 (15), 121 (70), 105 (11) and 91 (28.95).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-10,11-didehydro-11-(4-hydroxyphenyl)-6′-methoxycinchonan 21k.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 4-iodophenol (117 mg, 0.53 mmol, 1.5 eq.) to yield p-hydroxyphenyl-substituted alkyne 21k (85%, 138 mg, 0.30 mmol); νmax(CHCl3)/cm−1 3420, 2940, 2876, 1744, 1620, 1592, 1508, 1472, 1456, 1368, 1304, 1236, 1136, 1084, 1064, 1028, 984, 916 and 844; δH(400 MHz; CDCl3) 8.75 (d, 1 H, J 4.6, H-2′), 8.04 (d, 1 H, J 9.2, H-8′), 7.92 (d, 2 H, J 8.4, H-14, H-16), 7.59 (d, 2 H, J 8.4, H-13, H-17), 7.43 (d, 1 H, J 2.6, H-5′), 7.40–7.37 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.36 (d, 1 H, J 4.7, H-3′), 6.57 (d, 1 H, J 6.8, H-9), 3.97 (s, 3 H, H-11′), 3.32–3.26 (m, 1 H, H-8), 2.95–2.92 (d, 1 H, J 14.4, H-2), 2.93–2.69 (m, 3 H, H-2, H-6, H-6), 2.32–2.26 (m, 1 H, H-3), 2.15 (s, 3 H, H-19), 2.16–2.08 (m, 1 H, H-4), 1.94–1.78 (m, 2 H, H-7, H-7) and 1.57–1.44 (m, 2 H, H-5, H-5); δC(100 MHz; CDCl3) 169.93 (C, C-18), 166.34 (C, C-15), 157.93 (C, C-6′), 147.37 (CH, C-2′), 144.63 (C, C-10′), 143.85 (C, C-4′), 131.69 (CH, C-8′), 131.55 (CH, C-14, C-16), 129.48 (C, C-12), 128.19 (CH, C-13, C-17), 126.99 (C, C-9′), 121.88 (CH, C-7′), 118.51 (CH, C-3′), 101.39 (CH, C-5′), 96.13 (C, C-11), 81.97 (C, C-10), 73.59 (CH, C-9), 59.03 (CH, C-8), 55.62 (CH3, C-11′), 49.98 (CH2, C-2), 49.16 (CH2, C-6), 31.25 (CH, C-3), 27.87 (CH, C-4), 26.35 (CH2, C-7), 23.28 (CH2, C-5) and 21.14 (CH3, C-19); m/z (MAT, 200 °C) (EI) 456 (M+, 7%), 455 (25), 397 (6), 369 (10), 355 (5), 325 (17), 313 (5), 297 (3), 279 (17), 258 (7), 231 (17), 226 (12), 197 (100), 183 (12), 168 (18), 143 (13), 123 (4), 105 (12) and 91 (16).
)-9-Acetoxy-10,11-didehydro-11-(4-hydroxyphenyl)-6′-methoxycinchonan 21k.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 4-iodophenol (117 mg, 0.53 mmol, 1.5 eq.) to yield p-hydroxyphenyl-substituted alkyne 21k (85%, 138 mg, 0.30 mmol); νmax(CHCl3)/cm−1 3420, 2940, 2876, 1744, 1620, 1592, 1508, 1472, 1456, 1368, 1304, 1236, 1136, 1084, 1064, 1028, 984, 916 and 844; δH(400 MHz; CDCl3) 8.75 (d, 1 H, J 4.6, H-2′), 8.04 (d, 1 H, J 9.2, H-8′), 7.92 (d, 2 H, J 8.4, H-14, H-16), 7.59 (d, 2 H, J 8.4, H-13, H-17), 7.43 (d, 1 H, J 2.6, H-5′), 7.40–7.37 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.36 (d, 1 H, J 4.7, H-3′), 6.57 (d, 1 H, J 6.8, H-9), 3.97 (s, 3 H, H-11′), 3.32–3.26 (m, 1 H, H-8), 2.95–2.92 (d, 1 H, J 14.4, H-2), 2.93–2.69 (m, 3 H, H-2, H-6, H-6), 2.32–2.26 (m, 1 H, H-3), 2.15 (s, 3 H, H-19), 2.16–2.08 (m, 1 H, H-4), 1.94–1.78 (m, 2 H, H-7, H-7) and 1.57–1.44 (m, 2 H, H-5, H-5); δC(100 MHz; CDCl3) 169.93 (C, C-18), 166.34 (C, C-15), 157.93 (C, C-6′), 147.37 (CH, C-2′), 144.63 (C, C-10′), 143.85 (C, C-4′), 131.69 (CH, C-8′), 131.55 (CH, C-14, C-16), 129.48 (C, C-12), 128.19 (CH, C-13, C-17), 126.99 (C, C-9′), 121.88 (CH, C-7′), 118.51 (CH, C-3′), 101.39 (CH, C-5′), 96.13 (C, C-11), 81.97 (C, C-10), 73.59 (CH, C-9), 59.03 (CH, C-8), 55.62 (CH3, C-11′), 49.98 (CH2, C-2), 49.16 (CH2, C-6), 31.25 (CH, C-3), 27.87 (CH, C-4), 26.35 (CH2, C-7), 23.28 (CH2, C-5) and 21.14 (CH3, C-19); m/z (MAT, 200 °C) (EI) 456 (M+, 7%), 455 (25), 397 (6), 369 (10), 355 (5), 325 (17), 313 (5), 297 (3), 279 (17), 258 (7), 231 (17), 226 (12), 197 (100), 183 (12), 168 (18), 143 (13), 123 (4), 105 (12) and 91 (16).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-10,11-didehydro-11-(3-hydroxyphenyl)-6′-methoxycinchonan 21l.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-iodophenol (117 mg, 0.53 mmol, 1.5 eq.) to yield m-hydroxyphenyl-substituted alkyne 21l (82%, 134 mg, 0.30 mmol); νmax(CHCl3)/cm−1 3368, 2960, 2932, 2872, 1720, 1620, 1596, 1508, 1464, 1408, 1376, 1292, 1232, 1132, 1072, 1036, 992, 960 and 852; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.25 (d, 1 H, J 9.2, H-8′), 8.16 (d, 1 H, J 1.6, H-13), 7.77 (dd, 1 H, J 5.7 and 3.3, H-15), 7.64 (d, 1 H, J 4.8, H-3′), 7.59 (dd, 1 H, J 5.8 and 3.4, H-17), 7.55–7.51 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.26–7.18 (m, 2 H, H-5′, H-16), 6.52 (d, 1 H, J 6.4, H-9), 4.08 (s, 3 H, H-11′), 3.53–3.47 (m, 1 H, H-8), 3.34–3.27 (m, 1 H, H-2), 3.18–3.09 (m, 1 H, H-2), 2.92–2.74 (m, 2 H, H-6, H-6), 2.38–2.29 (m, 1 H, H-3), 2.24 (s, 3 H, H-19), 2.13–2.08 (m, 1 H, H-4), 1.76–1.68 (m, 3 H, H-7, H-7, H-5) and 1.53–1.46 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.79 (C, C-18), 166.90 (C, C-14), 157.22 (C, C-6′), 147.18 (CH, C-2′), 144.87 (C, C-10′), 143.91 (C, C-4′), 131.67 (CH, C-8′), 131.48 (CH, C-13), 131.39 (CH, C-15), 129.54 (C, C-12), 128.58 (CH, C-17), 126.93 (C, C-9′), 124.03 (CH, C-16), 121.38 (CH, C-7′), 117.85 (CH, C-3′), 101.66 (CH, C-5′), 95.62 (C, C-11), 81.32 (C, C-10), 73.02 (CH, C-9), 58.32 (CH, C-8), 55.19 (CH3, C-11′), 49.77 (CH2, C-2), 48.51 (CH2, C-6), 29.74 (CH, C-3), 28.29 (CH, C-4), 25.72 (CH2, C-7), 23.19 (CH2, C-5) and 22.31 (CH3, C-19); m/z (FAB) (EI) 457 (M+ + H, 17%), 413 (15), 391 (36), 279 (7), 167 (19), 149 (100) and 113 (21).
)-9-Acetoxy-10,11-didehydro-11-(3-hydroxyphenyl)-6′-methoxycinchonan 21l.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-iodophenol (117 mg, 0.53 mmol, 1.5 eq.) to yield m-hydroxyphenyl-substituted alkyne 21l (82%, 134 mg, 0.30 mmol); νmax(CHCl3)/cm−1 3368, 2960, 2932, 2872, 1720, 1620, 1596, 1508, 1464, 1408, 1376, 1292, 1232, 1132, 1072, 1036, 992, 960 and 852; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.25 (d, 1 H, J 9.2, H-8′), 8.16 (d, 1 H, J 1.6, H-13), 7.77 (dd, 1 H, J 5.7 and 3.3, H-15), 7.64 (d, 1 H, J 4.8, H-3′), 7.59 (dd, 1 H, J 5.8 and 3.4, H-17), 7.55–7.51 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.26–7.18 (m, 2 H, H-5′, H-16), 6.52 (d, 1 H, J 6.4, H-9), 4.08 (s, 3 H, H-11′), 3.53–3.47 (m, 1 H, H-8), 3.34–3.27 (m, 1 H, H-2), 3.18–3.09 (m, 1 H, H-2), 2.92–2.74 (m, 2 H, H-6, H-6), 2.38–2.29 (m, 1 H, H-3), 2.24 (s, 3 H, H-19), 2.13–2.08 (m, 1 H, H-4), 1.76–1.68 (m, 3 H, H-7, H-7, H-5) and 1.53–1.46 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.79 (C, C-18), 166.90 (C, C-14), 157.22 (C, C-6′), 147.18 (CH, C-2′), 144.87 (C, C-10′), 143.91 (C, C-4′), 131.67 (CH, C-8′), 131.48 (CH, C-13), 131.39 (CH, C-15), 129.54 (C, C-12), 128.58 (CH, C-17), 126.93 (C, C-9′), 124.03 (CH, C-16), 121.38 (CH, C-7′), 117.85 (CH, C-3′), 101.66 (CH, C-5′), 95.62 (C, C-11), 81.32 (C, C-10), 73.02 (CH, C-9), 58.32 (CH, C-8), 55.19 (CH3, C-11′), 49.77 (CH2, C-2), 48.51 (CH2, C-6), 29.74 (CH, C-3), 28.29 (CH, C-4), 25.72 (CH2, C-7), 23.19 (CH2, C-5) and 22.31 (CH3, C-19); m/z (FAB) (EI) 457 (M+ + H, 17%), 413 (15), 391 (36), 279 (7), 167 (19), 149 (100) and 113 (21).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(4-iodophenyl)-10,11-didehydro-6′-methoxycinchonan 21m.
10,11-Didehydroquinidine 9b (546 mg, 1.50 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (53 mg, 0.08 mmol, 0.05 eq.), CuI (29 mg, 0.15 mmol, 0.1 eq.) and 1,4-diiodobenzene (743 mg, 2.25 mmol, 1.5 eq.) to yield p-iodophenyl-substituted alkyne 21m (82%, 680 mg, 1.20 mmol); νmax(CHCl3)/cm−1 2944, 2876, 2224, 1744, 1624, 1592, 1508, 1484, 1456, 1432, 1372, 1300, 1232, 1172, 1136, 1092, 1032, 1004, 844 and 824; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.07 (d, 1 H, J 9.2, H-8′), 7.73 (d, 2 H, J 8.6, H-14, H-16), 7.52 (d, 1 H, J 2.6, H-5′), 7.43–7.38 (m, 2 H, H-3′, H-7′), 7.28 (d, 2 H, J 8.5, H-13, H-17), 6.73 (d, 1 H, J 7.3, H-9), 3.90 (s, 3 H, H-11′), 3.47–3.38 (m, 1 H, H-8), 3.22–3.07 (m, 2 H, H-2, H-2), 2.92–2.84 (m, 1 H, H-6), 2.80–2.71 (m, 2 H, H-6, H-3), 2.27–2.20 (m, 1 H, H-4), 2.15 (s, 3 H, H-20), 1.72–1.53 (m, 3 H, H-7, H-7, H-5) and 1.23–1.14 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.89 (C, C-18), 157.91 (C, C-6′), 147.44 (CH, C-2′), 144.77 (C, C-4′), 143.54 (C, C-10′), 137.47 (CH, C-14, C-16), 133.23 (CH, C-13, C-17), 131.78 (CH, C-8′), 126.99 (C, C-9′), 123.23 (C, C-12), 121.89 (CH, C-7′), 118.82 (CH, C-3′), 101.36 (CH, C-5′), 94.37 (C, C-15), 93.48 (C, C-10), 80.92 (C, C-11), 73.52 (CH, C-9), 58.98 (CH, C-8), 55.49 (CH3, C-11′), 50.41 (CH2, C-2), 49.47 (CH2, C-6), 28.98 (CH, C-3), 27.71 (CH, C-4), 25.10 (CH2, C-5), 24.48 (CH2, C-7) and 21.09 (CH3, C-20); m/z (FAB) (EI) 567 (M+ + H, 100%), 507 (6), 413 (17), 391 (29), 336 (15), 307 (7) and 279 (12).
)-9-Acetoxy-11-(4-iodophenyl)-10,11-didehydro-6′-methoxycinchonan 21m.
10,11-Didehydroquinidine 9b (546 mg, 1.50 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (53 mg, 0.08 mmol, 0.05 eq.), CuI (29 mg, 0.15 mmol, 0.1 eq.) and 1,4-diiodobenzene (743 mg, 2.25 mmol, 1.5 eq.) to yield p-iodophenyl-substituted alkyne 21m (82%, 680 mg, 1.20 mmol); νmax(CHCl3)/cm−1 2944, 2876, 2224, 1744, 1624, 1592, 1508, 1484, 1456, 1432, 1372, 1300, 1232, 1172, 1136, 1092, 1032, 1004, 844 and 824; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.07 (d, 1 H, J 9.2, H-8′), 7.73 (d, 2 H, J 8.6, H-14, H-16), 7.52 (d, 1 H, J 2.6, H-5′), 7.43–7.38 (m, 2 H, H-3′, H-7′), 7.28 (d, 2 H, J 8.5, H-13, H-17), 6.73 (d, 1 H, J 7.3, H-9), 3.90 (s, 3 H, H-11′), 3.47–3.38 (m, 1 H, H-8), 3.22–3.07 (m, 2 H, H-2, H-2), 2.92–2.84 (m, 1 H, H-6), 2.80–2.71 (m, 2 H, H-6, H-3), 2.27–2.20 (m, 1 H, H-4), 2.15 (s, 3 H, H-20), 1.72–1.53 (m, 3 H, H-7, H-7, H-5) and 1.23–1.14 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.89 (C, C-18), 157.91 (C, C-6′), 147.44 (CH, C-2′), 144.77 (C, C-4′), 143.54 (C, C-10′), 137.47 (CH, C-14, C-16), 133.23 (CH, C-13, C-17), 131.78 (CH, C-8′), 126.99 (C, C-9′), 123.23 (C, C-12), 121.89 (CH, C-7′), 118.82 (CH, C-3′), 101.36 (CH, C-5′), 94.37 (C, C-15), 93.48 (C, C-10), 80.92 (C, C-11), 73.52 (CH, C-9), 58.98 (CH, C-8), 55.49 (CH3, C-11′), 50.41 (CH2, C-2), 49.47 (CH2, C-6), 28.98 (CH, C-3), 27.71 (CH, C-4), 25.10 (CH2, C-5), 24.48 (CH2, C-7) and 21.09 (CH3, C-20); m/z (FAB) (EI) 567 (M+ + H, 100%), 507 (6), 413 (17), 391 (29), 336 (15), 307 (7) and 279 (12).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(2-aminophenyl)-10,11-didehydro-6′-methoxycinchonan 21n.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodoaniline (117 mg, 0.53 mmol, 1.5 eq.) to yield 2-aminophenyl-substituted alkyne 21n (92%, 150 mg, 0.33 mmol); νmax(CHCl3)/cm−1 3452 2960, 2868, 2836, 2216, 1740, 1620, 1572, 1508, 1472, 1432, 1368, 1304, 1236, 1144, 1084, 1032, 904 and 844; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.09 (d, 1 H, J 9.2, H-8′), 7.50 (d, 1 H, J 2.7, H-5′), 7.42 (d, 1 H, J 4.6, H-3′), 7.40 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.19 (dd, 1 H, J 7.8 and 1.7, H-14), 7.14–7.09 (m, 1 H, H-15), 6.71–6.66 (m, 2 H, H-16, H-17), 6.54 (d, 1 H, J 7.5, H-9), 4.08 (s, 2 H, NH2), 3.96 (s, 3 H, H-11′), 3.72–3.67 (m, 1 H, H-8), 3.28–3.21 (dd, 1 H, J 13.6 and 9.9, H-2endo), 3.20–3.14 (m, 1 H, H-2), 2.97–2.89 (m, 1 H, H-6), 2.86–2.79 (m, 1 H, H-6), 2.74–2.66 (m, 1 H, H-3), 2.31–2.23 (m, 1 H, H-4), 2.18 (s, 3 H, H-19), 1.85–1.76 (m, 1 H, H-7), 1.67–1.53 (m, 2 H, H-7, H-5) and 1.48–1.42 (m, 1 H, H-5); δC(100 MHz; CDCl3) 170.17 (C, C-18), 157.94 (C, C-6′), 147.58 (C, C-13), 147.45 (CH, C-2′), 144.75 (C, C-10′), 143.49 (C, C-4′), 132.14 (CH, C-14), 132.04 (CH, C-15), 131.77 (CH, C-8′), 129.13 (C, C-12), 127.04 (C, C-9′), 121.84 (CH, C-7′), 119.26 (CH, C-3′), 117.89 (CH, C-16), 114.24 (CH, C-17), 101.61 (CH, C-5′), 98.60 (C, C-11), 81.27 (C, C-10), 73.79 (CH, C-9), 58.66 (CH, C-8), 55.64 (CH3, C-11′), 50.32 (CH2, C-2), 48.91 (CH2, C-6), 28.62 (CH, C-3), 27.15 (CH, C-4), 26.24 (CH2, C-7), 25.10 (CH2, C-5) and 21.10 (CH3, C-19); m/z (MAT, 200 °C) (EI) 455.2199 (M+, C28H29N3O3 requires 455.2209), 396 (11%), 369 (5), 355 (6), 325 (9), 313 (8), 296 (5), 278 (4), 258 (7), 231 (17), 225 (56), 211 (6), 197 (100), 182 (19), 168 (30), 155 (10), 143 (42), 130 (21), 115 (15), 91 (16) and 77 (11).
)-9-Acetoxy-11-(2-aminophenyl)-10,11-didehydro-6′-methoxycinchonan 21n.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodoaniline (117 mg, 0.53 mmol, 1.5 eq.) to yield 2-aminophenyl-substituted alkyne 21n (92%, 150 mg, 0.33 mmol); νmax(CHCl3)/cm−1 3452 2960, 2868, 2836, 2216, 1740, 1620, 1572, 1508, 1472, 1432, 1368, 1304, 1236, 1144, 1084, 1032, 904 and 844; δH(400 MHz; CDCl3) 8.79 (d, 1 H, J 4.6, H-2′), 8.09 (d, 1 H, J 9.2, H-8′), 7.50 (d, 1 H, J 2.7, H-5′), 7.42 (d, 1 H, J 4.6, H-3′), 7.40 (dd, 1 H, J 9.2 and 2.6, H-7′), 7.19 (dd, 1 H, J 7.8 and 1.7, H-14), 7.14–7.09 (m, 1 H, H-15), 6.71–6.66 (m, 2 H, H-16, H-17), 6.54 (d, 1 H, J 7.5, H-9), 4.08 (s, 2 H, NH2), 3.96 (s, 3 H, H-11′), 3.72–3.67 (m, 1 H, H-8), 3.28–3.21 (dd, 1 H, J 13.6 and 9.9, H-2endo), 3.20–3.14 (m, 1 H, H-2), 2.97–2.89 (m, 1 H, H-6), 2.86–2.79 (m, 1 H, H-6), 2.74–2.66 (m, 1 H, H-3), 2.31–2.23 (m, 1 H, H-4), 2.18 (s, 3 H, H-19), 1.85–1.76 (m, 1 H, H-7), 1.67–1.53 (m, 2 H, H-7, H-5) and 1.48–1.42 (m, 1 H, H-5); δC(100 MHz; CDCl3) 170.17 (C, C-18), 157.94 (C, C-6′), 147.58 (C, C-13), 147.45 (CH, C-2′), 144.75 (C, C-10′), 143.49 (C, C-4′), 132.14 (CH, C-14), 132.04 (CH, C-15), 131.77 (CH, C-8′), 129.13 (C, C-12), 127.04 (C, C-9′), 121.84 (CH, C-7′), 119.26 (CH, C-3′), 117.89 (CH, C-16), 114.24 (CH, C-17), 101.61 (CH, C-5′), 98.60 (C, C-11), 81.27 (C, C-10), 73.79 (CH, C-9), 58.66 (CH, C-8), 55.64 (CH3, C-11′), 50.32 (CH2, C-2), 48.91 (CH2, C-6), 28.62 (CH, C-3), 27.15 (CH, C-4), 26.24 (CH2, C-7), 25.10 (CH2, C-5) and 21.10 (CH3, C-19); m/z (MAT, 200 °C) (EI) 455.2199 (M+, C28H29N3O3 requires 455.2209), 396 (11%), 369 (5), 355 (6), 325 (9), 313 (8), 296 (5), 278 (4), 258 (7), 231 (17), 225 (56), 211 (6), 197 (100), 182 (19), 168 (30), 155 (10), 143 (42), 130 (21), 115 (15), 91 (16) and 77 (11).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-3-benzofuryl-6′-methoxycinchonan 21o.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodophenol (117 mg, 0.53 mmol, 1.5 eq.) to yield benzofuran 21o (78%, 127 mg, 0.28 mmol); νmax(CHCl3)/cm−1 3032, 2936, 2872, 2220, 1744, 1620, 1508, 1472, 1452, 1372, 1300, 1228, 1132, 1088, 1068, 1028, 988 and 844; δH(400 MHz; CDCl3) 8.84 (d, 1 H, J 4.6, H-2′), 8.18 (d, 1 H, J 8.9, H-8′), 7.61 (d, 1 H, J 2.4, H-5′), 7.54–7.47 (m, 1 H, H-3′), 7.45–7.37 (m, 3 H, H-7′, H-13, H-16), 7.31–7.25 (m, 2 H, H-14, H-15), 6.77 (d, 1 H, J 7.2, H-9), 6.59 (s, 1 H, H-11), 3.96 (s, 3 H, H-11′), 3.44–3.37 (m, 1 H, H-8), 3.27–3.10 (m, 2 H, H-2, H-2), 2.98–2.84 (m, 2 H, H-6, H-6), 2.36–2.29 (m, 1 H, H-3), 2.24–2.20 (m, 1 H, H-4), 2.10 (s, 3 H, H-19), 2.07–1.98 (m, 1 H, H-7) and 1.78–1.52 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 170.30 (C, C-18), 160.35 (C, C-17), 158.01 (C, C-6′), 147.63 (CH, C-2′), 144.83 (C, C-10), 144.09 (C, C-10′), 143.65 (C, C-4′), 131.75 (CH, C-8′), 129.85 (C, C-12), 128.57 (C, C-9′), 123.58 (CH, C-15), 122.69 (CH, C-14), 121.97 (CH, C-7′), 120.49 (CH, C-13), 120.24 (CH, C-3′), 110.87 (CH, C-16), 102.20 (CH, C-11), 101.53 (CH, C-5′), 73.45 (CH, C-9), 59.15 (CH, C-8), 55.62 (CH3, C-11′), 49.89 (CH2, C-2), 47.57 (CH2, C-6), 29.27 (CH, C-3), 27.88 (CH, C-4), 26.12 (CH2, C-7), 25.69 (CH2, C-5) and 21.12 (CH3, C-19); m/z (MAT, 200 °C) (EI) 456.2050 (M+, C28H28N2O4 requires 456.2049), 441 (3%), 413 (3), 397 (9), 365 (4), 325 (5), 313 (2), 265 (2), 253 (6), 226 (100), 211 (7), 199 (22), 188 (51), 172 (22), 157 (29), 144 (82), 132 (23), 115 (6), 99 (7) and 86 (13).
)-9-Acetoxy-3-benzofuryl-6′-methoxycinchonan 21o.
10,11-Didehydroquinidine 9b (130 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodophenol (117 mg, 0.53 mmol, 1.5 eq.) to yield benzofuran 21o (78%, 127 mg, 0.28 mmol); νmax(CHCl3)/cm−1 3032, 2936, 2872, 2220, 1744, 1620, 1508, 1472, 1452, 1372, 1300, 1228, 1132, 1088, 1068, 1028, 988 and 844; δH(400 MHz; CDCl3) 8.84 (d, 1 H, J 4.6, H-2′), 8.18 (d, 1 H, J 8.9, H-8′), 7.61 (d, 1 H, J 2.4, H-5′), 7.54–7.47 (m, 1 H, H-3′), 7.45–7.37 (m, 3 H, H-7′, H-13, H-16), 7.31–7.25 (m, 2 H, H-14, H-15), 6.77 (d, 1 H, J 7.2, H-9), 6.59 (s, 1 H, H-11), 3.96 (s, 3 H, H-11′), 3.44–3.37 (m, 1 H, H-8), 3.27–3.10 (m, 2 H, H-2, H-2), 2.98–2.84 (m, 2 H, H-6, H-6), 2.36–2.29 (m, 1 H, H-3), 2.24–2.20 (m, 1 H, H-4), 2.10 (s, 3 H, H-19), 2.07–1.98 (m, 1 H, H-7) and 1.78–1.52 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 170.30 (C, C-18), 160.35 (C, C-17), 158.01 (C, C-6′), 147.63 (CH, C-2′), 144.83 (C, C-10), 144.09 (C, C-10′), 143.65 (C, C-4′), 131.75 (CH, C-8′), 129.85 (C, C-12), 128.57 (C, C-9′), 123.58 (CH, C-15), 122.69 (CH, C-14), 121.97 (CH, C-7′), 120.49 (CH, C-13), 120.24 (CH, C-3′), 110.87 (CH, C-16), 102.20 (CH, C-11), 101.53 (CH, C-5′), 73.45 (CH, C-9), 59.15 (CH, C-8), 55.62 (CH3, C-11′), 49.89 (CH2, C-2), 47.57 (CH2, C-6), 29.27 (CH, C-3), 27.88 (CH, C-4), 26.12 (CH2, C-7), 25.69 (CH2, C-5) and 21.12 (CH3, C-19); m/z (MAT, 200 °C) (EI) 456.2050 (M+, C28H28N2O4 requires 456.2049), 441 (3%), 413 (3), 397 (9), 365 (4), 325 (5), 313 (2), 265 (2), 253 (6), 226 (100), 211 (7), 199 (22), 188 (51), 172 (22), 157 (29), 144 (82), 132 (23), 115 (6), 99 (7) and 86 (13).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-Hydroxymethyl-5-phenylethynyl-1-azabicyclo[2.2.2]octane 23a.
10,11-Didehydroquincorine 18a (600 mg, 3.64 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (128 mg, 0.18 mmol, 0.05 eq.), CuI (69 mg, 0.36 mmol, 0.1 eq.) and iodobenzene (0.61 ml, 5.46 mmol, 1.5 eq.) to yield phenyl-substituted alkyne 23a (74%, 649 mg, 2.69 mmol); νmax(CHCl3)/cm−1 3380, 3054, 2955, 2868, 2224, 1599, 1489, 1454, 1412, 1378, 1338, 1265, 1230, 1099, 1069, 1021, 995 and 942; δH(400 MHz; CDCl3) 7.43–7.39 (m, 2 H, Ph-H), 7.32–7.27 (m, 3 H, Ph-H), 4.24 (s, 1 H, OH), 3.63–3.58 (m, 1 H, H-9), 3.54–3.47 (m, 1 H, H-9), 3.41–3.32 (m, 1 H, H-6), 3.20–3.07 (m, 2 H, H-2, H-6), 2.94–2.79 (m, 2 H, H-7, H-7), 2.28–2.19 (m, 1 H, H-5), 2.13–2.08 (m, 1 H, H-4), 1.71–1.55 (m, 3 H, H-3, H-8, H-8) and 1.04–0.97 (m, 1 H, H-3); δC(100 MHz; CDCl3) 131.65 (CH, C-13, C-17), 128.28 (CH, C-14, C-16), 127.94 (CH, C-15), 123.34 (C, C-12), 91.99 (C, C-10), 81.71 (C, C-11), 62.18 (CH2, C-9), 57.64 (CH, C-2), 56.60 (CH2, C-6), 40.14 (CH2, C-7), 28.23 (CH, C-5), 26.71 (CH, C-4), 25.51 (CH2, C-8) and 24.54 (CH2, C-3); m/z (MAT, 70 °C) (EI) 241.1468 (M+, C16H19N1O1 requires 241.1467), 210 (100%), 128 (27) and 85 (32).
)-2-Hydroxymethyl-5-phenylethynyl-1-azabicyclo[2.2.2]octane 23a.
10,11-Didehydroquincorine 18a (600 mg, 3.64 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (128 mg, 0.18 mmol, 0.05 eq.), CuI (69 mg, 0.36 mmol, 0.1 eq.) and iodobenzene (0.61 ml, 5.46 mmol, 1.5 eq.) to yield phenyl-substituted alkyne 23a (74%, 649 mg, 2.69 mmol); νmax(CHCl3)/cm−1 3380, 3054, 2955, 2868, 2224, 1599, 1489, 1454, 1412, 1378, 1338, 1265, 1230, 1099, 1069, 1021, 995 and 942; δH(400 MHz; CDCl3) 7.43–7.39 (m, 2 H, Ph-H), 7.32–7.27 (m, 3 H, Ph-H), 4.24 (s, 1 H, OH), 3.63–3.58 (m, 1 H, H-9), 3.54–3.47 (m, 1 H, H-9), 3.41–3.32 (m, 1 H, H-6), 3.20–3.07 (m, 2 H, H-2, H-6), 2.94–2.79 (m, 2 H, H-7, H-7), 2.28–2.19 (m, 1 H, H-5), 2.13–2.08 (m, 1 H, H-4), 1.71–1.55 (m, 3 H, H-3, H-8, H-8) and 1.04–0.97 (m, 1 H, H-3); δC(100 MHz; CDCl3) 131.65 (CH, C-13, C-17), 128.28 (CH, C-14, C-16), 127.94 (CH, C-15), 123.34 (C, C-12), 91.99 (C, C-10), 81.71 (C, C-11), 62.18 (CH2, C-9), 57.64 (CH, C-2), 56.60 (CH2, C-6), 40.14 (CH2, C-7), 28.23 (CH, C-5), 26.71 (CH, C-4), 25.51 (CH2, C-8) and 24.54 (CH2, C-3); m/z (MAT, 70 °C) (EI) 241.1468 (M+, C16H19N1O1 requires 241.1467), 210 (100%), 128 (27) and 85 (32).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-phenylethynyl-1-azabicyclo[2.2.2]octane 23b.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and iodobenzene (110 mg, 0.54 mmol, 1.5 eq.) to yield phenyl-substituted alkyne 23b (84%, 107 mg, 0.30 mmol); νmax(CHCl3)/cm−1 3056, 2952, 2928, 2884, 2856, 1596, 1468, 1388, 1360, 1324, 1264, 1228, 1172, 1116, 1080, 1028, 940 and 836; δH(400 MHz; CDCl3) 7.42–7.39 (m, 2 H, Ph-H), 7.31–7.28 (m, 3 H, Ph-H), 3.84–3.80 (dd, 1 H, J 10.6 and 5.6, H-9), 3.78–3.73 (dd, J 10.5 and 5.8, H-9), 3.47–3.40 (dd, 1 H, J 12.9 and 10.4, H-6endo), 3.24–3.17 (m, 1 H, H-2), 3.16–3.09 (m, 1 H, H-6exo), 2.87–2.76 (m, 2 H, H-7, H-7), 2.25–2.17 (m, 1 H, H-5), 2.12–2.08 (m, 1 H, H-4), 1.70–1.62 (m, 1 H, H-3), 1.59–1.50 (m, 1 H, H-8), 1.45–1.38 (m, 1 H, H-8), 1.32–1.26 (m, 1 H, H-3), 0.93 (s, 9 H, SiC(CH3)3), 0.11 (s, 3 H, SiCH3) and 0.10 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 131.96 (CH, C-13, C-17), 128.45 (CH, C-14, C-16), 127.75 (CH, C-15), 123.61 (C, C-12), 92.79 (C, C-10), 81.33 (C, C-11), 65.28 (CH2, C-9), 57.51 (CH2, C-6), 57.45 (CH, C-2), 42.29 (CH2, C-7), 28.24 (CH, C-5), 27.20 (CH, C-4), 25.84 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-phenylethynyl-1-azabicyclo[2.2.2]octane 23b.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and iodobenzene (110 mg, 0.54 mmol, 1.5 eq.) to yield phenyl-substituted alkyne 23b (84%, 107 mg, 0.30 mmol); νmax(CHCl3)/cm−1 3056, 2952, 2928, 2884, 2856, 1596, 1468, 1388, 1360, 1324, 1264, 1228, 1172, 1116, 1080, 1028, 940 and 836; δH(400 MHz; CDCl3) 7.42–7.39 (m, 2 H, Ph-H), 7.31–7.28 (m, 3 H, Ph-H), 3.84–3.80 (dd, 1 H, J 10.6 and 5.6, H-9), 3.78–3.73 (dd, J 10.5 and 5.8, H-9), 3.47–3.40 (dd, 1 H, J 12.9 and 10.4, H-6endo), 3.24–3.17 (m, 1 H, H-2), 3.16–3.09 (m, 1 H, H-6exo), 2.87–2.76 (m, 2 H, H-7, H-7), 2.25–2.17 (m, 1 H, H-5), 2.12–2.08 (m, 1 H, H-4), 1.70–1.62 (m, 1 H, H-3), 1.59–1.50 (m, 1 H, H-8), 1.45–1.38 (m, 1 H, H-8), 1.32–1.26 (m, 1 H, H-3), 0.93 (s, 9 H, SiC(CH3)3), 0.11 (s, 3 H, SiCH3) and 0.10 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 131.96 (CH, C-13, C-17), 128.45 (CH, C-14, C-16), 127.75 (CH, C-15), 123.61 (C, C-12), 92.79 (C, C-10), 81.33 (C, C-11), 65.28 (CH2, C-9), 57.51 (CH2, C-6), 57.45 (CH, C-2), 42.29 (CH2, C-7), 28.24 (CH, C-5), 27.20 (CH, C-4), 25.84 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.72 (CH2, C-8), 24.71 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.23 (CH3, SiC
H3)3), 25.72 (CH2, C-8), 24.71 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.23 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.27 (CH3, SiC
H3) and −5.27 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT, 50 °C) (EI) 355.2333 (M+, C22H33N1O1Si requires 355.2331), 340 (8%), 299 (71), 280 (2), 270 (6), 240 (7), 222 (11), 210 (1000), 194 (3), 184 (40), 170 (10), 156 (26), 128 (15), 115 (11), 98 (6) and 75 (39.03).
H3); m/z (MAT, 50 °C) (EI) 355.2333 (M+, C22H33N1O1Si requires 355.2331), 340 (8%), 299 (71), 280 (2), 270 (6), 240 (7), 222 (11), 210 (1000), 194 (3), 184 (40), 170 (10), 156 (26), 128 (15), 115 (11), 98 (6) and 75 (39.03).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-Hydroxymethyl-5-(4-nitrophenylethynyl)-1-azabicyclo[2.2.2]octane 23c.
10,11-Didehydroquincorine 18a (600 mg, 3.64 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (128 mg, 0.18 mmol, 0.05 eq.), CuI (69 mg, 0.36 mmol, 0.1 eq.) and p-nitrophenyl iodide (1358 mg, 5.46 mmol, 1.5 eq.) to yield p-nitrophenyl-substituted alkyne 23c (77%, 801 mg, 2.80 mmol); νmax(CHCl3)/cm−1 3424, 2955, 2868, 2222, 1595, 1519, 1490, 1454, 1410, 1344, 1308, 1285, 1230, 1193, 1175, 1108, 1019, 996, 942 and 855; δH(400 MHz; CDCl3) 8.17–8.13 (d, 2 H, J 8.9, H-14, H-16), 7.56–7.51 (d, 2 H, J 9.0, H-13, H-17), 4.17 (s, 1 H, OH), 3.62–3.57 (m, 1 H, H-9), 3.53–3.45 (m, 1 H, H-9), 3.37–3.29 (m, 1 H, H-6), 3.19–3.08 (m, 2 H, H-2, H-6), 2.96–2.90 (m, 1 H, H-7), 2.85–2.76 (m, 1 H, H-7), 2.22–2.15 (m, 1 H, H-5), 2.13–2.08 (m, 1 H, H-4), 1.66–1.58 (m, 2 H, H-3, H-8), 1.46–1.41 (m, 1 H, H-8) and 1.04–0.97 (m, 1 H, H-3); δC(100 MHz; CDCl3) 146.84 (C, C-15), 132.45 (CH, C-13, C-17), 130.48 (C, C-12), 123.55 (CH, C-14, C-16), 98.29 (C, C-10), 80.15 (C, C-11), 62.28 (CH2, C-9), 57.55 (CH, C-2), 56.37 (CH2, C-6), 40.04 (CH2, C-7), 28.52 (CH, C-5), 26.63 (CH, C-4), 25.62 (CH2, C-8) and 24.69 (CH2, C-3); m/z (MAT, 130 °C) (EI) 286.1317 (M+, C16H18N2O3 requires 286.1317), 255 (100%), 228 (6), 209 (6), 181 (4), 152 (7), 126 (10), 102 (2), 87 (26) and 72 (12).
)-2-Hydroxymethyl-5-(4-nitrophenylethynyl)-1-azabicyclo[2.2.2]octane 23c.
10,11-Didehydroquincorine 18a (600 mg, 3.64 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (128 mg, 0.18 mmol, 0.05 eq.), CuI (69 mg, 0.36 mmol, 0.1 eq.) and p-nitrophenyl iodide (1358 mg, 5.46 mmol, 1.5 eq.) to yield p-nitrophenyl-substituted alkyne 23c (77%, 801 mg, 2.80 mmol); νmax(CHCl3)/cm−1 3424, 2955, 2868, 2222, 1595, 1519, 1490, 1454, 1410, 1344, 1308, 1285, 1230, 1193, 1175, 1108, 1019, 996, 942 and 855; δH(400 MHz; CDCl3) 8.17–8.13 (d, 2 H, J 8.9, H-14, H-16), 7.56–7.51 (d, 2 H, J 9.0, H-13, H-17), 4.17 (s, 1 H, OH), 3.62–3.57 (m, 1 H, H-9), 3.53–3.45 (m, 1 H, H-9), 3.37–3.29 (m, 1 H, H-6), 3.19–3.08 (m, 2 H, H-2, H-6), 2.96–2.90 (m, 1 H, H-7), 2.85–2.76 (m, 1 H, H-7), 2.22–2.15 (m, 1 H, H-5), 2.13–2.08 (m, 1 H, H-4), 1.66–1.58 (m, 2 H, H-3, H-8), 1.46–1.41 (m, 1 H, H-8) and 1.04–0.97 (m, 1 H, H-3); δC(100 MHz; CDCl3) 146.84 (C, C-15), 132.45 (CH, C-13, C-17), 130.48 (C, C-12), 123.55 (CH, C-14, C-16), 98.29 (C, C-10), 80.15 (C, C-11), 62.28 (CH2, C-9), 57.55 (CH, C-2), 56.37 (CH2, C-6), 40.04 (CH2, C-7), 28.52 (CH, C-5), 26.63 (CH, C-4), 25.62 (CH2, C-8) and 24.69 (CH2, C-3); m/z (MAT, 130 °C) (EI) 286.1317 (M+, C16H18N2O3 requires 286.1317), 255 (100%), 228 (6), 209 (6), 181 (4), 152 (7), 126 (10), 102 (2), 87 (26) and 72 (12).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-Hydroxymethyl-5-(4-ethoxycarbonylphenylethynyl)-1-azabicyclo[2.2.2]octane 23d.
10,11-Didehydroquincorine 18a (600 mg, 3.64 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (128 mg, 0.18 mmol, 0.05 eq.), CuI (69 mg, 0.36 mmol, 0.1 eq.) and 4-ethoxycarbonylphenyl iodide (0.91 ml, 5.46 mmol, 1.5 eq.) to yield 4-ethoxycarbonylphenyl-substituted alkyne 23d (71%, 808 mg, 2.58 mmol); νmax(CHCl3)/cm−1 3366, 2957, 2871, 2222, 1712, 1606, 1508, 1454, 1406, 1369, 1344, 1307, 1275, 1231, 1176, 1108, 1020, 996 and 858; δH(400 MHz; CDCl3) 7.98–7.96 (d, 2 H, J 8.6, H-14, H-16), 7.47–7.45 (d, 2 H, J 8.5, H-13, H-17), 4.39 (q, 2 H, J 7.2, H-19), 4.35–4.28 (s, 1 H, OH), 3.68–3.53 (m, 2 H, H-9, H-9), 3.48–3.38 (m, 1 H, H-6), 3.27–3.13 (m, 2 H, H-2, H-6), 3.04–2.97 (m, 1 H, H-7), 2.96–2.86 (m, 1 H, H-7), 2.29–2.21 (m, 1 H, H-5), 2.17–2.12 (m, 1 H, H-4), 1.74–1.63 (m, 3 H, H-3, H-8, H-8), 1.41–1.37 (t, 3 H, J 7.2, H-20) and 1.11–1.04 (m, 1 H, H-3); δC(100 MHz; CDCl3) 166.07 (C, C-18), 131.59 (CH, C-13, C-17), 129.73 (C, C-12), 129.43 (CH, C-14, C-16), 127.83 (C, C-15), 94.73 (C, C-10), 81.41 (C, C-11), 61.99 (CH2, C-9), 61.13 (CH2, C-19), 57.91 (CH, C-2), 56.23 (CH2, C-6), 40.26 (CH2, C-7), 28.19 (CH, C-5), 26.64 (CH, C-4), 25.33 (CH2, C-8), 24.39 (CH2, C-3) and 14.32 (CH3, C-20); m/z (MAT, 110 °C) (EI) 313.1679 (M+, C19H23N1O3 requires 313.1768), 283 (100%), 269 (7), 255 (6), 227 (2), 210 (2), 200 (6), 181 (2), 155 (4), 127 (3), 91 (1) and 72 (2).
)-2-Hydroxymethyl-5-(4-ethoxycarbonylphenylethynyl)-1-azabicyclo[2.2.2]octane 23d.
10,11-Didehydroquincorine 18a (600 mg, 3.64 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (128 mg, 0.18 mmol, 0.05 eq.), CuI (69 mg, 0.36 mmol, 0.1 eq.) and 4-ethoxycarbonylphenyl iodide (0.91 ml, 5.46 mmol, 1.5 eq.) to yield 4-ethoxycarbonylphenyl-substituted alkyne 23d (71%, 808 mg, 2.58 mmol); νmax(CHCl3)/cm−1 3366, 2957, 2871, 2222, 1712, 1606, 1508, 1454, 1406, 1369, 1344, 1307, 1275, 1231, 1176, 1108, 1020, 996 and 858; δH(400 MHz; CDCl3) 7.98–7.96 (d, 2 H, J 8.6, H-14, H-16), 7.47–7.45 (d, 2 H, J 8.5, H-13, H-17), 4.39 (q, 2 H, J 7.2, H-19), 4.35–4.28 (s, 1 H, OH), 3.68–3.53 (m, 2 H, H-9, H-9), 3.48–3.38 (m, 1 H, H-6), 3.27–3.13 (m, 2 H, H-2, H-6), 3.04–2.97 (m, 1 H, H-7), 2.96–2.86 (m, 1 H, H-7), 2.29–2.21 (m, 1 H, H-5), 2.17–2.12 (m, 1 H, H-4), 1.74–1.63 (m, 3 H, H-3, H-8, H-8), 1.41–1.37 (t, 3 H, J 7.2, H-20) and 1.11–1.04 (m, 1 H, H-3); δC(100 MHz; CDCl3) 166.07 (C, C-18), 131.59 (CH, C-13, C-17), 129.73 (C, C-12), 129.43 (CH, C-14, C-16), 127.83 (C, C-15), 94.73 (C, C-10), 81.41 (C, C-11), 61.99 (CH2, C-9), 61.13 (CH2, C-19), 57.91 (CH, C-2), 56.23 (CH2, C-6), 40.26 (CH2, C-7), 28.19 (CH, C-5), 26.64 (CH, C-4), 25.33 (CH2, C-8), 24.39 (CH2, C-3) and 14.32 (CH3, C-20); m/z (MAT, 110 °C) (EI) 313.1679 (M+, C19H23N1O3 requires 313.1768), 283 (100%), 269 (7), 255 (6), 227 (2), 210 (2), 200 (6), 181 (2), 155 (4), 127 (3), 91 (1) and 72 (2).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-Benzoyloxymethyl-5-(4-ethoxycarbonylphenylethynyl)-1-azabicyclo[2.2.2]octane 23e.
10,11-Didehydroquincorine 18c (125 mg, 0.47 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (16 mg, 0.03 mmol, 0.05 eq.), CuI (9 mg, 0.05 mmol, 0.1 eq.) and ethyl 4-iodobenzoate (192 mg, 0.69 mmol, 1.5 eq.) to yield 4-ethoxycarbonylphenyl-substituted alkyne 23e (92%, 172 mg, 0.43 mmol); νmax(CHCl3)/cm−1 3060, 2944, 2868, 2220, 1712, 1604, 1508, 1452, 1404, 1368, 1276, 1176, 1108, 1068, 1048, 1024, 976 and 856; δH(400 MHz; CDCl3) 8.08–7.06 (d, 2 H, J 7.5, H-23, H-27), 7.99–7.96 (d, 2 H, J 8.2, H-14, H-16), 7.54–7.50 (m, 1 H, Ph-H), 7.48–7.45 (d, 2 H, J 8.2, H-13, H-17), 7.42–7.37 (m, 2 H, Ph-H), 4.53–4.46 (m, 1 H, H-9), 4.39 (q, 2 H, J 7.1, H-19), 4.35–4.28 (m, 1 H, H-9), 3.52–3.42 (m, 1 H, H-6), 3.21–3.07 (m, 2 H, H-2, H-6), 2.88–2.76 (m, 2 H, H-7, H-7), 2.33–2.27 (m, 1 H, H-5), 2.14–2.08 (m, 1 H, H-4), 1.70–1.51 (m, 3 H, H-3, H-8, H-8), 1.41–1.36 (t, 3 H, J 7.1, H-20) and 1.26–1.17 (m, 1 H, H-3); δC(100 MHz; CDCl3) 166.68 (C, C-21), 166.08 (C, C-18), 132.95 (CH, C-25), 131.52 (CH, C-13, C-17), 130.06 (C, C-12), 129.75 (CH, C-23, C-27), 129.39 (CH, C-14, C-16), 128.56 (CH, C-24, C-26), 128.30 (C, C-22), 127.84 (C, C-15), 96.22 (C, C-10), 80.82 (C, C-11), 65.48 (CH2, C-9), 61.07 (CH2, C-19), 56.72 (CH2, C-6), 54.44 (CH, C-2), 40.39 (CH2, C-7), 28.37 (CH, C-5), 26.93 (CH, C-4), 26.00 (CH2, C-8), 25.39 (CH2, C-3) and 14.31 (CH3, C-20); m/z (MAT, 120 °C) (EI) 417.1938 (M+, C16H27N1O4 requires 417.1940), 367 (2%), 324 (4), 307 (2), 277 (100), 262 (2), 225 (4), 201 (19), 183 (15), 152 (9), 122 (15), 105 (26) and 77 (27).
)-2-Benzoyloxymethyl-5-(4-ethoxycarbonylphenylethynyl)-1-azabicyclo[2.2.2]octane 23e.
10,11-Didehydroquincorine 18c (125 mg, 0.47 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (16 mg, 0.03 mmol, 0.05 eq.), CuI (9 mg, 0.05 mmol, 0.1 eq.) and ethyl 4-iodobenzoate (192 mg, 0.69 mmol, 1.5 eq.) to yield 4-ethoxycarbonylphenyl-substituted alkyne 23e (92%, 172 mg, 0.43 mmol); νmax(CHCl3)/cm−1 3060, 2944, 2868, 2220, 1712, 1604, 1508, 1452, 1404, 1368, 1276, 1176, 1108, 1068, 1048, 1024, 976 and 856; δH(400 MHz; CDCl3) 8.08–7.06 (d, 2 H, J 7.5, H-23, H-27), 7.99–7.96 (d, 2 H, J 8.2, H-14, H-16), 7.54–7.50 (m, 1 H, Ph-H), 7.48–7.45 (d, 2 H, J 8.2, H-13, H-17), 7.42–7.37 (m, 2 H, Ph-H), 4.53–4.46 (m, 1 H, H-9), 4.39 (q, 2 H, J 7.1, H-19), 4.35–4.28 (m, 1 H, H-9), 3.52–3.42 (m, 1 H, H-6), 3.21–3.07 (m, 2 H, H-2, H-6), 2.88–2.76 (m, 2 H, H-7, H-7), 2.33–2.27 (m, 1 H, H-5), 2.14–2.08 (m, 1 H, H-4), 1.70–1.51 (m, 3 H, H-3, H-8, H-8), 1.41–1.36 (t, 3 H, J 7.1, H-20) and 1.26–1.17 (m, 1 H, H-3); δC(100 MHz; CDCl3) 166.68 (C, C-21), 166.08 (C, C-18), 132.95 (CH, C-25), 131.52 (CH, C-13, C-17), 130.06 (C, C-12), 129.75 (CH, C-23, C-27), 129.39 (CH, C-14, C-16), 128.56 (CH, C-24, C-26), 128.30 (C, C-22), 127.84 (C, C-15), 96.22 (C, C-10), 80.82 (C, C-11), 65.48 (CH2, C-9), 61.07 (CH2, C-19), 56.72 (CH2, C-6), 54.44 (CH, C-2), 40.39 (CH2, C-7), 28.37 (CH, C-5), 26.93 (CH, C-4), 26.00 (CH2, C-8), 25.39 (CH2, C-3) and 14.31 (CH3, C-20); m/z (MAT, 120 °C) (EI) 417.1938 (M+, C16H27N1O4 requires 417.1940), 367 (2%), 324 (4), 307 (2), 277 (100), 262 (2), 225 (4), 201 (19), 183 (15), 152 (9), 122 (15), 105 (26) and 77 (27).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(3′-quinolylethynyl)-1-azabicyclo[2.2.2]octane 23f.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-bromoquinoline (67 μl, 0.54 mmol, 1.5 eq.) to yield quinolyl-substituted alkyne 23f (86%, 125 mg, 0.31 mmol); νmax(CHCl3)/cm−1 2940, 2928, 2856, 1596, 1468, 1388, 1344, 1320, 1256, 1188, 1116, 1084, 1028, 940 and 836; δH(400 MHz; CDCl3) 8.86 (d, 1 H, J 2.2, H-2′), 8.17 (d, 1 H, J 2.1, H-4′), 8.08 (d, 1 H, J 8.4, H-8′), 7.76 (d, 1 H, J 8.0, H-5′), 7.71–7.66 (ddd, 1 H, J 8.3, 7.0 and 1.4, H-7′), 7.56–7.51 (ddd, 1 H, J 7.9, 7.2 and 1.1, H-6′), 3.70–3.66 (dd, 1 H, J 10.2 and 6.0, H-9), 3.64–3.59 (dd, J 10.3 and 6.0, H-9), 3.24–3.18 (dd, 1 H, J 13.3 and 10.1, H-6endo), 3.11–2.89 (m, 3 H, H-2, H-6, H-7), 2.61–2.53 (m, 1 H, H-7), 2.34–2.30 (m, 1 H, H-5), 2.09–2.00 (m, 1 H, H-4), 1.96–1.92 (m, 1 H, H-3), 1.53–1.42 (m, 1 H, H-8), 1.32–1.21 (m, 2 H, H-8, H-3), 0.90 (s, 9 H, SiC(CH3)3) and 0.07 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 152.46 (CH, C-6′), 146.59 (C, C-10′), 138.01 (CH, C-2′), 129.67 (CH, C-8′), 129.36 (CH, C-7′), 127.39 (CH, C-5′), 127.34 (C, C-9′), 127.14 (CH, C-4′), 118.06 (C, C-3″), 97.43 (C, C-10), 81.49 (C, C-11), 65.92 (CH2, C-9), 57.86 (CH2, C-6), 57.13 (CH, C-2), 41.71 (CH2, C-7), 29.28 (CH, C-5), 27.61 (CH, C-4), 26.01 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(3′-quinolylethynyl)-1-azabicyclo[2.2.2]octane 23f.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-bromoquinoline (67 μl, 0.54 mmol, 1.5 eq.) to yield quinolyl-substituted alkyne 23f (86%, 125 mg, 0.31 mmol); νmax(CHCl3)/cm−1 2940, 2928, 2856, 1596, 1468, 1388, 1344, 1320, 1256, 1188, 1116, 1084, 1028, 940 and 836; δH(400 MHz; CDCl3) 8.86 (d, 1 H, J 2.2, H-2′), 8.17 (d, 1 H, J 2.1, H-4′), 8.08 (d, 1 H, J 8.4, H-8′), 7.76 (d, 1 H, J 8.0, H-5′), 7.71–7.66 (ddd, 1 H, J 8.3, 7.0 and 1.4, H-7′), 7.56–7.51 (ddd, 1 H, J 7.9, 7.2 and 1.1, H-6′), 3.70–3.66 (dd, 1 H, J 10.2 and 6.0, H-9), 3.64–3.59 (dd, J 10.3 and 6.0, H-9), 3.24–3.18 (dd, 1 H, J 13.3 and 10.1, H-6endo), 3.11–2.89 (m, 3 H, H-2, H-6, H-7), 2.61–2.53 (m, 1 H, H-7), 2.34–2.30 (m, 1 H, H-5), 2.09–2.00 (m, 1 H, H-4), 1.96–1.92 (m, 1 H, H-3), 1.53–1.42 (m, 1 H, H-8), 1.32–1.21 (m, 2 H, H-8, H-3), 0.90 (s, 9 H, SiC(CH3)3) and 0.07 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 152.46 (CH, C-6′), 146.59 (C, C-10′), 138.01 (CH, C-2′), 129.67 (CH, C-8′), 129.36 (CH, C-7′), 127.39 (CH, C-5′), 127.34 (C, C-9′), 127.14 (CH, C-4′), 118.06 (C, C-3″), 97.43 (C, C-10), 81.49 (C, C-11), 65.92 (CH2, C-9), 57.86 (CH2, C-6), 57.13 (CH, C-2), 41.71 (CH2, C-7), 29.28 (CH, C-5), 27.61 (CH, C-4), 26.01 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.07 (CH2, C-8), 22.68 (CH2, C-3), 18.42 (C, SiC(CH3)3), −5.27 (CH3, SiC
H3)3), 25.07 (CH2, C-8), 22.68 (CH2, C-3), 18.42 (C, SiC(CH3)3), −5.27 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.34 (CH3, SiC
H3) and −5.34 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 407 (M+ + H, 8%), 355 (14), 325 (10), 281 (41), 221 (55), 207 (43) and 147 (100).
H3); m/z (FAB) (EI) 407 (M+ + H, 8%), 355 (14), 325 (10), 281 (41), 221 (55), 207 (43) and 147 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(2-aminophenylethynyl)-1-azabicyclo[2.2.2]octane 23g.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodoaniline (118 mg, 0.54 mmol, 1.5 eq.) to yield o-aminophenyl-substituted alkyne 23g (84%, 111 mg, 0.30 mmol); νmax(CHCl3)/cm−1 2940, 2928, 2856, 1612, 1492, 1456, 1388, 1360, 1304, 1256, 1228, 1116, 1084, 1028, 936, 836 and 808; δH(400 MHz; CDCl3) 7.25 (dd, 1 H, J 7.5 and 1.5, H-17), 7.10–7.05 (ddd, 1 H, J 8.9, 7.6 and 1.5, H-14), 6.69–6.63 (m, 2 H, H-15, H-16), 4.23–4.10 (m, 2 H, NH2), 3.73–3.63 (m, 2 H, H-9, H-9), 3.38–3.31 (dd, 1 H, J 13.2 and 10.0, H-6endo), 3.12–2.98 (m, 2 H, H-2, H-6), 2.81–2.57 (m, 2 H, H-7, H-7), 2.47–2.42 (m, 1 H, H-5), 2.18–2.10 (m, 1 H, H-3), 2.07–2.02 (m, 1 H, H-4), 1.59–1.46 (m, 1 H, H-8), 1.32–1.24 (m, 2 H, H-8, H-3), 0.91 (s, 9 H, SiC(CH3)3) and 0.07 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 147.62 (C, C-13), 132.12 (CH, C-14), 128.96 (CH, C-15), 117.83 (CH, C-17), 114.18 (CH, C-16), 108.62 (C, C-12), 98.96 (C, C-10), 81.42 (C, C-11), 65.78 (CH2, C-9), 57.52 (CH2, C-6), 57.05 (CH, C-2), 41.84 (CH2, C-7), 29.55 (CH, C-5), 27.46 (CH, C-4), 26.30 (CH2, C-8), 26.00 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(2-aminophenylethynyl)-1-azabicyclo[2.2.2]octane 23g.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodoaniline (118 mg, 0.54 mmol, 1.5 eq.) to yield o-aminophenyl-substituted alkyne 23g (84%, 111 mg, 0.30 mmol); νmax(CHCl3)/cm−1 2940, 2928, 2856, 1612, 1492, 1456, 1388, 1360, 1304, 1256, 1228, 1116, 1084, 1028, 936, 836 and 808; δH(400 MHz; CDCl3) 7.25 (dd, 1 H, J 7.5 and 1.5, H-17), 7.10–7.05 (ddd, 1 H, J 8.9, 7.6 and 1.5, H-14), 6.69–6.63 (m, 2 H, H-15, H-16), 4.23–4.10 (m, 2 H, NH2), 3.73–3.63 (m, 2 H, H-9, H-9), 3.38–3.31 (dd, 1 H, J 13.2 and 10.0, H-6endo), 3.12–2.98 (m, 2 H, H-2, H-6), 2.81–2.57 (m, 2 H, H-7, H-7), 2.47–2.42 (m, 1 H, H-5), 2.18–2.10 (m, 1 H, H-3), 2.07–2.02 (m, 1 H, H-4), 1.59–1.46 (m, 1 H, H-8), 1.32–1.24 (m, 2 H, H-8, H-3), 0.91 (s, 9 H, SiC(CH3)3) and 0.07 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 147.62 (C, C-13), 132.12 (CH, C-14), 128.96 (CH, C-15), 117.83 (CH, C-17), 114.18 (CH, C-16), 108.62 (C, C-12), 98.96 (C, C-10), 81.42 (C, C-11), 65.78 (CH2, C-9), 57.52 (CH2, C-6), 57.05 (CH, C-2), 41.84 (CH2, C-7), 29.55 (CH, C-5), 27.46 (CH, C-4), 26.30 (CH2, C-8), 26.00 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 22.67 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.30 (CH3, SiC
H3)3), 22.67 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.30 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.32 (CH3, SiC
H3) and −5.32 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 371 (M+ + H, 16), 313 (11), 240 (9), 201 (8), 189 (15) and 147 (100).
H3); m/z (FAB) (EI) 371 (M+ + H, 16), 313 (11), 240 (9), 201 (8), 189 (15) and 147 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-benzofuryl-1-azabicyclo[2.2.2]octane 23h.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodoaniline (119 mg, 0.54 mmol, 1.5 eq.) to yield benzofuran 23h (78%, 104 mg, 0.28 mmol); νmax(CHCl3)/cm−1 2940, 2928, 2856, 1612, 1460, 1388, 1344, 1324, 1256, 1188, 1116, 1084, 1028, 1004, 936, 836 and 808; δH(400 MHz; CDCl3) 7.32–7.29 (dd, 1 H, J 7.7 and 1.6, H-13), 7.23–7.18 (ddd, J 8.7, 7.1 and 1.6, H-14), 6.86–6.82 (m, 1 H, H-15), 6.81–6.79 (dd, 1 H, J 8.6 and 1.6, H-16), 6.51 (m, 1 H, H-11), 3.73–3.61 (m, 2 H, H-9, H-9), 3.26–3.19 (dd, 1 H, J 13.4 and 10.0, H-6endo), 3.13–2.91 (m, 2 H, H-2, H-6), 2.81–2.57 (m, 2 H, H-7, H-7), 2.37–2.32 (m, 1 H, H-5), 2.09–2.01 (m, 1 H, H-3), 1.99–1.95 (m, 1 H, H-4), 1.68–1.61 (m, 1 H, H-8), 1.53–1.43 (m, 2 H, H-8, H-3), 0.91 (s, 9 H, SiC(CH3)3) and 0.07 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 161.36 (C, C-17), 154.71 (C, C-10), 129.44 (CH, C-13), 128.65 (C, C-12), 122.50 (CH, C-14), 120.38 (CH, C-15), 110.78 (CH, C-16), 101.75 (CH, C-11), 65.80 (CH2, C-9), 57.82 (CH2, C-6), 56.99 (CH, C-2), 41.67 (CH2, C-7), 35.77 (CH, C-5), 28.46 (CH, C-4), 27.07 (CH2, C-8), 26.01 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-benzofuryl-1-azabicyclo[2.2.2]octane 23h.
10,11-Didehydroquincorine 18b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-iodoaniline (119 mg, 0.54 mmol, 1.5 eq.) to yield benzofuran 23h (78%, 104 mg, 0.28 mmol); νmax(CHCl3)/cm−1 2940, 2928, 2856, 1612, 1460, 1388, 1344, 1324, 1256, 1188, 1116, 1084, 1028, 1004, 936, 836 and 808; δH(400 MHz; CDCl3) 7.32–7.29 (dd, 1 H, J 7.7 and 1.6, H-13), 7.23–7.18 (ddd, J 8.7, 7.1 and 1.6, H-14), 6.86–6.82 (m, 1 H, H-15), 6.81–6.79 (dd, 1 H, J 8.6 and 1.6, H-16), 6.51 (m, 1 H, H-11), 3.73–3.61 (m, 2 H, H-9, H-9), 3.26–3.19 (dd, 1 H, J 13.4 and 10.0, H-6endo), 3.13–2.91 (m, 2 H, H-2, H-6), 2.81–2.57 (m, 2 H, H-7, H-7), 2.37–2.32 (m, 1 H, H-5), 2.09–2.01 (m, 1 H, H-3), 1.99–1.95 (m, 1 H, H-4), 1.68–1.61 (m, 1 H, H-8), 1.53–1.43 (m, 2 H, H-8, H-3), 0.91 (s, 9 H, SiC(CH3)3) and 0.07 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 161.36 (C, C-17), 154.71 (C, C-10), 129.44 (CH, C-13), 128.65 (C, C-12), 122.50 (CH, C-14), 120.38 (CH, C-15), 110.78 (CH, C-16), 101.75 (CH, C-11), 65.80 (CH2, C-9), 57.82 (CH2, C-6), 56.99 (CH, C-2), 41.67 (CH2, C-7), 35.77 (CH, C-5), 28.46 (CH, C-4), 27.07 (CH2, C-8), 26.01 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.01 (CH2, C-3), 18.42 (C, SiC(CH3)3), −5.31 (CH3, SiC
H3)3), 25.01 (CH2, C-3), 18.42 (C, SiC(CH3)3), −5.31 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.33 (CH3, SiC
H3) and −5.33 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT) (EI) 372 (M+ + H, 44%), 341 (13), 281 (43), 221 (47), 207 (41), 184 (16) and 147 (100).
H3); m/z (MAT) (EI) 372 (M+ + H, 44%), 341 (13), 281 (43), 221 (47), 207 (41), 184 (16) and 147 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-Hydroxymethyl-5-(phenylethynyl)-1-azabicyclo[2.2.2]octane 24a.
10,11-Didehydroquincoridine 20a (800 mg, 4.97 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (174 mg, 0.25 mmol, 0.05 eq.), CuI (92 mg, 0.50 mmol, 0.1 eq.) and phenyl iodide (1483 mg, 7.27 mmol, 1.5 eq.) to yield phenyl-substituted alkyne 24a (65%, 759 mg, 3.15 mmol); νmax(CHCl3)/cm−1 3375, 3059, 2950, 2879, 2225, 1599, 1490, 1463, 1411, 1337, 1324, 1255, 1234, 1137, 1099, 1068, 1029, 1015 and 999; δH(400 MHz; CDCl3) 7.42–7.39 (m, 2 H, Ph-H), 7.31–7.28 (m, 3 H, Ph-H), 4.62–4.45 (s, 1 H, OH), 3.83–3.63 (m, 2 H, H-9, H-9), 3.43–3.28 (m, 2 H, H-6, H-6), 3.26–3.07 (m, 3 H, H-2, H-7, H-7), 2.95–2.89 (m, 1 H, H-5), 2.14–2.10 (m, 1 H, H-4), 1.87–1.68 (m, 3 H, H-3, H-8, H-8) and 1.66–1.58 (m, 1 H, H-3); δC(100 MHz; CDCl3) 131.67 (CH, C-15), 128.32 (CH, C-13, C-17), 128.07 (CH, C-14, C-16), 123.10 (C, C-12), 90.84 (C, C-10), 82.54 (C, C-11), 67.09 (CH2, C-9), 58.15 (CH, C-2), 48.36 (CH2, C-6), 47.91 (CH2, C-7), 28.31 (CH, C-5), 27.18 (CH, C-4), 24.49 (CH2, C-8) and 23.67 (CH2, C-3); m/z (MAT, 60 °C) (EI) 241.1467 (M+, C16H19N1O1 requires 241.1467), 224 (3%), 210 (100), 194 (2), 182 (12), 167 (5), 154 (7), 141 (6), 128 (46), 115 (11), 102 (3) and 84 (14).
)-2-Hydroxymethyl-5-(phenylethynyl)-1-azabicyclo[2.2.2]octane 24a.
10,11-Didehydroquincoridine 20a (800 mg, 4.97 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (174 mg, 0.25 mmol, 0.05 eq.), CuI (92 mg, 0.50 mmol, 0.1 eq.) and phenyl iodide (1483 mg, 7.27 mmol, 1.5 eq.) to yield phenyl-substituted alkyne 24a (65%, 759 mg, 3.15 mmol); νmax(CHCl3)/cm−1 3375, 3059, 2950, 2879, 2225, 1599, 1490, 1463, 1411, 1337, 1324, 1255, 1234, 1137, 1099, 1068, 1029, 1015 and 999; δH(400 MHz; CDCl3) 7.42–7.39 (m, 2 H, Ph-H), 7.31–7.28 (m, 3 H, Ph-H), 4.62–4.45 (s, 1 H, OH), 3.83–3.63 (m, 2 H, H-9, H-9), 3.43–3.28 (m, 2 H, H-6, H-6), 3.26–3.07 (m, 3 H, H-2, H-7, H-7), 2.95–2.89 (m, 1 H, H-5), 2.14–2.10 (m, 1 H, H-4), 1.87–1.68 (m, 3 H, H-3, H-8, H-8) and 1.66–1.58 (m, 1 H, H-3); δC(100 MHz; CDCl3) 131.67 (CH, C-15), 128.32 (CH, C-13, C-17), 128.07 (CH, C-14, C-16), 123.10 (C, C-12), 90.84 (C, C-10), 82.54 (C, C-11), 67.09 (CH2, C-9), 58.15 (CH, C-2), 48.36 (CH2, C-6), 47.91 (CH2, C-7), 28.31 (CH, C-5), 27.18 (CH, C-4), 24.49 (CH2, C-8) and 23.67 (CH2, C-3); m/z (MAT, 60 °C) (EI) 241.1467 (M+, C16H19N1O1 requires 241.1467), 224 (3%), 210 (100), 194 (2), 182 (12), 167 (5), 154 (7), 141 (6), 128 (46), 115 (11), 102 (3) and 84 (14).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-Hydroxymethyl-5-(3′-quinolylethynyl)-1-azabicyclo[2.2.2]octane 24b.
10,11-Didehydroquincoridine 20a (82 mg, 0.50 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (35 mg, 0.05 mmol, 0.1 eq.), CuI (19 mg, 0.10 mmol, 0.2 eq.) and 3-bromoquinoline (90 μl, 0.75 mmol, 1.5 eq.) to yield quinolyl-substituted alkyne 24b (62%, 90 mg, 0.31 mmol) (Found: C, 77.72; H, 7.36; N, 9.40. C19H20N2O1 requires C, 78.05; H, 6.89; N, 9.58%); νmax(CHCl3)/cm−1 3416, 3068, 2948, 2876, 2200, 1600, 1568, 1488, 1412, 1372, 1336, 1256, 1228, 1136, 1100, 1064, 1028, 1012 and 908; δH(400 MHz; CDCl3) 8.87 (d, 1 H, J 2.0, H-2′), 8.18 (d, 1 H, J 2.0, H-4′), 8.09 (d, 1 H, J 8.4, H-8′), 7.78 (d, 1 H, J 8.3, H-5′), 7.74–7.68 (ddd, 1 H, J 8.4, 7.0 and 1.5, H-7′), 7.58–7.53 (ddd, 1 H, J 8.2, 7.0 and 1.3, H-6′), 4.12–3.95 (s, 1 H, OH), 3.77–3.71 (dd, 1 H, J 11.4 and 10.4, H-9), 3.62–3.55 (dd, J 11.6 and 4.8, H-9), 3.17–3.05 (m, 3 H, H-6, H-6, H-2), 2.99–2.91 (m, 1 H, H-7), 2.86–2.80 (m, 1 H, H-7), 2.11–2.08 (m, 1 H, H-5), 1.76–1.70 (m, 1 H, H-4), 1.69–1.54 (m, 3 H, H-3, H-8, H-8) and 1.33–1.23 (m, 1 H, H-3); δC(100 MHz; CDCl3) 152.31 (CH, C-2′), 146.60 (C, C-10′), 138.16 (CH, C-8′), 133.61 (C, C-9′), 129.83 (CH, C-4′), 129.29 (CH, C-7′), 127.43 (CH, C-6′), 127.22 (CH, C-5′), 117.67 (C, C-3′), 95.87 (C, C-10), 79.13 (CH, C-11), 62.10 (CH2, C-9), 57.43 (CH, C-2), 48.38 (CH2, C-6), 47.82 (CH2, C-7), 29.13 (CH, C-5), 27.45 (CH, C-4), 25.38 (CH2, C-8) and 23.34 (CH2, C-3); m/z (MAT, 70 °C) (EI) 292.1576 (M+, C19H20N2O1 requires 292.1576), 261 (6%), 237 (3), 224 (8), 207 (100), 175 (5), 166 (8), 149 (29), 128 (71), 105 (35), 101 (33), 86 (55) and 75 (78).
)-2-Hydroxymethyl-5-(3′-quinolylethynyl)-1-azabicyclo[2.2.2]octane 24b.
10,11-Didehydroquincoridine 20a (82 mg, 0.50 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (35 mg, 0.05 mmol, 0.1 eq.), CuI (19 mg, 0.10 mmol, 0.2 eq.) and 3-bromoquinoline (90 μl, 0.75 mmol, 1.5 eq.) to yield quinolyl-substituted alkyne 24b (62%, 90 mg, 0.31 mmol) (Found: C, 77.72; H, 7.36; N, 9.40. C19H20N2O1 requires C, 78.05; H, 6.89; N, 9.58%); νmax(CHCl3)/cm−1 3416, 3068, 2948, 2876, 2200, 1600, 1568, 1488, 1412, 1372, 1336, 1256, 1228, 1136, 1100, 1064, 1028, 1012 and 908; δH(400 MHz; CDCl3) 8.87 (d, 1 H, J 2.0, H-2′), 8.18 (d, 1 H, J 2.0, H-4′), 8.09 (d, 1 H, J 8.4, H-8′), 7.78 (d, 1 H, J 8.3, H-5′), 7.74–7.68 (ddd, 1 H, J 8.4, 7.0 and 1.5, H-7′), 7.58–7.53 (ddd, 1 H, J 8.2, 7.0 and 1.3, H-6′), 4.12–3.95 (s, 1 H, OH), 3.77–3.71 (dd, 1 H, J 11.4 and 10.4, H-9), 3.62–3.55 (dd, J 11.6 and 4.8, H-9), 3.17–3.05 (m, 3 H, H-6, H-6, H-2), 2.99–2.91 (m, 1 H, H-7), 2.86–2.80 (m, 1 H, H-7), 2.11–2.08 (m, 1 H, H-5), 1.76–1.70 (m, 1 H, H-4), 1.69–1.54 (m, 3 H, H-3, H-8, H-8) and 1.33–1.23 (m, 1 H, H-3); δC(100 MHz; CDCl3) 152.31 (CH, C-2′), 146.60 (C, C-10′), 138.16 (CH, C-8′), 133.61 (C, C-9′), 129.83 (CH, C-4′), 129.29 (CH, C-7′), 127.43 (CH, C-6′), 127.22 (CH, C-5′), 117.67 (C, C-3′), 95.87 (C, C-10), 79.13 (CH, C-11), 62.10 (CH2, C-9), 57.43 (CH, C-2), 48.38 (CH2, C-6), 47.82 (CH2, C-7), 29.13 (CH, C-5), 27.45 (CH, C-4), 25.38 (CH2, C-8) and 23.34 (CH2, C-3); m/z (MAT, 70 °C) (EI) 292.1576 (M+, C19H20N2O1 requires 292.1576), 261 (6%), 237 (3), 224 (8), 207 (100), 175 (5), 166 (8), 149 (29), 128 (71), 105 (35), 101 (33), 86 (55) and 75 (78).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(4-ethoxycarbonylphenylethynyl)-1-azabicyclo[2.2.2]octane 24c.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and ethyl 4-iodobenzoate (148 mg, 0.54 mmol, 1.5 eq.) to yield 4-ethoxycarbonylphenyl-substituted alkyne 24c (91%, 139 mg, 0.33 mmol); νmax(CHCl3)/cm−1 2952, 2880, 2856, 2220, 1712, 1604, 1508, 1464, 1404, 1368, 1344, 1276, 1176, 1108, 1052, 1020, 940, 856 and 836; δH(400 MHz; CDCl3) 7.97–7.95 (d, 2 H, J 8.4, H-14, H-16), 7.45–7.42 (d, 2 H, J 8.4, H-13, H-17), 4.40–4.34 (q, 2 H, J 7.1, H-19, H-19), 3.79–3.76 (m, 2 H, H-9, H-9), 3.19–3.13 (m, 2 H, H-6, H-6), 3.05–2.83 (m, 3 H, H-2, H-7, H-7), 2.78–2.73 (m, 1 H, H-5), 2.09–2.05 (m, 1 H, H-4), 1.89–1.83 (m, 1 H, H-3), 1.71–1.58 (m, 3 H, H-8, H-8, H-3), 1.41–1.37 (t, 3 H, J 7.1, H-20), 0.89 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.06 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 166.12 (C, C-18), 135.74 (C, C-15), 131.49 (CH, C-14, C-16), 129.35 (CH, C-14, C-16), 128.44 (C, C-12), 96.03 (C, C-10), 81.15 (C, C-11), 65.21 (CH2, C-9), 61.05 (CH2, C-19), 57.38 (CH, C-2), 49.69 (CH2, C-6), 49.02 (CH2, C-7), 29.08 (CH, C-5), 27.83 (CH, C-4), 26.00 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(4-ethoxycarbonylphenylethynyl)-1-azabicyclo[2.2.2]octane 24c.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and ethyl 4-iodobenzoate (148 mg, 0.54 mmol, 1.5 eq.) to yield 4-ethoxycarbonylphenyl-substituted alkyne 24c (91%, 139 mg, 0.33 mmol); νmax(CHCl3)/cm−1 2952, 2880, 2856, 2220, 1712, 1604, 1508, 1464, 1404, 1368, 1344, 1276, 1176, 1108, 1052, 1020, 940, 856 and 836; δH(400 MHz; CDCl3) 7.97–7.95 (d, 2 H, J 8.4, H-14, H-16), 7.45–7.42 (d, 2 H, J 8.4, H-13, H-17), 4.40–4.34 (q, 2 H, J 7.1, H-19, H-19), 3.79–3.76 (m, 2 H, H-9, H-9), 3.19–3.13 (m, 2 H, H-6, H-6), 3.05–2.83 (m, 3 H, H-2, H-7, H-7), 2.78–2.73 (m, 1 H, H-5), 2.09–2.05 (m, 1 H, H-4), 1.89–1.83 (m, 1 H, H-3), 1.71–1.58 (m, 3 H, H-8, H-8, H-3), 1.41–1.37 (t, 3 H, J 7.1, H-20), 0.89 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.06 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 166.12 (C, C-18), 135.74 (C, C-15), 131.49 (CH, C-14, C-16), 129.35 (CH, C-14, C-16), 128.44 (C, C-12), 96.03 (C, C-10), 81.15 (C, C-11), 65.21 (CH2, C-9), 61.05 (CH2, C-19), 57.38 (CH, C-2), 49.69 (CH2, C-6), 49.02 (CH2, C-7), 29.08 (CH, C-5), 27.83 (CH, C-4), 26.00 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.26 (CH2, C-8), 25.09 (CH2, C-3), 18.44 (C, SiC(CH3)3), 14.31 (CH3, C-20), −5.23 (CH3, SiC
H3)3), 25.26 (CH2, C-8), 25.09 (CH2, C-3), 18.44 (C, SiC(CH3)3), 14.31 (CH3, C-20), −5.23 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.26 (CH3, SiC
H3) and −5.26 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 428 (M+ + H, 100), 370 (45), 355 (14), 281 (22), 267 (10), 221 (35) and 147 (53).
H3); m/z (FAB) (EI) 428 (M+ + H, 100), 370 (45), 355 (14), 281 (22), 267 (10), 221 (35) and 147 (53).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(4-formylphenylethynyl)-1-azabicyclo[2.2.2]octane 24d.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 4-bromobenzaldehyde (100 mg, 0.54 mmol, 1.5 eq.) to yield p-formylphenyl-substituted alkyne 24d (82%, 113 mg, 0.29 mmol); νmax(CHCl3)/cm−1 2952, 2928, 2880, 2856, 2220, 1700, 1600, 1560, 1460, 1392, 1360, 1344, 1320, 1256, 1164, 1116, 1100, 1008 and 836; δH(400 MHz; CDCl3) 10.01 (s, 1 H, H-18), 7.84–7.81 (d, 2 H, J 8.5, H-14, H-16), 7.56–7.53 (d, 2 H, J 8.2, H-13, H-17), 3.91–3.76 (m, 2 H, H-9, H-9), 3.31–3.28 (d, 1 H, J 13.2, H-6endo), 3.18–3.15 (d, 1 H, J 13.1, H-6exo), 3.12–3.03 (m, 1 H, H-2), 2.99–2.82 (m, 2 H, H-7, H-7), 2.66–2.61 (m, 1 H, H-5), 2.17–2.13 (m, 1 H, H-4), 2.08–1.97 (m, 1 H, H-3), 1.78–1.57 (m, 3 H, H-8, H-8, H-3), 0.89 (s, 9 H, SiC(CH3)3), 0.09 (s, 3 H, SiCH3) and 0.07 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 191.41 (C, C-18), 135.20 (C, C-15), 132.19 (CH, C-14, C-16), 129.96 (C, C-12), 129.49 (CH, C-14, C-16), 96.82 (C, C-10), 81.34 (C, C-11), 64.59 (CH2, C-9), 57.20 (CH, C-2), 49.09 (CH2, C-6), 48.49 (CH2, C-7), 28.65 (CH, C-5), 27.79 (CH, C-4), 25.97 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(4-formylphenylethynyl)-1-azabicyclo[2.2.2]octane 24d.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 4-bromobenzaldehyde (100 mg, 0.54 mmol, 1.5 eq.) to yield p-formylphenyl-substituted alkyne 24d (82%, 113 mg, 0.29 mmol); νmax(CHCl3)/cm−1 2952, 2928, 2880, 2856, 2220, 1700, 1600, 1560, 1460, 1392, 1360, 1344, 1320, 1256, 1164, 1116, 1100, 1008 and 836; δH(400 MHz; CDCl3) 10.01 (s, 1 H, H-18), 7.84–7.81 (d, 2 H, J 8.5, H-14, H-16), 7.56–7.53 (d, 2 H, J 8.2, H-13, H-17), 3.91–3.76 (m, 2 H, H-9, H-9), 3.31–3.28 (d, 1 H, J 13.2, H-6endo), 3.18–3.15 (d, 1 H, J 13.1, H-6exo), 3.12–3.03 (m, 1 H, H-2), 2.99–2.82 (m, 2 H, H-7, H-7), 2.66–2.61 (m, 1 H, H-5), 2.17–2.13 (m, 1 H, H-4), 2.08–1.97 (m, 1 H, H-3), 1.78–1.57 (m, 3 H, H-8, H-8, H-3), 0.89 (s, 9 H, SiC(CH3)3), 0.09 (s, 3 H, SiCH3) and 0.07 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 191.41 (C, C-18), 135.20 (C, C-15), 132.19 (CH, C-14, C-16), 129.96 (C, C-12), 129.49 (CH, C-14, C-16), 96.82 (C, C-10), 81.34 (C, C-11), 64.59 (CH2, C-9), 57.20 (CH, C-2), 49.09 (CH2, C-6), 48.49 (CH2, C-7), 28.65 (CH, C-5), 27.79 (CH, C-4), 25.97 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.94 (CH2, C-8), 24.79 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.33 (CH3, SiC
H3)3), 25.94 (CH2, C-8), 24.79 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.33 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.36 (CH3, SiC
H3) and −5.36 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT, 80 °C) (EI) 383.2279 (M+, C23H33N1O2Si requires 383.2281), 326 (3%), 280 (4), 264 (4), 238 (3), 222 (41), 202 (14), 184 (45), 156 (100), 135 (6), 110 (4), 83 (28) and 75 (64).
H3); m/z (MAT, 80 °C) (EI) 383.2279 (M+, C23H33N1O2Si requires 383.2281), 326 (3%), 280 (4), 264 (4), 238 (3), 222 (41), 202 (14), 184 (45), 156 (100), 135 (6), 110 (4), 83 (28) and 75 (64).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(3-hydroxyphenylethynyl)-1-azabicyclo[2.2.2]octane 24e.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-iodophenol (119 mg, 0.54 mmol, 1.5 eq.) to yield m-hydroxyphenyl-substituted alkyne 24e (89%, 118 mg, 0.32 mmol); νmax(CHCl3)/cm−1 2952, 2932, 2880, 2856, 2220, 1592, 1452, 1388, 1360, 1344, 1288, 1256, 1156, 1116, 1096, 1052, 1024 and 836; δH(400 MHz; CDCl3) 7.14–7.09 (m, 1 H, H-16), 6.89 (m, 1 H, H-15), 6.82 (s, 1 H, H-13), 6.76 (m, 1 H, H-17), 3.88–3.83 (dd, 1 H, J 10.5 and 6.9, H-9), 3.77–3.72 (dd, 1 H, J 10.5 and 6.0, H-9), 3.26–3.18 (m, 2 H, H-6, H-6), 3.08–2.99 (m, 2 H, H-2, H-7), 2.96–2.86 (m, 1 H, H-7), 2.82–2.76 (m, 1 H, H-5), 2.12–2.08 (m, 1 H, H-4), 1.88–1.80 (m, 1 H, H-3), 1.77–1.60 (m, 3 H, H-8, H-8, H-3), 0.87 (s, 9 H, SiC(CH3)3), 0.05 (s, 3 H, SiCH3) and 0.02 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 157.00 (C, C-14), 129.30 (CH, C-15), 124.28 (C, C-12), 122.79 (CH, C-13), 118.99 (CH, C-16), 116.02 (CH, C-17), 91.27 (C, C-10), 82.12 (C, C-11), 64.31 (CH2, C-9), 57.66 (CH, C-2), 49.44 (CH2, C-6), 48.49 (CH2, C-7), 28.80 (CH, C-5), 27.62 (CH, C-4), 26.05 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(3-hydroxyphenylethynyl)-1-azabicyclo[2.2.2]octane 24e.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 3-iodophenol (119 mg, 0.54 mmol, 1.5 eq.) to yield m-hydroxyphenyl-substituted alkyne 24e (89%, 118 mg, 0.32 mmol); νmax(CHCl3)/cm−1 2952, 2932, 2880, 2856, 2220, 1592, 1452, 1388, 1360, 1344, 1288, 1256, 1156, 1116, 1096, 1052, 1024 and 836; δH(400 MHz; CDCl3) 7.14–7.09 (m, 1 H, H-16), 6.89 (m, 1 H, H-15), 6.82 (s, 1 H, H-13), 6.76 (m, 1 H, H-17), 3.88–3.83 (dd, 1 H, J 10.5 and 6.9, H-9), 3.77–3.72 (dd, 1 H, J 10.5 and 6.0, H-9), 3.26–3.18 (m, 2 H, H-6, H-6), 3.08–2.99 (m, 2 H, H-2, H-7), 2.96–2.86 (m, 1 H, H-7), 2.82–2.76 (m, 1 H, H-5), 2.12–2.08 (m, 1 H, H-4), 1.88–1.80 (m, 1 H, H-3), 1.77–1.60 (m, 3 H, H-8, H-8, H-3), 0.87 (s, 9 H, SiC(CH3)3), 0.05 (s, 3 H, SiCH3) and 0.02 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 157.00 (C, C-14), 129.30 (CH, C-15), 124.28 (C, C-12), 122.79 (CH, C-13), 118.99 (CH, C-16), 116.02 (CH, C-17), 91.27 (C, C-10), 82.12 (C, C-11), 64.31 (CH2, C-9), 57.66 (CH, C-2), 49.44 (CH2, C-6), 48.49 (CH2, C-7), 28.80 (CH, C-5), 27.62 (CH, C-4), 26.05 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.02 (CH2, C-8), 24.78 (CH2, C-3), 18.44 (C, SiC(CH3)3), −5.29 (CH3, SiC
H3)3), 25.02 (CH2, C-8), 24.78 (CH2, C-3), 18.44 (C, SiC(CH3)3), −5.29 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.34 (CH3, SiC
H3) and −5.34 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT, 80 °C) (EI) 371 (M+, 6%), 356 (4), 328 (3), 314 (100), 301 (7), 290 (43), 278 (5), 226 (1), 129 (2), 115 (1) and 86 (3).
H3); m/z (MAT, 80 °C) (EI) 371 (M+, 6%), 356 (4), 328 (3), 314 (100), 301 (7), 290 (43), 278 (5), 226 (1), 129 (2), 115 (1) and 86 (3).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(1,3-thiazolin-2-ylethynyl)-1-azabicyclo[2.2.2]octane 24f.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiazole (48 μl, 0.54 mmol, 1.5 eq.) to yield thiazolinyl-substituted alkyne 24f (83%, 108 mg, 0.30 mmol); νmax(CHCl3)/cm−1 2952, 2928, 2880, 2856, 2220, 1480, 1472, 1408, 1360, 1344, 1320, 1264, 1136, 1116, 1096, 1056, 1020, 936 and 836; δH(400 MHz; CDCl3) 7.78 (d, 1 H, J 3.3, H-14), 7.30 (d, 1 H, J 3.4, H-15), 3.79–3.70 (m, 2 H, H-9, H-9), 3.18–3.15 (m, 2 H, H-6, H-6), 3.05–2.96 (m, 1 H, H-2), 2.94–2.82 (m, 2 H, H-7, H-7), 2.80–2.75 (m, 1 H, H-5), 2.13–2.09 (m, 1 H, H-4), 1.84–1.77 (m, 1 H, H-3), 1.70–1.56 (m, 3 H, H-8, H-8, H-3), 0.89 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.06 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 149.18 (C, C-12), 143.14 (CH, C-14), 119.96 (CH, C-15), 98.39 (C, C-10), 79.91 (C, C-11), 65.13 (CH2, C-9), 57.37 (CH, C-2), 49.00 (CH2, C-6), 48.92 (CH2, C-7), 29.19 (CH, C-5), 27.58 (CH, C-4), 26.04 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(1,3-thiazolin-2-ylethynyl)-1-azabicyclo[2.2.2]octane 24f.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiazole (48 μl, 0.54 mmol, 1.5 eq.) to yield thiazolinyl-substituted alkyne 24f (83%, 108 mg, 0.30 mmol); νmax(CHCl3)/cm−1 2952, 2928, 2880, 2856, 2220, 1480, 1472, 1408, 1360, 1344, 1320, 1264, 1136, 1116, 1096, 1056, 1020, 936 and 836; δH(400 MHz; CDCl3) 7.78 (d, 1 H, J 3.3, H-14), 7.30 (d, 1 H, J 3.4, H-15), 3.79–3.70 (m, 2 H, H-9, H-9), 3.18–3.15 (m, 2 H, H-6, H-6), 3.05–2.96 (m, 1 H, H-2), 2.94–2.82 (m, 2 H, H-7, H-7), 2.80–2.75 (m, 1 H, H-5), 2.13–2.09 (m, 1 H, H-4), 1.84–1.77 (m, 1 H, H-3), 1.70–1.56 (m, 3 H, H-8, H-8, H-3), 0.89 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.06 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 149.18 (C, C-12), 143.14 (CH, C-14), 119.96 (CH, C-15), 98.39 (C, C-10), 79.91 (C, C-11), 65.13 (CH2, C-9), 57.37 (CH, C-2), 49.00 (CH2, C-6), 48.92 (CH2, C-7), 29.19 (CH, C-5), 27.58 (CH, C-4), 26.04 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.71 (CH2, C-8), 25.16 (CH2, C-3), 18.44 (C, SiC(CH3)3), −5.24 (CH3, SiC
H3)3), 25.71 (CH2, C-8), 25.16 (CH2, C-3), 18.44 (C, SiC(CH3)3), −5.24 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.27 (CH3, SiC
H3) and −5.27 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT, 50 °C) (EI) 362.1848 (M+, C19H30N2O1S1Si requires 362.1848), 348 (3%), 321 (1), 305 (33), 280 (10), 264 (9), 240 (11), 222 (100), 195 (2), 184 (8), 156 (12), 134 (16), 110 (10), 91 (7), 82 (7) and 75 (42).
H3); m/z (MAT, 50 °C) (EI) 362.1848 (M+, C19H30N2O1S1Si requires 362.1848), 348 (3%), 321 (1), 305 (33), 280 (10), 264 (9), 240 (11), 222 (100), 195 (2), 184 (8), 156 (12), 134 (16), 110 (10), 91 (7), 82 (7) and 75 (42).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(2-thienylethynyl)-1-azabicyclo[2.2.2]octane 24g.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiophene (52 μl, 0.54 mmol, 1.5 eq.) to yield thiophenyl-substituted alkyne 24g (80%, 104 mg, 0.29 mmol); νmax(CHCl3)/cm−1 2948, 2928, 2880, 2856, 2220, 1468, 1388, 1360, 1340, 1320, 1256, 1116, 1080, 1052, 1020, 936 and 908; δH(400 MHz; CDCl3) 7.21 (dd, 1 H, J 5.2 and 1.2, H-15), 7.14 (dd, 1 H, J 3.6 and 1.0, H-13), 6.97 (dd, 1 H, J 5.2 and 3.6, H-14), 3.82–3.67 (m, 2 H, H-9, H-9), 3.15–3.09 (m, 1 H, H-6), 3.06–3.01 (m, 1 H, H-6), 2.98–2.72 (m, 3 H, H-2, H-7, H-7), 2.58–2.54 (m, 1 H, H-5), 1.99–1.95 (m, 1 H, H-4), 1.82–1.72 (m, 1 H, H-3), 1.69–1.49 (m, 3 H, H-8, H-8, H-3), 0.93 (s, 9 H, SiC(CH3)3), 0.10 (s, 3 H, SiCH3) and 0.09 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 131.13 (CH, C-15), 126.76 (CH, C-14), 126.06 (CH, C-13), 123.96 (C, C-12), 96.86 (C, C-10), 80.73 (C, C-11), 65.37 (CH2, C-9), 57.37 (CH, C-2), 49.33 (CH2, C-6), 48.96 (CH2, C-7), 29.08 (CH, C-5), 27.81 (CH, C-4), 26.07 (CH3, SiC(C
)-2-tert-Butyldimethylsilanyloxymethyl-5-(2-thienylethynyl)-1-azabicyclo[2.2.2]octane 24g.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and 2-bromothiophene (52 μl, 0.54 mmol, 1.5 eq.) to yield thiophenyl-substituted alkyne 24g (80%, 104 mg, 0.29 mmol); νmax(CHCl3)/cm−1 2948, 2928, 2880, 2856, 2220, 1468, 1388, 1360, 1340, 1320, 1256, 1116, 1080, 1052, 1020, 936 and 908; δH(400 MHz; CDCl3) 7.21 (dd, 1 H, J 5.2 and 1.2, H-15), 7.14 (dd, 1 H, J 3.6 and 1.0, H-13), 6.97 (dd, 1 H, J 5.2 and 3.6, H-14), 3.82–3.67 (m, 2 H, H-9, H-9), 3.15–3.09 (m, 1 H, H-6), 3.06–3.01 (m, 1 H, H-6), 2.98–2.72 (m, 3 H, H-2, H-7, H-7), 2.58–2.54 (m, 1 H, H-5), 1.99–1.95 (m, 1 H, H-4), 1.82–1.72 (m, 1 H, H-3), 1.69–1.49 (m, 3 H, H-8, H-8, H-3), 0.93 (s, 9 H, SiC(CH3)3), 0.10 (s, 3 H, SiCH3) and 0.09 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 131.13 (CH, C-15), 126.76 (CH, C-14), 126.06 (CH, C-13), 123.96 (C, C-12), 96.86 (C, C-10), 80.73 (C, C-11), 65.37 (CH2, C-9), 57.37 (CH, C-2), 49.33 (CH2, C-6), 48.96 (CH2, C-7), 29.08 (CH, C-5), 27.81 (CH, C-4), 26.07 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.69 (CH2, C-8), 25.16 (CH2, C-3), 18.47 (C, SiC(CH3)3), −5.21 (CH3, SiC
H3)3), 25.69 (CH2, C-8), 25.16 (CH2, C-3), 18.47 (C, SiC(CH3)3), −5.21 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.23 (CH3, SiC
H3) and −5.23 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT, 80 °C) (EI) 361.1885 (M+, C20H31N1O1S1Si requires 361.1895), 346 (8%), 318 (3), 304 (100), 276 (2), 264 (4), 240 (9), 216 (58), 203 (3), 184 (9), 156 (16), 134 (21), 115 (91), 94 (9), 85 (49) and 73 (36).
H3); m/z (MAT, 80 °C) (EI) 361.1885 (M+, C20H31N1O1S1Si requires 361.1895), 346 (8%), 318 (3), 304 (100), 276 (2), 264 (4), 240 (9), 216 (58), 203 (3), 184 (9), 156 (16), 134 (21), 115 (91), 94 (9), 85 (49) and 73 (36).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(2-(E
)-2-tert-Butyldimethylsilanyloxymethyl-5-(2-(E![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-chlorovinylethynyl)-1-azabicyclo[2.2.2]octane 24h.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and (E
)-chlorovinylethynyl)-1-azabicyclo[2.2.2]octane 24h.
10,11-Didehydroquincoridine 20b (100 mg, 0.36 mmol, 1 eq.) was allowed to react with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-1,2-dichloroethene (27 μl, 0.36 mmol, 1 eq.) in Pri2NH–THF (3∶1) to yield (E
)-1,2-dichloroethene (27 μl, 0.36 mmol, 1 eq.) in Pri2NH–THF (3∶1) to yield (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-chlorovinyl-substituted alkyne 24h (78%, 95 mg, 0.28 mmol); νmax(CHCl3)/cm−1 2936, 2880, 2856, 2210, 1468, 1380, 1360, 1320, 1256, 1228, 1176, 1116, 1092, 1052, 1024, 1004, 936, 916 and 836; δH(400 MHz; CDCl3) 6.45 (dd, 1 H, J 13.6 and 0.8, H-13), 5.94 (dd, 1 H, J 13.6 and 2.2, H-12), 3.74–3.70 (dd, 1 H, J 10.2 and 6.0, H-9), 3.69–3.64 (dd, 1 H, J 10.3 and 6.8, H-9), 3.08–2.80 (m, 2 H, H-6, H-6), 2.69–2.65 (m, 1 H, H-2), 2.61–2.51 (m, 2 H, H-7, H-7), 2.43–2.37 (m, 1 H, H-5), 1.94–1.90 (m, 1 H, H-4), 1.74–1.68 (m, 1 H, H-3), 1.63–1.50 (m, 3 H, H-8, H-8, H-3), 0.91 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.07 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 129.02 (CH, C-13), 114.13 (CH, C-12), 95.96 (C, C-10), 77.39 (C, C-11), 65.34 (CH2, C-9), 57.23 (CH, C-2), 49.63 (CH2, C-6), 48.99 (CH2, C-7), 29.12 (CH, C-5), 27.75 (CH, C-4), 25.99 (CH3, SiC(C
)-chlorovinyl-substituted alkyne 24h (78%, 95 mg, 0.28 mmol); νmax(CHCl3)/cm−1 2936, 2880, 2856, 2210, 1468, 1380, 1360, 1320, 1256, 1228, 1176, 1116, 1092, 1052, 1024, 1004, 936, 916 and 836; δH(400 MHz; CDCl3) 6.45 (dd, 1 H, J 13.6 and 0.8, H-13), 5.94 (dd, 1 H, J 13.6 and 2.2, H-12), 3.74–3.70 (dd, 1 H, J 10.2 and 6.0, H-9), 3.69–3.64 (dd, 1 H, J 10.3 and 6.8, H-9), 3.08–2.80 (m, 2 H, H-6, H-6), 2.69–2.65 (m, 1 H, H-2), 2.61–2.51 (m, 2 H, H-7, H-7), 2.43–2.37 (m, 1 H, H-5), 1.94–1.90 (m, 1 H, H-4), 1.74–1.68 (m, 1 H, H-3), 1.63–1.50 (m, 3 H, H-8, H-8, H-3), 0.91 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.07 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 129.02 (CH, C-13), 114.13 (CH, C-12), 95.96 (C, C-10), 77.39 (C, C-11), 65.34 (CH2, C-9), 57.23 (CH, C-2), 49.63 (CH2, C-6), 48.99 (CH2, C-7), 29.12 (CH, C-5), 27.75 (CH, C-4), 25.99 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.08 (CH2, C-8), 24.25 (CH2, C-3), 18.45 (C, SiC(CH3)3), −5.25 (CH3, SiC
H3)3), 25.08 (CH2, C-8), 24.25 (CH2, C-3), 18.45 (C, SiC(CH3)3), −5.25 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.29 (CH3, SiC
H3) and −5.29 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT) (EI) 339.1786 (M+, C18H30N1O1SiCl requires 339.1785), 324 (9%), 304 (11), 282 (100), 269 (1), 240 (18), 222 (4), 194 (22), 170 (8), 132 (7), 115 (8), 98 (32) and 73 (31).
H3); m/z (MAT) (EI) 339.1786 (M+, C18H30N1O1SiCl requires 339.1785), 324 (9%), 304 (11), 282 (100), 269 (1), 240 (18), 222 (4), 194 (22), 170 (8), 132 (7), 115 (8), 98 (32) and 73 (31).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-tert-Butyldimethylsilanyloxymethyl-5-(2-(Z
)-2-tert-Butyldimethylsilanyloxymethyl-5-(2-(Z![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-chlorovinylethynyl)-1-azabicyclo[2.2.2]octane 24i.
10,11-Didehydroquincoridine 20b (165 mg, 0.59 mmol, 1 eq.) was allowed to react with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (11 mg, 0.06 mmol, 0.1 eq.) and (Z
)-chlorovinylethynyl)-1-azabicyclo[2.2.2]octane 24i.
10,11-Didehydroquincoridine 20b (165 mg, 0.59 mmol, 1 eq.) was allowed to react with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (11 mg, 0.06 mmol, 0.1 eq.) and (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-1,2-dichloroethene (86 mg, 0.89 mmol, 1.5 eq.) in piperidine–THF (3∶1) to yield (Z
)-1,2-dichloroethene (86 mg, 0.89 mmol, 1.5 eq.) in piperidine–THF (3∶1) to yield (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-chlorovinyl-substituted alkyne 24i (83%, 166 mg, 0.49 mmol); νmax(CHCl3)/cm−1 2948, 2880, 2856, 2208, 1464, 1388, 1360, 1324, 1256, 1116, 1084, 1052, 1020, 936, 912 and 836; δH(400 MHz; CDCl3) 6.33 (dd, 1 H, J 7.4 and 0.5, H-13), 5.94 (dd, 1 H, J 7.4 and 2.2, H-12), 3.77–3.72 (dd, 1 H, J 10.2 and 6.2, H-9), 3.68–3.63 (dd, 1 H, J 10.3 and 7.1, H-9), 3.12–2.96 (m, 2 H, H-6, H-6), 2.94–2.76 (m, 3 H, H-2, H-7, H-7), 2.68–2.63 (m, 1 H, H-5), 2.00–1.96 (m, 1 H, H-4), 1.79–1.72 (m, 1 H, H-3), 1.63–1.49 (m, 3 H, H-8, H-8, H-3), 0.90 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.07 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 127.25 (CH, C-13), 112.31 (CH, C-12), 96.05 (C, C-10), 75.84 (C, C-11), 65.42 (CH2, C-9), 57.30 (CH, C-2), 49.77 (CH2, C-6), 48.96 (CH2, C-7), 29.19 (CH, C-5), 27.82 (CH, C-4), 25.98 (CH3, SiC(C
)-chlorovinyl-substituted alkyne 24i (83%, 166 mg, 0.49 mmol); νmax(CHCl3)/cm−1 2948, 2880, 2856, 2208, 1464, 1388, 1360, 1324, 1256, 1116, 1084, 1052, 1020, 936, 912 and 836; δH(400 MHz; CDCl3) 6.33 (dd, 1 H, J 7.4 and 0.5, H-13), 5.94 (dd, 1 H, J 7.4 and 2.2, H-12), 3.77–3.72 (dd, 1 H, J 10.2 and 6.2, H-9), 3.68–3.63 (dd, 1 H, J 10.3 and 7.1, H-9), 3.12–2.96 (m, 2 H, H-6, H-6), 2.94–2.76 (m, 3 H, H-2, H-7, H-7), 2.68–2.63 (m, 1 H, H-5), 2.00–1.96 (m, 1 H, H-4), 1.79–1.72 (m, 1 H, H-3), 1.63–1.49 (m, 3 H, H-8, H-8, H-3), 0.90 (s, 9 H, SiC(CH3)3), 0.08 (s, 3 H, SiCH3) and 0.07 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 127.25 (CH, C-13), 112.31 (CH, C-12), 96.05 (C, C-10), 75.84 (C, C-11), 65.42 (CH2, C-9), 57.30 (CH, C-2), 49.77 (CH2, C-6), 48.96 (CH2, C-7), 29.19 (CH, C-5), 27.82 (CH, C-4), 25.98 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.02 (CH2, C-8), 24.36 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.31 (CH3, SiC
H3)3), 25.02 (CH2, C-8), 24.36 (CH2, C-3), 18.39 (C, SiC(CH3)3), −5.31 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.34 (CH3, SiC
H3) and −5.34 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT) (EI) 339.1784 (M+, C18H30NOSiCl requires 339.1785), 324 (9%), 304 (11), 282 (100), 261 (1), 249 (1), 240 (20), 194 (22), 170 (7), 131 (7), 116 (8), 98 (14) and 73 (30).
H3); m/z (MAT) (EI) 339.1784 (M+, C18H30NOSiCl requires 339.1785), 324 (9%), 304 (11), 282 (100), 261 (1), 249 (1), 240 (20), 194 (22), 170 (7), 131 (7), 116 (8), 98 (14) and 73 (30).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(Z
)-9-Acetoxy-11-(Z![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-phenylethynyl-6′-methoxycinchonan 25a.
(Z
)-phenylethynyl-6′-methoxycinchonan 25a.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (92 mg, 0.19 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.1 eq.), CuI (7 mg, 0.04 mmol, 0.2 eq.) and phenylacetylene (19 mg, 0.19 mmol, 1 eq.) to yield (Z
)-11-(2-Iodovinyl)quinidine 11b (92 mg, 0.19 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.1 eq.), CuI (7 mg, 0.04 mmol, 0.2 eq.) and phenylacetylene (19 mg, 0.19 mmol, 1 eq.) to yield (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enyne 25a (86%, 74 mg, 0.16 mmol); νmax(CHCl3)/cm−1 2940, 2876, 1744, 1664, 1620, 1592, 1508, 1472, 1456, 1372, 1356, 1304, 1236, 1176, 1120, 1084, 1068, 1028, 988 and 844; δH(400 MHz; CDCl3) 8.81 (d, 1 H, J 4.6, H-2′), 8.12 (d, 1 H, J 9.2, H-8′), 7.74 (dd, 1 H, J 9.2 and 2.4, H-7′), 7.61 (m, 2 H, H-3′, H-5′), 7.54–7.43 (m, 2 H, Ph-H), 7.37–7.33 (m, 3 H, Ph-H), 6.64 (d, 1 H, J 6.8, H-9), 6.61–6.57 (dd, 1 H, J 9.7 and 8.6, H-10), 6.40–6.38 (d, 1 H, J 10.1, H-11), 3.99 (s, 3 H, H-11′), 3.78–3.69 (m, 1 H, H-8), 3.44–3.34 (m, 1 H, H-2), 3.24–3.12 (m, 1 H, H-2), 3.08–2.96 (m, 2 H, H-6, H-6), 2.89–2.85 (m, 1 H, H-3), 2.21 (s, 3 H, H-21), 2.01–1.88 (m, 2 H, H-4, H-7) and 1.74–1.57 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 169.99 (C, C-20), 158.03 (C, C-6′), 145.99 (CH, C-2′), 144.02 (C, C-10′), 143.62 (C, C-4′), 132.14 (CH, C-15, C-19), 131.94 (CH, C-8′), 131.38 (CH, C-17), 129.21 (CH, C-10), 128.56 (CH, C-16, C-18), 128.24 (C, C-9′), 123.33 (C, C-14), 121.83 (CH, C-7′), 118.05 (CH, C-3′), 110.07 (CH, C-11), 101.59 (CH, C-5′), 93.96 (C, C-13), 86.26 (C, C-12), 73.58 (CH, C-9), 58.44 (CH, C-8), 55.68 (CH3, C-11′), 49.70 (CH2, C-2), 47.23 (CH2, C-6), 29.69 (CH, C-3), 27.90 (CH, C-4), 26.24 (CH2, C-7), 25.31 (CH2, C-5) and 21.17 (CH3, C-21); m/z (FAB) (EI) 467 (M+ + H, 28%), 429 (7), 401 (10), 355 (26), 341 (20), 325 (13), 295 (16), 281 (52), 221 (78), 207 (51) and 147 (100).
)-enyne 25a (86%, 74 mg, 0.16 mmol); νmax(CHCl3)/cm−1 2940, 2876, 1744, 1664, 1620, 1592, 1508, 1472, 1456, 1372, 1356, 1304, 1236, 1176, 1120, 1084, 1068, 1028, 988 and 844; δH(400 MHz; CDCl3) 8.81 (d, 1 H, J 4.6, H-2′), 8.12 (d, 1 H, J 9.2, H-8′), 7.74 (dd, 1 H, J 9.2 and 2.4, H-7′), 7.61 (m, 2 H, H-3′, H-5′), 7.54–7.43 (m, 2 H, Ph-H), 7.37–7.33 (m, 3 H, Ph-H), 6.64 (d, 1 H, J 6.8, H-9), 6.61–6.57 (dd, 1 H, J 9.7 and 8.6, H-10), 6.40–6.38 (d, 1 H, J 10.1, H-11), 3.99 (s, 3 H, H-11′), 3.78–3.69 (m, 1 H, H-8), 3.44–3.34 (m, 1 H, H-2), 3.24–3.12 (m, 1 H, H-2), 3.08–2.96 (m, 2 H, H-6, H-6), 2.89–2.85 (m, 1 H, H-3), 2.21 (s, 3 H, H-21), 2.01–1.88 (m, 2 H, H-4, H-7) and 1.74–1.57 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 169.99 (C, C-20), 158.03 (C, C-6′), 145.99 (CH, C-2′), 144.02 (C, C-10′), 143.62 (C, C-4′), 132.14 (CH, C-15, C-19), 131.94 (CH, C-8′), 131.38 (CH, C-17), 129.21 (CH, C-10), 128.56 (CH, C-16, C-18), 128.24 (C, C-9′), 123.33 (C, C-14), 121.83 (CH, C-7′), 118.05 (CH, C-3′), 110.07 (CH, C-11), 101.59 (CH, C-5′), 93.96 (C, C-13), 86.26 (C, C-12), 73.58 (CH, C-9), 58.44 (CH, C-8), 55.68 (CH3, C-11′), 49.70 (CH2, C-2), 47.23 (CH2, C-6), 29.69 (CH, C-3), 27.90 (CH, C-4), 26.24 (CH2, C-7), 25.31 (CH2, C-5) and 21.17 (CH3, C-21); m/z (FAB) (EI) 467 (M+ + H, 28%), 429 (7), 401 (10), 355 (26), 341 (20), 325 (13), 295 (16), 281 (52), 221 (78), 207 (51) and 147 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(Z
)-9-Acetoxy-11-(Z![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-hept-1-ynyl-6′-methoxycinchonan 25b.
(Z
)-hept-1-ynyl-6′-methoxycinchonan 25b.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (110 mg, 0.22 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (8 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and hept-1-yne (38 μl, 0.29 mmol, 1.3 eq.) to yield (Z
)-11-(2-Iodovinyl)quinidine 11b (110 mg, 0.22 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (8 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and hept-1-yne (38 μl, 0.29 mmol, 1.3 eq.) to yield (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enyne 25b (82%, 84 mg, 0.18 mmol); νmax(CHCl3)/cm−1 2936, 2872, 1744, 1664, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1304, 1236, 1172, 1112, 1084, 1068, 1032, 988 and 844; δH(400 MHz; CDCl3) 8.84 (d, 1 H, J 4.4, H-2′), 8.16 (d, 1 H, J 9.2, H-8′), 7.43–7.37 (m, 3 H, H-7′, H-3′, H-5′), 6.61 (d, 1 H, J 6.8, H-9), 6.17–6.13 (dd, 1 H, J 10.7 and 8.1, H-10), 5.61–5.57 (d, 1 H, J 10.8, H-11), 3.98 (s, 3 H, H-11′), 3.77–3.68 (m, 1 H, H-8), 3.39–3.29 (m, 1 H, H-2endo), 3.13–3.05 (dd, 1 H, J 15.6 and 12.7, H-2exo), 2.94–2.78 (m, 2 H, H-6, H-6), 2.38–2.34 (m, 1 H, H-3), 2.18 (s, 3 H, H-20), 1.92–1.85 (m, 3 H, H-4, H-14, H-14), 1.64–1.53 (m, 4 H, 2 H-7, 2 H-15), 1.45–1.28 (m, 6 H, 2 H-5, 2 H-16, 2 H-17) and 0.98–0.90 (t, 3 H, J 7.1, H-18); δC(100 MHz; CDCl3) 169.99 (C, C-19), 158.01 (C, C-6′), 147.67 (CH, C-2′), 144.09 (C, C-10′), 143.94 (C, C-4′), 131.77 (CH, C-8′), 128.75 (CH, C-10), 128.46 (C, C-9′), 121.96 (CH, C-7′), 118.55 (CH, C-3′), 110.48 (CH, C-11), 101.41 (CH, C-5′), 95.15 (C, C-13), 84.59 (C, C-12), 73.50 (CH, C-9), 58.85 (CH, C-8), 55.67 (CH3, C-11′), 49.88 (CH2, C-2), 49.74 (CH2, C-6), 36.68 (CH, C-3), 31.07 (CH2, C-16), 28.45 (CH2, C-15), 27.72 (CH, C-4), 26.17 (CH2, C-7), 23.62 (CH2, C-5), 22.19 (CH2, C-17), 21.16 (CH3, C-20), 19.50 (CH2, C-14) and 14.01 (CH3, C-18); m/z (FAB) (EI) 461 (M+ + H, 100%), 401 (29), 282 (21), 230 (83), 207 (30), 189 (35) and 147 (72).
)-enyne 25b (82%, 84 mg, 0.18 mmol); νmax(CHCl3)/cm−1 2936, 2872, 1744, 1664, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1304, 1236, 1172, 1112, 1084, 1068, 1032, 988 and 844; δH(400 MHz; CDCl3) 8.84 (d, 1 H, J 4.4, H-2′), 8.16 (d, 1 H, J 9.2, H-8′), 7.43–7.37 (m, 3 H, H-7′, H-3′, H-5′), 6.61 (d, 1 H, J 6.8, H-9), 6.17–6.13 (dd, 1 H, J 10.7 and 8.1, H-10), 5.61–5.57 (d, 1 H, J 10.8, H-11), 3.98 (s, 3 H, H-11′), 3.77–3.68 (m, 1 H, H-8), 3.39–3.29 (m, 1 H, H-2endo), 3.13–3.05 (dd, 1 H, J 15.6 and 12.7, H-2exo), 2.94–2.78 (m, 2 H, H-6, H-6), 2.38–2.34 (m, 1 H, H-3), 2.18 (s, 3 H, H-20), 1.92–1.85 (m, 3 H, H-4, H-14, H-14), 1.64–1.53 (m, 4 H, 2 H-7, 2 H-15), 1.45–1.28 (m, 6 H, 2 H-5, 2 H-16, 2 H-17) and 0.98–0.90 (t, 3 H, J 7.1, H-18); δC(100 MHz; CDCl3) 169.99 (C, C-19), 158.01 (C, C-6′), 147.67 (CH, C-2′), 144.09 (C, C-10′), 143.94 (C, C-4′), 131.77 (CH, C-8′), 128.75 (CH, C-10), 128.46 (C, C-9′), 121.96 (CH, C-7′), 118.55 (CH, C-3′), 110.48 (CH, C-11), 101.41 (CH, C-5′), 95.15 (C, C-13), 84.59 (C, C-12), 73.50 (CH, C-9), 58.85 (CH, C-8), 55.67 (CH3, C-11′), 49.88 (CH2, C-2), 49.74 (CH2, C-6), 36.68 (CH, C-3), 31.07 (CH2, C-16), 28.45 (CH2, C-15), 27.72 (CH, C-4), 26.17 (CH2, C-7), 23.62 (CH2, C-5), 22.19 (CH2, C-17), 21.16 (CH3, C-20), 19.50 (CH2, C-14) and 14.01 (CH3, C-18); m/z (FAB) (EI) 461 (M+ + H, 100%), 401 (29), 282 (21), 230 (83), 207 (30), 189 (35) and 147 (72).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(Z
)-9-Acetoxy-11-(Z![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-(5-hydroxypent-1-ynyl)-6′-methoxycinchonan 25c.
(Z
)-(5-hydroxypent-1-ynyl)-6′-methoxycinchonan 25c.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (110 mg, 0.22 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (8 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and pent-4-yn-1-ol (20 μl, 0.22 mmol, 1 eq.) to yield (Z
)-11-(2-Iodovinyl)quinidine 11b (110 mg, 0.22 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (8 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and pent-4-yn-1-ol (20 μl, 0.22 mmol, 1 eq.) to yield (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enyne 25c (78%, 78 mg, 0.17 mmol); νmax(CHCl3)/cm−1 3624, 2940, 2876, 2212, 1744, 1664, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1228, 1172, 1136, 1032, 988 and 844; δH(400 MHz; CDCl3) 8.75 (d, 1 H, J 4.6, H-2′), 8.06 (d, 1 H, J 9.0, H-8′), 7.42–7.40 (m, 1 H, H-7′), 7.39 (d, 1 H, J 2.7, H-5′), 7.38 (d, 1 H, J 4.6, H-3′), 6.59 (d, 1 H, J 6.4, H-9), 6.18–6.13 (dd, 1 H, J 10.6 and 8.4, H-10), 5.59–5.56 (d, 1 H, J 10.5, H-11), 3.99 (s, 3 H, H-11′), 3.79–3.71 (m, 2 H, H-16, H-16), 3.35–3.28 (m, 1 H, H-8), 3.11–3.04 (dd, 1 H, J 12.7 and 9.7, H-2endo), 2.92–2.78 (m, 3 H, H-2exo, H-6, H-6), 2.55–2.39 (m, 3 H, H-3, H-14, H-14), 2.18 (s, 3 H, H-18), 1.92–1.85 (m, 1 H, H-4), 1.84–1.76 (m, 2 H, H-15, H-15), 1.67–1.49 (m, 3 H, H-7, H-7, H-5) and 1.38–1.30 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.98 (C, C-17), 158.04 (C, C-6′), 147.27 (CH, C-2′), 144.55 (C, C-10′), 144.41 (CH, C-10), 143.76 (C, C-4′), 131.61 (CH, C-8′), 126.94 (C, C-9′), 122.01 (CH, C-7′), 118.46 (CH, C-3′), 110.31 (CH, C-11), 101.39 (CH, C-5′), 94.28 (C, C-13), 84.11 (C, C-12), 73.57 (CH, C-9), 61.37 (CH2, C-16), 58.76 (CH, C-8), 55.71 (CH3, C-11′), 49.79 (CH2, C-2), 49.64 (CH2, C-6), 36.67 (CH, C-3), 31.54 (CH2, C-15), 27.72 (CH, C-4), 26.09 (CH2, C-7), 23.74 (CH2, C-5), 21.16 (CH3, C-18) and 16.15 (CH2, C-14); m/z (FAB) (EI) 449 (M+ + H, 73%), 413 (12), 391 (23), 279 (8), 167 (19) and 149 (100).
)-enyne 25c (78%, 78 mg, 0.17 mmol); νmax(CHCl3)/cm−1 3624, 2940, 2876, 2212, 1744, 1664, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1228, 1172, 1136, 1032, 988 and 844; δH(400 MHz; CDCl3) 8.75 (d, 1 H, J 4.6, H-2′), 8.06 (d, 1 H, J 9.0, H-8′), 7.42–7.40 (m, 1 H, H-7′), 7.39 (d, 1 H, J 2.7, H-5′), 7.38 (d, 1 H, J 4.6, H-3′), 6.59 (d, 1 H, J 6.4, H-9), 6.18–6.13 (dd, 1 H, J 10.6 and 8.4, H-10), 5.59–5.56 (d, 1 H, J 10.5, H-11), 3.99 (s, 3 H, H-11′), 3.79–3.71 (m, 2 H, H-16, H-16), 3.35–3.28 (m, 1 H, H-8), 3.11–3.04 (dd, 1 H, J 12.7 and 9.7, H-2endo), 2.92–2.78 (m, 3 H, H-2exo, H-6, H-6), 2.55–2.39 (m, 3 H, H-3, H-14, H-14), 2.18 (s, 3 H, H-18), 1.92–1.85 (m, 1 H, H-4), 1.84–1.76 (m, 2 H, H-15, H-15), 1.67–1.49 (m, 3 H, H-7, H-7, H-5) and 1.38–1.30 (m, 1 H, H-5); δC(100 MHz; CDCl3) 169.98 (C, C-17), 158.04 (C, C-6′), 147.27 (CH, C-2′), 144.55 (C, C-10′), 144.41 (CH, C-10), 143.76 (C, C-4′), 131.61 (CH, C-8′), 126.94 (C, C-9′), 122.01 (CH, C-7′), 118.46 (CH, C-3′), 110.31 (CH, C-11), 101.39 (CH, C-5′), 94.28 (C, C-13), 84.11 (C, C-12), 73.57 (CH, C-9), 61.37 (CH2, C-16), 58.76 (CH, C-8), 55.71 (CH3, C-11′), 49.79 (CH2, C-2), 49.64 (CH2, C-6), 36.67 (CH, C-3), 31.54 (CH2, C-15), 27.72 (CH, C-4), 26.09 (CH2, C-7), 23.74 (CH2, C-5), 21.16 (CH3, C-18) and 16.15 (CH2, C-14); m/z (FAB) (EI) 449 (M+ + H, 73%), 413 (12), 391 (23), 279 (8), 167 (19) and 149 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-hept-1-ynyl-6′-methoxycinchonan 26.
(Z
)-hept-1-ynyl-6′-methoxycinchonan 26.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinine 12 (100 mg, 0.20 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (7 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and hept-1-yne (37 μl, 0.28 mmol, 1.4 eq.) to yield (Z
)-11-(2-Iodovinyl)quinine 12 (100 mg, 0.20 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (7 mg, 0.01 mmol, 0.05 eq.), CuI (4 mg, 0.02 mmol, 0.1 eq.) and hept-1-yne (37 μl, 0.28 mmol, 1.4 eq.) to yield (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enyne 26 (86%, 81 mg, 0.17 mmol); νmax(CHCl3)/cm−1 2936, 2869, 1743, 1622, 1593, 1510, 1474, 1434, 1372, 1265, 1238, 1086, 1031 and 850; δH(400 MHz; CDCl3) 8.75 (d, 1 H, J 4.4, H-2′), 8.03 (d, 1 H, J 9.2, H-8′), 7.44 (d, 1 H, J 2.8, H-5′), 7.37 (dd, 1 H, J 9.3 and 2.6, H-7′), 7.36 (d, 1 H, J 4.6, H-3′), 6.53 (d, 1 H, J 6.9, H-9), 5.85 (dd, 1 H, J 10.5 and 9.3, H-10), 5.44 (dd, 1 H, J 10.5 and 1.0, H-11), 3.97 (s, 3 H, H-11′), 3.38 (ddd, 1 H, J 8.8, 7.7 and 7.6, H-8), 3.17 (dd, 1 H, J 13.7 and 10.0, H-2exo), 3.16–3.08 (m, 1 H, H-6endo), 2.91–2.83 (m, 1 H, H-2endo), 2.74–2.66 (m, 1 H, H-6exo), 2.57–2.50 (m, 1 H, H-3), 2.31 (m, 2 H, H-14, H-14), 2.13 (s, 3 H, H-20), 1.92 (m, 1 H, H-4), 1.89–1.81 (m, 1 H, H-7), 1.77–1.68 (m, 1 H, H-7), 1.65–1.49 (m, 4 H, 2 H-5, 2 H-15), 1.41–1.27 (m, 4 H, 2 H-16, 2 H-17) and 0.90 (t, 3 H, J 7.2, H-18); δC(100 MHz; CDCl3) 170.00 (C, C-19), 157.99 (C, C-6′), 147.47 (CH, C-2′), 145.24 (C, C-10′), 144.76 (C, C-4′), 131.92 (CH, C-8′), 130.95 (CH, C-10), 127.04 (C, C-9′), 121.83 (CH, C-7′), 118.78 (CH, C-3′), 110.18 (CH, C-11), 101.47 (CH, C-5′), 95.28 (C, C-13), 77.19 (C, C-12), 73.64 (CH, C-9), 59.29 (CH, C-8), 57.70 (CH2, C-2), 55.71 (CH3, C-11′), 42.38 (CH2, C-6), 36.66 (CH, C-3), 31.05 (CH2, C-16), 28.44 (CH2, C-15), 27.52 (CH2, C-5), 27.05 (CH, C-4), 24.48 (CH2, C-7), 22.20 (CH2, C-17), 21.10 (CH3, C-20), 19.49 (CH2, C-14) and 14.02 (CH3, C-18); m/z (MAT, 140 °C) (EI) 460.2726 (M+ + H, C29H36N2O3 requires 460.2725), 412 (4%), 367 (5), 308 (37), 294 (3), 277 (100), 230 (29), 201 (19), 183 (14), 152 (8) and 91 (35).
)-enyne 26 (86%, 81 mg, 0.17 mmol); νmax(CHCl3)/cm−1 2936, 2869, 1743, 1622, 1593, 1510, 1474, 1434, 1372, 1265, 1238, 1086, 1031 and 850; δH(400 MHz; CDCl3) 8.75 (d, 1 H, J 4.4, H-2′), 8.03 (d, 1 H, J 9.2, H-8′), 7.44 (d, 1 H, J 2.8, H-5′), 7.37 (dd, 1 H, J 9.3 and 2.6, H-7′), 7.36 (d, 1 H, J 4.6, H-3′), 6.53 (d, 1 H, J 6.9, H-9), 5.85 (dd, 1 H, J 10.5 and 9.3, H-10), 5.44 (dd, 1 H, J 10.5 and 1.0, H-11), 3.97 (s, 3 H, H-11′), 3.38 (ddd, 1 H, J 8.8, 7.7 and 7.6, H-8), 3.17 (dd, 1 H, J 13.7 and 10.0, H-2exo), 3.16–3.08 (m, 1 H, H-6endo), 2.91–2.83 (m, 1 H, H-2endo), 2.74–2.66 (m, 1 H, H-6exo), 2.57–2.50 (m, 1 H, H-3), 2.31 (m, 2 H, H-14, H-14), 2.13 (s, 3 H, H-20), 1.92 (m, 1 H, H-4), 1.89–1.81 (m, 1 H, H-7), 1.77–1.68 (m, 1 H, H-7), 1.65–1.49 (m, 4 H, 2 H-5, 2 H-15), 1.41–1.27 (m, 4 H, 2 H-16, 2 H-17) and 0.90 (t, 3 H, J 7.2, H-18); δC(100 MHz; CDCl3) 170.00 (C, C-19), 157.99 (C, C-6′), 147.47 (CH, C-2′), 145.24 (C, C-10′), 144.76 (C, C-4′), 131.92 (CH, C-8′), 130.95 (CH, C-10), 127.04 (C, C-9′), 121.83 (CH, C-7′), 118.78 (CH, C-3′), 110.18 (CH, C-11), 101.47 (CH, C-5′), 95.28 (C, C-13), 77.19 (C, C-12), 73.64 (CH, C-9), 59.29 (CH, C-8), 57.70 (CH2, C-2), 55.71 (CH3, C-11′), 42.38 (CH2, C-6), 36.66 (CH, C-3), 31.05 (CH2, C-16), 28.44 (CH2, C-15), 27.52 (CH2, C-5), 27.05 (CH, C-4), 24.48 (CH2, C-7), 22.20 (CH2, C-17), 21.10 (CH3, C-20), 19.49 (CH2, C-14) and 14.02 (CH3, C-18); m/z (MAT, 140 °C) (EI) 460.2726 (M+ + H, C29H36N2O3 requires 460.2725), 412 (4%), 367 (5), 308 (37), 294 (3), 277 (100), 230 (29), 201 (19), 183 (14), 152 (8) and 91 (35).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-2-(Hydroxymethyl)-5-phenylethynyl-1-azabicyclo[2.2.2]oct-5-ene 27.
Vinyltin precursor 15 (152 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and phenylacetylene (55 mg, 0.54 mmol, 1.5 eq.) to yield enyne 27 (69%, 59 mg, 0.25 mmol); νmax(CHCl3)/cm−1 3590, 2999, 2954, 2876, 2220, 1599, 1447, 1409, 1330, 1260, 1138, 1067 and 1024; δH(400 MHz; CDCl3; CD3OD) 7.41–7.37 (m, 2 H, Ar-H), 7.36–7.31 (m, 3 H, Ar-H), 6.68 (s, 1 H, H-6), 3.89–3.82 (m, 1 H, H-9), 3.76–3.69 (m, 1 H, H-9), 3.64–3.56 (m, 1 H, H-7), 3.30–3.04 (m, 2 H, H-7, H-2), 2.39–2.24 (m, 2 H, H-4, H-3), 2.01–1.89 (m, 1 H, H-8) and 1.73–1.64 (m, 2 H, H-8, H-3); δC(100 MHz; CDCl3, CD3OD) 131.54 (CH, Ph), 131.17 (C, C-5), 128.96 (CH, C-6), 128.74 (CH, Ph), 121.82 (C, Ph), 90.05 (C, C-10), 83.97 (C, C-11), 62.14 (CH2, C-9), 58.41 (CH, C-2), 43.85 (CH2, C-7), 34.07 (CH, C-4), 21.39 (CH2, C-8) and 20.54 (CH2, C-3); m/z (MAT, 180 °C) (EI) 239.1308 (M+, C16H17N1O1 requires 239.1310), 226 (100%), 208 (21), 198 (33), 180 (30), 171 (17), 155 (52), 140 (17), 127 (22), 113 (23) and 96 (36).
)-2-(Hydroxymethyl)-5-phenylethynyl-1-azabicyclo[2.2.2]oct-5-ene 27.
Vinyltin precursor 15 (152 mg, 0.36 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (13 mg, 0.02 mmol, 0.05 eq.), CuI (7 mg, 0.04 mmol, 0.1 eq.) and phenylacetylene (55 mg, 0.54 mmol, 1.5 eq.) to yield enyne 27 (69%, 59 mg, 0.25 mmol); νmax(CHCl3)/cm−1 3590, 2999, 2954, 2876, 2220, 1599, 1447, 1409, 1330, 1260, 1138, 1067 and 1024; δH(400 MHz; CDCl3; CD3OD) 7.41–7.37 (m, 2 H, Ar-H), 7.36–7.31 (m, 3 H, Ar-H), 6.68 (s, 1 H, H-6), 3.89–3.82 (m, 1 H, H-9), 3.76–3.69 (m, 1 H, H-9), 3.64–3.56 (m, 1 H, H-7), 3.30–3.04 (m, 2 H, H-7, H-2), 2.39–2.24 (m, 2 H, H-4, H-3), 2.01–1.89 (m, 1 H, H-8) and 1.73–1.64 (m, 2 H, H-8, H-3); δC(100 MHz; CDCl3, CD3OD) 131.54 (CH, Ph), 131.17 (C, C-5), 128.96 (CH, C-6), 128.74 (CH, Ph), 121.82 (C, Ph), 90.05 (C, C-10), 83.97 (C, C-11), 62.14 (CH2, C-9), 58.41 (CH, C-2), 43.85 (CH2, C-7), 34.07 (CH, C-4), 21.39 (CH2, C-8) and 20.54 (CH2, C-3); m/z (MAT, 180 °C) (EI) 239.1308 (M+, C16H17N1O1 requires 239.1310), 226 (100%), 208 (21), 198 (33), 180 (30), 171 (17), 155 (52), 140 (17), 127 (22), 113 (23) and 96 (36).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-11,11″-Bi(10,11-didehydro-9-hydroxy-6′-methoxycinchonan) 28a.
10,11-Didehydroquinidine 9a (161 mg, 0.50 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (18 mg, 0.025 mmol, 0.05 eq.), CuI (10 mg, 0.05 mmol, 0.1 eq.) and iodine (64 mg, 0.25 mmol, 0.5 eq.) to yield dimeric alkyne 28a (71%, 114 mg, 0.18 mmol); νmax(CHCl3)/cm−1 3132, 2944, 2872, 1620, 1592, 1508, 1472, 1432, 1360, 1320, 1300, 1240, 1174, 1136, 1092, 1032, 1000, 932 and 832; δH(400 MHz; CDCl3) 8.43 (d, 2 H, J 4.6, H-2′, H-2‴), 7.91 (d, 2 H, J 9.3, H-8′, H-8‴), 7.43 (d, 2 H, J 4.6, H-3′, H-3‴), 7.31 (dd, 2 H, J 9.3 and 2.6, H-7′, H-7‴), 7.18 (d, 2 H, J 2.6, H-5′, H-5‴), 5.68 (m, 2 H, H-9, H-9″), 3.89 (s, 6 H, H-11′, H-11‴), 3.69–3.61 (m, 2 H, H-8, H-8″), 3.19–3.12 (m, 2 H, H-2, H-2″), 2.91–2.84 (m, 2 H, H-2, H-2″), 2.75–2.51 (m, 4 H, H-6, H-6, H-6″, H-6″), 2.01–1.97 (m, 2 H, H-3, H-3″), 1.88–1.82 (m, 2 H, H-4, H-4″), 1.88–1.64 (m, 6 H, H-5, H-7, H-7, H-5″, H-7″, H-7″) and 1.41–1.33 (m, 2 H, H-5, H-5″); δC(100 MHz; CDCl3) 157.77 (C, C-6′, C-6‴), 148.31 (C, C-10′, C-10‴), 147.43 (CH, C-2′, C-2‴), 143.73 (C, C-4′, C-4‴), 131.14 (CH, C-8′, C-8‴), 124.81 (C, C-9′, C-9‴), 121.36 (CH, C-7′, C-7‴), 119.07 (CH, C-3′, C-3‴), 101.32 (CH, C-5′, C-5‴), 81.28 (C, C-10, C-10″), 77.27 (CH, C-9, C-9″), 66.22 (C, C-11, C-11″), 60.39 (CH, C-8, C-8″), 55.95 (CH3, C-11′, C-11‴), 49.76 (CH2, C-2, C-2″), 49.33 (CH2, C-6, C-6″), 29.69 (CH, C-3, C-3″), 28.79 (CH, C-4, C-4″), 24.91 (CH2, C-7, C-7″) and 21.04 (CH2, C-5, C-5″); m/z (FAB) (EI) 643 (M+ + H, 5%), 356 (10), 341 (11), 295 (9), 281 (41), 267 (19), 207 (48), 189 (28), 159 (29) and 147 (100).
)-11,11″-Bi(10,11-didehydro-9-hydroxy-6′-methoxycinchonan) 28a.
10,11-Didehydroquinidine 9a (161 mg, 0.50 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (18 mg, 0.025 mmol, 0.05 eq.), CuI (10 mg, 0.05 mmol, 0.1 eq.) and iodine (64 mg, 0.25 mmol, 0.5 eq.) to yield dimeric alkyne 28a (71%, 114 mg, 0.18 mmol); νmax(CHCl3)/cm−1 3132, 2944, 2872, 1620, 1592, 1508, 1472, 1432, 1360, 1320, 1300, 1240, 1174, 1136, 1092, 1032, 1000, 932 and 832; δH(400 MHz; CDCl3) 8.43 (d, 2 H, J 4.6, H-2′, H-2‴), 7.91 (d, 2 H, J 9.3, H-8′, H-8‴), 7.43 (d, 2 H, J 4.6, H-3′, H-3‴), 7.31 (dd, 2 H, J 9.3 and 2.6, H-7′, H-7‴), 7.18 (d, 2 H, J 2.6, H-5′, H-5‴), 5.68 (m, 2 H, H-9, H-9″), 3.89 (s, 6 H, H-11′, H-11‴), 3.69–3.61 (m, 2 H, H-8, H-8″), 3.19–3.12 (m, 2 H, H-2, H-2″), 2.91–2.84 (m, 2 H, H-2, H-2″), 2.75–2.51 (m, 4 H, H-6, H-6, H-6″, H-6″), 2.01–1.97 (m, 2 H, H-3, H-3″), 1.88–1.82 (m, 2 H, H-4, H-4″), 1.88–1.64 (m, 6 H, H-5, H-7, H-7, H-5″, H-7″, H-7″) and 1.41–1.33 (m, 2 H, H-5, H-5″); δC(100 MHz; CDCl3) 157.77 (C, C-6′, C-6‴), 148.31 (C, C-10′, C-10‴), 147.43 (CH, C-2′, C-2‴), 143.73 (C, C-4′, C-4‴), 131.14 (CH, C-8′, C-8‴), 124.81 (C, C-9′, C-9‴), 121.36 (CH, C-7′, C-7‴), 119.07 (CH, C-3′, C-3‴), 101.32 (CH, C-5′, C-5‴), 81.28 (C, C-10, C-10″), 77.27 (CH, C-9, C-9″), 66.22 (C, C-11, C-11″), 60.39 (CH, C-8, C-8″), 55.95 (CH3, C-11′, C-11‴), 49.76 (CH2, C-2, C-2″), 49.33 (CH2, C-6, C-6″), 29.69 (CH, C-3, C-3″), 28.79 (CH, C-4, C-4″), 24.91 (CH2, C-7, C-7″) and 21.04 (CH2, C-5, C-5″); m/z (FAB) (EI) 643 (M+ + H, 5%), 356 (10), 341 (11), 295 (9), 281 (41), 267 (19), 207 (48), 189 (28), 159 (29) and 147 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-11,11″-Bi(9-acetoxy-10,11-didehydro-6′-methoxycinchonan) 28b.
10,11-Didehydroquinidine 9a (100 mg, 0.27 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (10 mg, 0.02 mmol, 0.05 eq.), CuI (5 mg, 0.03 mmol, 0.1 eq.) and iodine (35 mg, 0.14 mmol, 0.5 eq.) to yield dimeric alkyne 28b (86%, 86 mg, 0.12 mmol); νmax(CHCl3)/cm−1 2948, 2876, 1744, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1320, 1300, 1228, 1136, 1092, 1032, 988 and 844; δH(400 MHz; CDCl3) 8.81 (d, 2 H, J 4.4, H-2′, H-2‴), 8.11 (d, 2 H, J 9.4, H-8′, H-8‴), 7.48 (d, 2 H, J 2.7, H-5′, H-5‴), 7.43–7.40 (m, 4 H, H-3′, H-3‴, H-7′, H-7‴), 6.61 (d, 2 H, J 6.3, H-9, H-9″), 4.02 (s, 6 H, H-11′, H-11‴), 3.37–3.30 (m, 2 H, H-8, H-8″), 3.21–3.07 (m, 4 H, H-2, H-2, H-2″, H-2″), 2.89–2.80 (m, 2 H, H-6, H-6″), 2.75–2.64 (m, 4 H, H-6, H-6″, H-3, H-3″), 2.24 (s, 6 H, H-13, H-13″), 2.13–2.09 (m, 2 H, H-4, H-4″), 1.67–1.49 (m, 6 H, H-5, H-7, H-7, H-5″, H-7″, H-7″) and 1.38–1.27 (m, 2 H, H-5, H-5″); δC(100 MHz; CDCl3) 169.84 (C, C-12, C-12″), 158.01 (C, C-6′, C-6‴), 147.53 (C, C-10′, C-10‴), 144.59 (CH, C-2′, C-2‴), 143.92 (C, C-4′, C-4‴), 131.81 (CH, C-8′, C-8‴), 126.89 (C, C-9′, C-9‴), 121.87 (CH, C-7′, C-7‴), 118.46 (CH, C-3′, C-3‴), 101.50 (CH, C-5′, C-5‴), 80.74 (C, C-10, C-10″), 73.92 (CH, C-9, C-9″), 66.39 (C, C-11, C-11″), 59.05 (CH, C-8, C-8″), 55.69 (CH3, C-11′, C-11‴), 49.98 (CH2, C-2, C-2″), 49.41 (CH2, C-6, C-6″), 28.95 (CH, C-3, C-3″), 27.91 (CH, C-4, C-4″), 24.96 (CH2, C-7, C-7″), 23.99 (CH2, C-5, C-5″) and 21.12 (CH3, C-13, C-13″); m/z (FAB) (EI) 727.1104 (M+, C44H46N4O6 requires 727.1118), 663 (9%), 496 (5), 391 (41), 279 (8) and 149 (86).
)-11,11″-Bi(9-acetoxy-10,11-didehydro-6′-methoxycinchonan) 28b.
10,11-Didehydroquinidine 9a (100 mg, 0.27 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (10 mg, 0.02 mmol, 0.05 eq.), CuI (5 mg, 0.03 mmol, 0.1 eq.) and iodine (35 mg, 0.14 mmol, 0.5 eq.) to yield dimeric alkyne 28b (86%, 86 mg, 0.12 mmol); νmax(CHCl3)/cm−1 2948, 2876, 1744, 1620, 1592, 1508, 1472, 1456, 1432, 1372, 1320, 1300, 1228, 1136, 1092, 1032, 988 and 844; δH(400 MHz; CDCl3) 8.81 (d, 2 H, J 4.4, H-2′, H-2‴), 8.11 (d, 2 H, J 9.4, H-8′, H-8‴), 7.48 (d, 2 H, J 2.7, H-5′, H-5‴), 7.43–7.40 (m, 4 H, H-3′, H-3‴, H-7′, H-7‴), 6.61 (d, 2 H, J 6.3, H-9, H-9″), 4.02 (s, 6 H, H-11′, H-11‴), 3.37–3.30 (m, 2 H, H-8, H-8″), 3.21–3.07 (m, 4 H, H-2, H-2, H-2″, H-2″), 2.89–2.80 (m, 2 H, H-6, H-6″), 2.75–2.64 (m, 4 H, H-6, H-6″, H-3, H-3″), 2.24 (s, 6 H, H-13, H-13″), 2.13–2.09 (m, 2 H, H-4, H-4″), 1.67–1.49 (m, 6 H, H-5, H-7, H-7, H-5″, H-7″, H-7″) and 1.38–1.27 (m, 2 H, H-5, H-5″); δC(100 MHz; CDCl3) 169.84 (C, C-12, C-12″), 158.01 (C, C-6′, C-6‴), 147.53 (C, C-10′, C-10‴), 144.59 (CH, C-2′, C-2‴), 143.92 (C, C-4′, C-4‴), 131.81 (CH, C-8′, C-8‴), 126.89 (C, C-9′, C-9‴), 121.87 (CH, C-7′, C-7‴), 118.46 (CH, C-3′, C-3‴), 101.50 (CH, C-5′, C-5‴), 80.74 (C, C-10, C-10″), 73.92 (CH, C-9, C-9″), 66.39 (C, C-11, C-11″), 59.05 (CH, C-8, C-8″), 55.69 (CH3, C-11′, C-11‴), 49.98 (CH2, C-2, C-2″), 49.41 (CH2, C-6, C-6″), 28.95 (CH, C-3, C-3″), 27.91 (CH, C-4, C-4″), 24.96 (CH2, C-7, C-7″), 23.99 (CH2, C-5, C-5″) and 21.12 (CH3, C-13, C-13″); m/z (FAB) (EI) 727.1104 (M+, C44H46N4O6 requires 727.1118), 663 (9%), 496 (5), 391 (41), 279 (8) and 149 (86).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,4-Bis(2-hydroxymethyl-1-azabicyclo[2.2.2]octan-5-yl)buta-1,3-diyne 30.
10,11-Didehydroquincorine 18a (100 mg, 0.61 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (12 mg, 0.06 mmol, 0.1 eq.) and iodine (77 mg, 0.30 mmol, 0.5 eq.) to yield dimeric alkyne 30 (66%, 66 mg, 0.20 mmol); νmax(CHCl3)/cm−1 3456, 3000, 2944, 2868, 1480, 1452, 1412, 1376, 1344, 1324, 1260, 1236, 1132, 1100, 1020, 996 and 936; δH(400 MHz; CDCl3) 3.52–3.44 (m, 4 H, H-9, H-9, H-9′, H-9′), 3.32–3.23 (m, 2 H, H-6, H-6′), 3.19–3.06 (m, 2 H, H-2, H-2′), 2.98–2.86 (m, 4 H, H-6, H-6′, H-7, H-7′), 2.68–2.53 (m, 4 H, H-7, H-7′, H-5, H-5′), 2.12–2.03 (m, 2 H, H-3, H-3′), 1.97–1.92 (m, 2 H, H-4, H-4′), 1.54–1.37 (m, 4 H, H-8, H-8, H-8′, H-8′) and 0.89–0.80 (m, 2 H, H-3, H-3′); δC(100 MHz; CDCl3) 81.18 (C, C-10, C-10′), 65.68 (C, C-11, C-11′), 62.71 (CH2, C-9, C-9′), 56.99 (CH, C-2, C-2′), 56.44 (CH2, C-6, C-6′), 39.59 (CH2, C-7, C-7′), 28.67 (CH, C-5, C-5′), 26.75 (CH, C-4, C-4′), 26.16 (CH2, C-8, C-8′) and 24.98 (CH2, C-3, C-3′); m/z (MAT, 170 °C) (EI) 328.2152 (M+, C20H28N2O2 requires 328.2151), 311 (7%), 297 (91), 270 (40), 255 (11), 239 (49), 212 (42), 196 (14), 184 (34), 166 (17), 112 (37) and 86 (81).
)-1,4-Bis(2-hydroxymethyl-1-azabicyclo[2.2.2]octan-5-yl)buta-1,3-diyne 30.
10,11-Didehydroquincorine 18a (100 mg, 0.61 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (12 mg, 0.06 mmol, 0.1 eq.) and iodine (77 mg, 0.30 mmol, 0.5 eq.) to yield dimeric alkyne 30 (66%, 66 mg, 0.20 mmol); νmax(CHCl3)/cm−1 3456, 3000, 2944, 2868, 1480, 1452, 1412, 1376, 1344, 1324, 1260, 1236, 1132, 1100, 1020, 996 and 936; δH(400 MHz; CDCl3) 3.52–3.44 (m, 4 H, H-9, H-9, H-9′, H-9′), 3.32–3.23 (m, 2 H, H-6, H-6′), 3.19–3.06 (m, 2 H, H-2, H-2′), 2.98–2.86 (m, 4 H, H-6, H-6′, H-7, H-7′), 2.68–2.53 (m, 4 H, H-7, H-7′, H-5, H-5′), 2.12–2.03 (m, 2 H, H-3, H-3′), 1.97–1.92 (m, 2 H, H-4, H-4′), 1.54–1.37 (m, 4 H, H-8, H-8, H-8′, H-8′) and 0.89–0.80 (m, 2 H, H-3, H-3′); δC(100 MHz; CDCl3) 81.18 (C, C-10, C-10′), 65.68 (C, C-11, C-11′), 62.71 (CH2, C-9, C-9′), 56.99 (CH, C-2, C-2′), 56.44 (CH2, C-6, C-6′), 39.59 (CH2, C-7, C-7′), 28.67 (CH, C-5, C-5′), 26.75 (CH, C-4, C-4′), 26.16 (CH2, C-8, C-8′) and 24.98 (CH2, C-3, C-3′); m/z (MAT, 170 °C) (EI) 328.2152 (M+, C20H28N2O2 requires 328.2151), 311 (7%), 297 (91), 270 (40), 255 (11), 239 (49), 212 (42), 196 (14), 184 (34), 166 (17), 112 (37) and 86 (81).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,4-Bis(2-hydroxymethyl-1-azabicyclo[2.2.2]octan-5-yl)buta-1,3-diyne 31a.
10,11-Didehydroquincoridine 20a (100 mg, 0.61 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (12 mg, 0.06 mmol, 0.1 eq.) and iodine (77 mg, 0.30 mmol, 0.5 eq.) to yield dimeric alkyne 31a (64%, 64 mg, 0.19 mmol); νmax(CHCl3)/cm−1 3412, 3000, 2948, 2876, 1452, 1412, 1376, 1320, 1296, 1252, 1236, 1188, 1136, 1100, 1060, 1028, 940 and 864; δH(400 MHz; CDCl3) 5.36–5.21 (s, 2 H, OH, OH′), 3.75–3.67 (dd, 2 H, J 12.2 and 10.3, H-9, H-9′), 3.56–3.52 (dd, 2 H, J 12.1 and 4.5, H-9, H-9′), 3.13–2.86 (m, 10 H, H-6, H-6′, H-2, H-2′, H-6, H-6′, H-7, H-7′, H-7, H-7′), 2.72–2.65 (m, 2 H, H-5, H-5′), 2.06–1.99 (m, 2 H, H-4, H-4′), 1.75–1.57 (m, 6 H, H-3, H-3′, H-8, H-8′, H-8, H-8′) and 1.51–1.45 (m, 2 H, H-3, H-3′); δC(100 MHz; CDCl3) 79.91 (C, C-10, C-10′), 66.43 (C, C-11, C-11′), 61.89 (CH2, C-9, C-9′), 57.86 (CH, C-2, C-2′), 48.06 (CH2, C-6, C-6′), 46.99 (CH2, C-7, C-7′), 28.72 (CH, C-5, C-5′), 27.29 (CH, C-4, C-4′), 24.86 (CH2, C-8, C-8′) and 24.07 (CH2, C-3, C-3′); m/z (MAT, 170 °C) (EI) 328.2143 (M+, C20H28N2O2 requires 328.2151), 311 (7%), 298 (62), 270 (22), 256 (9), 239 (18), 212 (12), 202 (22), 184 (26), 170 (9), 149 (11), 126 (23), 112 (21) and 82 (21).
)-1,4-Bis(2-hydroxymethyl-1-azabicyclo[2.2.2]octan-5-yl)buta-1,3-diyne 31a.
10,11-Didehydroquincoridine 20a (100 mg, 0.61 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (12 mg, 0.06 mmol, 0.1 eq.) and iodine (77 mg, 0.30 mmol, 0.5 eq.) to yield dimeric alkyne 31a (64%, 64 mg, 0.19 mmol); νmax(CHCl3)/cm−1 3412, 3000, 2948, 2876, 1452, 1412, 1376, 1320, 1296, 1252, 1236, 1188, 1136, 1100, 1060, 1028, 940 and 864; δH(400 MHz; CDCl3) 5.36–5.21 (s, 2 H, OH, OH′), 3.75–3.67 (dd, 2 H, J 12.2 and 10.3, H-9, H-9′), 3.56–3.52 (dd, 2 H, J 12.1 and 4.5, H-9, H-9′), 3.13–2.86 (m, 10 H, H-6, H-6′, H-2, H-2′, H-6, H-6′, H-7, H-7′, H-7, H-7′), 2.72–2.65 (m, 2 H, H-5, H-5′), 2.06–1.99 (m, 2 H, H-4, H-4′), 1.75–1.57 (m, 6 H, H-3, H-3′, H-8, H-8′, H-8, H-8′) and 1.51–1.45 (m, 2 H, H-3, H-3′); δC(100 MHz; CDCl3) 79.91 (C, C-10, C-10′), 66.43 (C, C-11, C-11′), 61.89 (CH2, C-9, C-9′), 57.86 (CH, C-2, C-2′), 48.06 (CH2, C-6, C-6′), 46.99 (CH2, C-7, C-7′), 28.72 (CH, C-5, C-5′), 27.29 (CH, C-4, C-4′), 24.86 (CH2, C-8, C-8′) and 24.07 (CH2, C-3, C-3′); m/z (MAT, 170 °C) (EI) 328.2143 (M+, C20H28N2O2 requires 328.2151), 311 (7%), 298 (62), 270 (22), 256 (9), 239 (18), 212 (12), 202 (22), 184 (26), 170 (9), 149 (11), 126 (23), 112 (21) and 82 (21).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,4-Bis(2-tert-butyldimethylsilanyloxymethyl-1-azabicyclo[2.2.2]octan-5-yl)buta-1,3-diyne 31b.
10,11-Didehydroquincoridine 20b (200 mg, 0.72 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (33 mg, 0.036 mmol, 0.05 eq.), CuI (14 mg, 0.07 mmol, 0.1 eq.) and iodine (182 mg, 0.31 mmol, 0.5 eq.) to yield dimeric alkyne 31b (85%, 169 mg, 0.30 mmol); νmax(CHCl3)/cm−1 2948, 2880, 2856, 1468, 1388, 1360, 1320, 1300, 1256, 1148, 1116, 1080, 1052, 1020, 936 and 836; δH(400 MHz; CDCl3) 3.77–3.67 (m, 4 H, H-9, H-9′, H-9, H-9′), 3.07–3.02 (m, 4 H, H-6, H-6′, H-2, H-2′), 2.99–2.92 (m, 2 H, H-6, H-6′), 2.89–2.74 (m, 4 H, H-7, H-7′, H-7, H-7′), 2.59–2.54 (m, 2 H, H-5, H-5′), 2.00–1.95 (m, 2 H, H-4, H-4′), 1.79–1.72 (m, 2 H, H-3, H-3′), 1.66–1.48 (m, 6 H, H-8, H-8, H-8′, H-8′, H-3, H-3′), 0.93 (s, 9 H, SiC(CH3)3), 0.92 (s, 9 H, SiC(CH3)3), 0.10 (s, 6 H, SiCH3) and 0.09 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 80.63 (C, C-10, C-10′), 66.19 (C, C-11, C-11′), 65.22 (CH2, C-9, C-9′), 57.30 (CH, C-2, C-2′), 49.18 (CH2, C-6, C-6′), 48.82 (CH2, C-7, C-7′), 29.02 (CH, C-5, C-5′), 27.82 (CH, C-4, C-4′), 26.03 (CH3, SiC(C
)-1,4-Bis(2-tert-butyldimethylsilanyloxymethyl-1-azabicyclo[2.2.2]octan-5-yl)buta-1,3-diyne 31b.
10,11-Didehydroquincoridine 20b (200 mg, 0.72 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (33 mg, 0.036 mmol, 0.05 eq.), CuI (14 mg, 0.07 mmol, 0.1 eq.) and iodine (182 mg, 0.31 mmol, 0.5 eq.) to yield dimeric alkyne 31b (85%, 169 mg, 0.30 mmol); νmax(CHCl3)/cm−1 2948, 2880, 2856, 1468, 1388, 1360, 1320, 1300, 1256, 1148, 1116, 1080, 1052, 1020, 936 and 836; δH(400 MHz; CDCl3) 3.77–3.67 (m, 4 H, H-9, H-9′, H-9, H-9′), 3.07–3.02 (m, 4 H, H-6, H-6′, H-2, H-2′), 2.99–2.92 (m, 2 H, H-6, H-6′), 2.89–2.74 (m, 4 H, H-7, H-7′, H-7, H-7′), 2.59–2.54 (m, 2 H, H-5, H-5′), 2.00–1.95 (m, 2 H, H-4, H-4′), 1.79–1.72 (m, 2 H, H-3, H-3′), 1.66–1.48 (m, 6 H, H-8, H-8, H-8′, H-8′, H-3, H-3′), 0.93 (s, 9 H, SiC(CH3)3), 0.92 (s, 9 H, SiC(CH3)3), 0.10 (s, 6 H, SiCH3) and 0.09 (s, 6 H, SiCH3); δC(100 MHz; CDCl3) 80.63 (C, C-10, C-10′), 66.19 (C, C-11, C-11′), 65.22 (CH2, C-9, C-9′), 57.30 (CH, C-2, C-2′), 49.18 (CH2, C-6, C-6′), 48.82 (CH2, C-7, C-7′), 29.02 (CH, C-5, C-5′), 27.82 (CH, C-4, C-4′), 26.03 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.22 (CH2, C-8, C-8′), 25.05 (CH2, C-3, C-3′), 18.45 (C, SiC(CH3)3), −5.23 (CH3, SiC
H3)3), 25.22 (CH2, C-8, C-8′), 25.05 (CH2, C-3, C-3′), 18.45 (C, SiC(CH3)3), −5.23 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.27 (CH3, SiC
H3) and −5.27 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (MAT, 150 °C) (EI) 556.3889 (M+, C32H56N2O2Si2 requires 556.3880), 541 (11%), 499 (100), 443 (3), 425 (3), 411 (21), 384 (20), 368 (7), 326 (72), 298 (2), 279 (9), 238 (26), 222 (85), 202 (53), 185 (37), 155 (17), 110 (14), 89 (8) and 73 (60).
H3); m/z (MAT, 150 °C) (EI) 556.3889 (M+, C32H56N2O2Si2 requires 556.3880), 541 (11%), 499 (100), 443 (3), 425 (3), 411 (21), 384 (20), 368 (7), 326 (72), 298 (2), 279 (9), 238 (26), 222 (85), 202 (53), 185 (37), 155 (17), 110 (14), 89 (8) and 73 (60).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-(Z
)-(Z![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Bis(2-tert-butyldimethylsilanyloxymethyl-1-azabicyclo[2.2.2]octan-5-ylethynyl)ethene 32.
10,11-Didehydroquincorine 18b (200 mg, 0.72 mmol, 1 eq.) was allowed to react with (Ph3P)2PdCl2 (33 mg, 0.036 mmol, 0.05 eq.), CuI (14 mg, 0.072 mmol, 0.1 eq.) and (Z
)-1,2-Bis(2-tert-butyldimethylsilanyloxymethyl-1-azabicyclo[2.2.2]octan-5-ylethynyl)ethene 32.
10,11-Didehydroquincorine 18b (200 mg, 0.72 mmol, 1 eq.) was allowed to react with (Ph3P)2PdCl2 (33 mg, 0.036 mmol, 0.05 eq.), CuI (14 mg, 0.072 mmol, 0.1 eq.) and (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-1,2-dichloroethene (27 μl, 0.36 mmol, 0.5 eq.) in Pri2NH–THF (3∶1) to yield (Z
)-1,2-dichloroethene (27 μl, 0.36 mmol, 0.5 eq.) in Pri2NH–THF (3∶1) to yield (Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enediyne 32 (79%, 144 mg, 0.25 mmol) which partially isomerized to the corresponding (E
)-enediyne 32 (79%, 144 mg, 0.25 mmol) which partially isomerized to the corresponding (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-isomer upon storage (1 d) in CHCl3 at rt (E∶Z = 18∶32); νmax(CHCl3)/cm−1 2928, 2856, 1468, 1320, 1264, 1116, 1084, 1028 and 836; δH(400 MHz; CDCl3) 6.34 (d, 2 H, Jcis 7.4, H-12, H-13), 5.89 (dd, 2 H, Jtrans 13.2 and 1.6, H-12, H-13), 3.69–3.65 (dd, 2 H, J 10.3 and 6.0, H-9′, H-9″), 3.63–3.59 (dd, 2 H, J 10.4 and 6.1, H-9′, H-9″), 3.24–3.17 (dd, 2 H, J 13.4 and 10.0, H-6endo′, H-6endo″), 3.07–2.87 (m, 4 H, H-2′, H-2″, H-6′, H-6″), 2.62–2.53 (m, 4 H, H-7′, H-7″, H-7′, H-7″), 2.32–2.27 (m, 2 H, H-5′, H-5″), 2.07–1.99 (m, 2 H, H-4′, H-4″), 1.96–1.91 (m, 2 H, H-3′, H-3″), 1.54–1.32 (m, 6 H, H-8′, H-8″, H-8′, H-8″, H-3′, H-3″), 0.89 (s, 18 H, SiC(CH3)3) and 0.06 (s, 12 H, SiCH3); δC(100 MHz; CDCl3) 112.41 (CH, C-12, C-13), 81.48 (C, C-10′, C-10″), 65.89 (C, C-11′, C-11″), 65.52 (CH2, C-9′, C-9″), 57.92 (CH, C-2′, C-2″), 57.02 (CH2, C-6′, C-6″), 41.70 (CH2, C-7′, C-7″), 29.30 (CH, C-5′, C-5″), 27.64 (CH, C-4′, C-4″), 26.01 (CH3, SiC(C
)-isomer upon storage (1 d) in CHCl3 at rt (E∶Z = 18∶32); νmax(CHCl3)/cm−1 2928, 2856, 1468, 1320, 1264, 1116, 1084, 1028 and 836; δH(400 MHz; CDCl3) 6.34 (d, 2 H, Jcis 7.4, H-12, H-13), 5.89 (dd, 2 H, Jtrans 13.2 and 1.6, H-12, H-13), 3.69–3.65 (dd, 2 H, J 10.3 and 6.0, H-9′, H-9″), 3.63–3.59 (dd, 2 H, J 10.4 and 6.1, H-9′, H-9″), 3.24–3.17 (dd, 2 H, J 13.4 and 10.0, H-6endo′, H-6endo″), 3.07–2.87 (m, 4 H, H-2′, H-2″, H-6′, H-6″), 2.62–2.53 (m, 4 H, H-7′, H-7″, H-7′, H-7″), 2.32–2.27 (m, 2 H, H-5′, H-5″), 2.07–1.99 (m, 2 H, H-4′, H-4″), 1.96–1.91 (m, 2 H, H-3′, H-3″), 1.54–1.32 (m, 6 H, H-8′, H-8″, H-8′, H-8″, H-3′, H-3″), 0.89 (s, 18 H, SiC(CH3)3) and 0.06 (s, 12 H, SiCH3); δC(100 MHz; CDCl3) 112.41 (CH, C-12, C-13), 81.48 (C, C-10′, C-10″), 65.89 (C, C-11′, C-11″), 65.52 (CH2, C-9′, C-9″), 57.92 (CH, C-2′, C-2″), 57.02 (CH2, C-6′, C-6″), 41.70 (CH2, C-7′, C-7″), 29.30 (CH, C-5′, C-5″), 27.64 (CH, C-4′, C-4″), 26.01 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.08 (CH2, C-8′, C-8″), 22.67 (CH2, C-3′, C-3″), 18.41 (C, SiC(CH3)3), −5.32 (CH3, SiC
H3)3), 25.08 (CH2, C-8′, C-8″), 22.67 (CH2, C-3′, C-3″), 18.41 (C, SiC(CH3)3), −5.32 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.35 (CH3, SiC
H3) and −5.35 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 584 (M+ + 2 H, 2), 557 (100), 542 (8), 500 (37), 411 (5), 310 (6) and 282 (16).
H3); m/z (FAB) (EI) 584 (M+ + 2 H, 2), 557 (100), 542 (8), 500 (37), 411 (5), 310 (6) and 282 (16).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,4-Bis(2-hydroxymethyl-1-azabicyclo[2.2.2]octan-5-ylethynyl)benzene 33.
10,11-Didehydroquincoridine 20a (100 mg, 0.61 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (12 mg, 0.06 mmol, 0.1 eq.) and 1,4–diiodobenzene (99 mg, 0.30 mmol, 0.5 eq.) to yield dimeric alkyne 33 (64%, 64 mg, 0.19 mmol); νmax(CHCl3)/cm−1 3416, 3000, 2948, 2876, 2224, 1484, 1464, 1412, 1388, 1324, 1256, 1236, 1160, 1136, 1100, 1056, 1028, 1008, 944 and 820; δH(400 MHz; CDCl3) 7.63 (d, 4 H, J 8.4, H-2, H-3, H-5, H-6), 5.42–5.33 (s, 2 H, OH, OH′), 3.73–3.56 (m, 4 H, H-9′, H-9″, H-9′, H-9″), 3.18–2.81 (m, 10 H, H-6′, H-6″, H-2′, H-2″, H-6′, H-6″, H-7′, H-7″, H-7′, H-7″), 2.69–2.61 (m, 2 H, H-5′, H-5″), 2.00–1.92 (m, 2 H, H-4′, H-4″), 1.79–1.52 (m, 6 H, H-3′, H-3″, H-8′, H-8″, H-8′, H-8″) and 1.46–1.33 (m, 2 H, H-3′, H-3″); δC(100 MHz; CDCl3) 133.17 (CH, C-2, C-3, C-5, C-6), 122.95 (C, C-1, C-4), 93.52 (C, C-10′, C-10″), 81.08 (C, C-11′, C-11″), 61.91 (CH2, C-9′, C-9″), 57.48 (CH, C-2′, C-2″), 48.35 (CH2, C-6′, C-6″), 47.76 (CH2, C-7′, C-7″), 29.11 (CH, C-5′, C-5″), 27.31 (CH, C-4′, C-4″), 24.16 (CH2, C-8′, C-8″) and 22.57 (CH2, C-3′, C-3″); m/z (FAB) (EI) 425 (M+ − 2 H + Na, 9%), 397 (18), 368 (100), 336 (14), 281 (15), 221 (20) and 147 (35).
)-1,4-Bis(2-hydroxymethyl-1-azabicyclo[2.2.2]octan-5-ylethynyl)benzene 33.
10,11-Didehydroquincoridine 20a (100 mg, 0.61 mmol, 1 eq.) was allowed to react according to the general procedure with (Ph3P)2PdCl2 (21 mg, 0.03 mmol, 0.05 eq.), CuI (12 mg, 0.06 mmol, 0.1 eq.) and 1,4–diiodobenzene (99 mg, 0.30 mmol, 0.5 eq.) to yield dimeric alkyne 33 (64%, 64 mg, 0.19 mmol); νmax(CHCl3)/cm−1 3416, 3000, 2948, 2876, 2224, 1484, 1464, 1412, 1388, 1324, 1256, 1236, 1160, 1136, 1100, 1056, 1028, 1008, 944 and 820; δH(400 MHz; CDCl3) 7.63 (d, 4 H, J 8.4, H-2, H-3, H-5, H-6), 5.42–5.33 (s, 2 H, OH, OH′), 3.73–3.56 (m, 4 H, H-9′, H-9″, H-9′, H-9″), 3.18–2.81 (m, 10 H, H-6′, H-6″, H-2′, H-2″, H-6′, H-6″, H-7′, H-7″, H-7′, H-7″), 2.69–2.61 (m, 2 H, H-5′, H-5″), 2.00–1.92 (m, 2 H, H-4′, H-4″), 1.79–1.52 (m, 6 H, H-3′, H-3″, H-8′, H-8″, H-8′, H-8″) and 1.46–1.33 (m, 2 H, H-3′, H-3″); δC(100 MHz; CDCl3) 133.17 (CH, C-2, C-3, C-5, C-6), 122.95 (C, C-1, C-4), 93.52 (C, C-10′, C-10″), 81.08 (C, C-11′, C-11″), 61.91 (CH2, C-9′, C-9″), 57.48 (CH, C-2′, C-2″), 48.35 (CH2, C-6′, C-6″), 47.76 (CH2, C-7′, C-7″), 29.11 (CH, C-5′, C-5″), 27.31 (CH, C-4′, C-4″), 24.16 (CH2, C-8′, C-8″) and 22.57 (CH2, C-3′, C-3″); m/z (FAB) (EI) 425 (M+ − 2 H + Na, 9%), 397 (18), 368 (100), 336 (14), 281 (15), 221 (20) and 147 (35).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-1,2-Bis(2-tert-butyldimethylsilanyloxymethyl-1-azabicyclo[2.2.2]octanyl)benzene 34.
Enediyne 32 (58 mg, 0.10 mmol) cycloaromatized upon refluxing in CHCl3 for 4 h furnishing aromatic dimer 34 (86%, 50 mg, 0.09 mmol); νmax(CHCl3)/cm−1 2952, 2928, 2856, 1592, 1468, 1388, 1360, 1332, 1304, 1256, 1120, 1084, 1028, 1004, 936 and 836; δH(400 MHz; CDCl3) 7.71–7.65 (m, 2 H, H-3, H-6), 7.51–7.46 (m, 2 H, H-4, H-5), 3.73–3.62 (m, 4 H, H-9′, H-9″, H-9′, H-9″), 3.31–3.25 (dd, 2 H, J 13.2 and 10.0, H-6endo′, H-6endo″), 3.12–2.90 (m, 4 H, H-2′, H-2″, H-6′, H-6″), 2.75–2.59 (m, 4 H, H-7′, H-7″, H-7′, H-7″), 2.40–2.36 (m, 2 H, H-5′, H-5″), 2.18–2.10 (m, 2 H, H-4′, H-4″), 2.03–1.95 (m, 2 H, H-3′, H-3″), 1.61–1.37 (m, 6 H, H-8′, H-8″, H-8′, H-8″, H-3′, H-3″), 0.92 (s, 18 H, SiC(CH3)3) and 0.09 (s, 12 H, SiCH3); δC(100 MHz; CDCl3) 132.17 (CH, C-3, C-6), 128.58 (CH, C-4, C-5), 127.25 (C, C-1, C-2), 65.86 (CH2, C-9′, C-9″), 57.72 (CH, C-2′, C-2″), 57.09 (CH2, C-6′, C-6″), 41.75 (CH2, C-7′, C-7″), 28.88 (CH, C-5′, C-5″), 27.59 (CH, C-4′, C-4″), 26.02 (CH3, SiC(C
)-1,2-Bis(2-tert-butyldimethylsilanyloxymethyl-1-azabicyclo[2.2.2]octanyl)benzene 34.
Enediyne 32 (58 mg, 0.10 mmol) cycloaromatized upon refluxing in CHCl3 for 4 h furnishing aromatic dimer 34 (86%, 50 mg, 0.09 mmol); νmax(CHCl3)/cm−1 2952, 2928, 2856, 1592, 1468, 1388, 1360, 1332, 1304, 1256, 1120, 1084, 1028, 1004, 936 and 836; δH(400 MHz; CDCl3) 7.71–7.65 (m, 2 H, H-3, H-6), 7.51–7.46 (m, 2 H, H-4, H-5), 3.73–3.62 (m, 4 H, H-9′, H-9″, H-9′, H-9″), 3.31–3.25 (dd, 2 H, J 13.2 and 10.0, H-6endo′, H-6endo″), 3.12–2.90 (m, 4 H, H-2′, H-2″, H-6′, H-6″), 2.75–2.59 (m, 4 H, H-7′, H-7″, H-7′, H-7″), 2.40–2.36 (m, 2 H, H-5′, H-5″), 2.18–2.10 (m, 2 H, H-4′, H-4″), 2.03–1.95 (m, 2 H, H-3′, H-3″), 1.61–1.37 (m, 6 H, H-8′, H-8″, H-8′, H-8″, H-3′, H-3″), 0.92 (s, 18 H, SiC(CH3)3) and 0.09 (s, 12 H, SiCH3); δC(100 MHz; CDCl3) 132.17 (CH, C-3, C-6), 128.58 (CH, C-4, C-5), 127.25 (C, C-1, C-2), 65.86 (CH2, C-9′, C-9″), 57.72 (CH, C-2′, C-2″), 57.09 (CH2, C-6′, C-6″), 41.75 (CH2, C-7′, C-7″), 28.88 (CH, C-5′, C-5″), 27.59 (CH, C-4′, C-4″), 26.02 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 25.23 (CH2, C-8′, C-8″), 22.70 (CH2, C-3′, C-3″), 18.44 (C, SiC(CH3)3), −5.30 (CH3, SiC
H3)3), 25.23 (CH2, C-8′, C-8″), 22.70 (CH2, C-3′, C-3″), 18.44 (C, SiC(CH3)3), −5.30 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.32 (CH3, SiC
H3) and −5.32 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 586 (M+ + 2 H, 13), 578 (71), 552 (73), 519 (100), 437 (43), 397 (45), 355 (63), 341 (63) and 327 (85).
H3); m/z (FAB) (EI) 586 (M+ + 2 H, 13), 578 (71), 552 (73), 519 (100), 437 (43), 397 (45), 355 (63), 341 (63) and 327 (85).
General procedure for the Heck reaction of Cinchona alkaloid precursors with α,β-unsaturated carbonyl compounds
Pd(OAc)2 (0.05 eq.), K2CO3 (2.50 eq.) and TBAI (1.00 eq.) were dissolved in absolute DMF under argon. The reaction mixture was stirred for 15 min at rt, the α,β-unsaturated carbonyl compound (4.00 eq.) was added and stirring was continued for 15 min, followed by dropwise addition of a solution of vinyl iodide precursor (1.50 eq.) in absolute DMF. Subsequent to stirring at rt for 12 h, the dark-red reaction mixture was treated with sat. aq. NaHCO3 and sat. aq. NaCl. The aqueous layer was thorougly extracted with CH2Cl2 and the combined organic layer was dried (MgSO4) and concentrated under reduced pressure. DMF was then removed at elevated temperature under reduced pressure. The resulting crude product was purified by column chromatography (EtOAc–MeOH 20∶1) to yield the desired coupling product.![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-[(4E
)-9-Acetoxy-11-[(4E![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-3-oxobutylidene]-6′-methoxycinchonan 35a.
(Z
)-3-oxobutylidene]-6′-methoxycinchonan 35a.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and methylvinyl ketone (82 μl, 0.99 mmol, 4.0 eq.) to afford (Z,E
)-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and methylvinyl ketone (82 μl, 0.99 mmol, 4.0 eq.) to afford (Z,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene 35a (92%, 98 mg, 0.23 mmol); νmax(CHCl3)/cm−1 2944, 2876, 1744, 1668, 1620, 1588, 1508, 1472, 1456, 1432, 1360, 1296, 1236, 1176, 1136, 1084, 1028, 992 and 844; δH(400 MHz; CDCl3) 8.73 (d, 1 H, J 4.5, H-2′), 8.02 (d, 1 H, J 9.9, H-8′), 7.41–7.35 (m, 3 H, H-7′, H-5′, H-12), 7.33 (d, 1 H, J 4.6, H-3′), 6.57 (d, 1 H, J 6.8, H-9), 6.24 (d, 1 H, J 9.2, H-10), 6.23 (d, 1 H, J 15.3, H-13), 6.16 (m, 1 H, H-11), 3.93 (s, 3 H, H-11′), 3.35–3.30 (m, 2 H, H-8, H-2), 3.06–2.97 (m, 1 H, H-2), 2.89–2.78 (m, 3 H, H-6, H-6, H-3), 2.28 (s, 3 H, H-15), 2.14 (s, 3 H, H-17), 1.94–1.88 (m, 1 H, H-4), 1.81–1.76 (m, 1 H, H-7) and 1.71–1.42 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 198.45 (C, C-14), 169.93 (C, C-16), 157.97 (C, C-6′), 147.41 (CH, C-2′), 144.72 (C, C-10′), 143.66 (C, C-4′), 143.45 (CH, C-12), 137.88 (CH, C-13), 131.83 (CH, C-8′), 130.83 (CH, C-11), 127.54 (CH, C-10), 126.92 (C, C-9′), 121.81 (CH, C-7′), 118.53 (CH, C-3′), 101.43 (CH, C-5′), 73.42 (CH, C-9), 58.82 (CH, C-8), 55.68 (CH3, C-11′), 50.07 (CH2, C-2), 49.67 (CH2, C-6), 34.88 (CH, C-3), 28.26 (CH, C-4), 25.91 (CH2, C-7), 23.13 (CH2, C-5), 21.17 (CH3, C-17) and 13.73 (CH3, C-15); m/z (MAT, 140 °C) (EI) 434.2206 (M+, C26H30N2O4 requires 434.2205), 391 (1%), 373 (1), 315 (1), 285 (2), 258 (8), 204 (6), 167 (4), 155 (7), 139 (5), 111 (6), 99 (37) and 83 (100).
)-diene 35a (92%, 98 mg, 0.23 mmol); νmax(CHCl3)/cm−1 2944, 2876, 1744, 1668, 1620, 1588, 1508, 1472, 1456, 1432, 1360, 1296, 1236, 1176, 1136, 1084, 1028, 992 and 844; δH(400 MHz; CDCl3) 8.73 (d, 1 H, J 4.5, H-2′), 8.02 (d, 1 H, J 9.9, H-8′), 7.41–7.35 (m, 3 H, H-7′, H-5′, H-12), 7.33 (d, 1 H, J 4.6, H-3′), 6.57 (d, 1 H, J 6.8, H-9), 6.24 (d, 1 H, J 9.2, H-10), 6.23 (d, 1 H, J 15.3, H-13), 6.16 (m, 1 H, H-11), 3.93 (s, 3 H, H-11′), 3.35–3.30 (m, 2 H, H-8, H-2), 3.06–2.97 (m, 1 H, H-2), 2.89–2.78 (m, 3 H, H-6, H-6, H-3), 2.28 (s, 3 H, H-15), 2.14 (s, 3 H, H-17), 1.94–1.88 (m, 1 H, H-4), 1.81–1.76 (m, 1 H, H-7) and 1.71–1.42 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 198.45 (C, C-14), 169.93 (C, C-16), 157.97 (C, C-6′), 147.41 (CH, C-2′), 144.72 (C, C-10′), 143.66 (C, C-4′), 143.45 (CH, C-12), 137.88 (CH, C-13), 131.83 (CH, C-8′), 130.83 (CH, C-11), 127.54 (CH, C-10), 126.92 (C, C-9′), 121.81 (CH, C-7′), 118.53 (CH, C-3′), 101.43 (CH, C-5′), 73.42 (CH, C-9), 58.82 (CH, C-8), 55.68 (CH3, C-11′), 50.07 (CH2, C-2), 49.67 (CH2, C-6), 34.88 (CH, C-3), 28.26 (CH, C-4), 25.91 (CH2, C-7), 23.13 (CH2, C-5), 21.17 (CH3, C-17) and 13.73 (CH3, C-15); m/z (MAT, 140 °C) (EI) 434.2206 (M+, C26H30N2O4 requires 434.2205), 391 (1%), 373 (1), 315 (1), 285 (2), 258 (8), 204 (6), 167 (4), 155 (7), 139 (5), 111 (6), 99 (37) and 83 (100).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-11-(3-Oxopropylidene-6′-methoxycinchonan-9-ol 35b.
(Z
)-11-(3-Oxopropylidene-6′-methoxycinchonan-9-ol 35b.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11a (111 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and methyl acrylate (90 μl, 0.99 mmol, 4.0 eq.) to afford (Z,E
)-11-(2-Iodovinyl)quinidine 11a (111 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and methyl acrylate (90 μl, 0.99 mmol, 4.0 eq.) to afford (Z,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene 35b (68%, 69 mg, 0.17 mmol); νmax(CHCl3)/cm−1 3336, 2964, 2876, 1620, 1592, 1508, 1460, 1436, 1384, 1296, 1276, 1228, 1176, 1140, 1104, 1064, 1028, 992 and 872; δH(400 MHz; CDCl3) 8.76 (d, 1 H, J 4.6, H-2′), 7.90 (d, 1 H, J 9.2, H-8′), 7.76 (d, 1 H, J 4.6, H-3′), 7.25–7.19 (m, 2 H, H-7′, H-5′), 6.83 (s, 1 H, H-9), 6.37–6.30 (m, 2 H, H-11, H-12), 6.26 (d, 1 H, J 9.2, H-10), 5.99 (d, 1 H, J 15.2, H-13), 3.87 (s, 3 H, H-11′), 3.76 (s, 3 H, H-15), 3.71–3.63 (m, 1 H, H-8), 3.56–3.42 (m, 2 H, H-2, H-2), 3.31–3.18 (m, 2 H, H-6, H-6), 2.52–2.45 (m, 1 H, H-3), 2.14–1.98 (m, 2 H, H-4, H-7), 1.73–1.65 (m, 1 H, H-7) and 1.50–1.42 (m, 2 H, H-5, H-5); δC(100 MHz; CDCl3) 167.26 (C, C-14), 158.73 (C, C-6′), 147.23 (CH, C-2′), 144.93 (C, C-10′), 143.84 (C, C-4′), 143.31 (CH, C-12), 137.94 (CH, C-13), 131.30 (CH, C-8′), 130.93 (CH, C-11), 129.29 (CH, C-10), 125.66 (C, C-9′), 123.60 (CH, C-7′), 119.19 (CH, C-3′), 100.48 (CH, C-5′), 73.90 (CH, C-9), 60.16 (CH, C-8), 59.23 (CH3, C-15), 58.58 (CH3, C-11′), 51.82 (CH2, C-2), 49.89 (CH2, C-6), 38.72 (CH, C-3), 28.92 (CH, C-4), 24.34 (CH2, C-7) and 23.74 (CH2, C-5); m/z (MAT, 140 °C) (EI) 408.3244 (M+, C24H28N2O4 requires 408.3239), 377 (2%), 335 (8), 323 (5), 279 (15), 254 (2), 220 (14), 167 (32), 149 (100), 127 (19), 113 (11), 87 (13), 71 (21).
)-diene 35b (68%, 69 mg, 0.17 mmol); νmax(CHCl3)/cm−1 3336, 2964, 2876, 1620, 1592, 1508, 1460, 1436, 1384, 1296, 1276, 1228, 1176, 1140, 1104, 1064, 1028, 992 and 872; δH(400 MHz; CDCl3) 8.76 (d, 1 H, J 4.6, H-2′), 7.90 (d, 1 H, J 9.2, H-8′), 7.76 (d, 1 H, J 4.6, H-3′), 7.25–7.19 (m, 2 H, H-7′, H-5′), 6.83 (s, 1 H, H-9), 6.37–6.30 (m, 2 H, H-11, H-12), 6.26 (d, 1 H, J 9.2, H-10), 5.99 (d, 1 H, J 15.2, H-13), 3.87 (s, 3 H, H-11′), 3.76 (s, 3 H, H-15), 3.71–3.63 (m, 1 H, H-8), 3.56–3.42 (m, 2 H, H-2, H-2), 3.31–3.18 (m, 2 H, H-6, H-6), 2.52–2.45 (m, 1 H, H-3), 2.14–1.98 (m, 2 H, H-4, H-7), 1.73–1.65 (m, 1 H, H-7) and 1.50–1.42 (m, 2 H, H-5, H-5); δC(100 MHz; CDCl3) 167.26 (C, C-14), 158.73 (C, C-6′), 147.23 (CH, C-2′), 144.93 (C, C-10′), 143.84 (C, C-4′), 143.31 (CH, C-12), 137.94 (CH, C-13), 131.30 (CH, C-8′), 130.93 (CH, C-11), 129.29 (CH, C-10), 125.66 (C, C-9′), 123.60 (CH, C-7′), 119.19 (CH, C-3′), 100.48 (CH, C-5′), 73.90 (CH, C-9), 60.16 (CH, C-8), 59.23 (CH3, C-15), 58.58 (CH3, C-11′), 51.82 (CH2, C-2), 49.89 (CH2, C-6), 38.72 (CH, C-3), 28.92 (CH, C-4), 24.34 (CH2, C-7) and 23.74 (CH2, C-5); m/z (MAT, 140 °C) (EI) 408.3244 (M+, C24H28N2O4 requires 408.3239), 377 (2%), 335 (8), 323 (5), 279 (15), 254 (2), 220 (14), 167 (32), 149 (100), 127 (19), 113 (11), 87 (13), 71 (21).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(3-tert-butoxycarbonylpropylidene)-6′-methoxycinchonan 35c.
(Z
)-9-Acetoxy-11-(3-tert-butoxycarbonylpropylidene)-6′-methoxycinchonan 35c.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and isobutyl acrylate (0.14 ml, 0.99 mmol, 4.0 eq.) to afford (Z,E
)-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and isobutyl acrylate (0.14 ml, 0.99 mmol, 4.0 eq.) to afford (Z,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene 35c (88%, 106 mg, 0.22 mmol); νmax(CHCl3)/cm−1 2964, 2876, 1744, 1672, 1632, 1508, 1472, 1432, 1376, 1308, 1268, 1228, 1176, 1140, 1112, 1084, 1028, 992 and 872; δH(400 MHz; CDCl3) 8.69 (d, 1 H, J 4.5, H-2′), 7.97 (d, 1 H, J 9.2, H-8′), 7.49 (dd, 1 H, J 15.2 and 11.6,H-12), 7.38–7.35 (m, 2 H, H-7′, H-5′), 7.32 (d, 1 H, J 4.5, H-3′), 6.74 (d, 1 H, J 6.6, H-9), 6.26 (dd, 1 H, J 11.6 and 9.2, H-11), 6.14 (d, 1 H, J 9.2, H-10), 5.95 (d, 1 H, J 15.2, H-13), 3.94 (s, 3 H, H-11′), 3.91 (d, 2 H, J 6.7, H-15), 3.69–3.61 (m, 1 H, H-8), 3.42–3.23 (m, 2 H, H-2, H-2), 2.99–2.82 (m, 3 H, H-6, H-6, H-3), 2.14 (s, 3 H, H-20), 2.02–1.93 (m, 1 H, H-4), 1.83–1.79 (m, 1 H, H-7), 1.69–1.57 (m, 3 H, H-7, H-5, H-5), 1.45–1.37 (m, 1 H, H-16) and 0.97–0.89 (m, 6 H, H-17, H-18); δC(100 MHz; CDCl3) 169.42 (C, C-19), 167.51 (C, C-14), 158.09 (C, C-6′), 147.34 (CH, C-2′), 144.84 (C, C-10′), 143.39 (C, C-4′), 143.02 (CH, C-12), 138.71 (CH, C-13), 131.82 (CH, C-8′), 127.78 (CH, C-11), 126.55 (C, C-9′), 122.76 (CH, C-10), 122.15 (CH, C-7′), 118.32 (CH, C-3′), 101.29 (CH, C-5′), 72.69 (CH, C-9), 70.61 (CH2, C-15), 58.81 (CH, C-8), 55.61 (CH3, C-11′), 49.82 (CH2, C-2), 49.27 (CH2, C-6), 34.07 (CH, C-3), 27.78 (CH, C-4), 25.68 (CH2, C-7), 24.30 (CH2, C-5), 21.13 (CH3, C-20), 19.12 (CH, C-16) and 13.76 (CH3, C-17, C-18); m/z (FAB) (EI) 493 (M+ + H, 17), 242 (100), 184 (8) and 142 (9).
)-diene 35c (88%, 106 mg, 0.22 mmol); νmax(CHCl3)/cm−1 2964, 2876, 1744, 1672, 1632, 1508, 1472, 1432, 1376, 1308, 1268, 1228, 1176, 1140, 1112, 1084, 1028, 992 and 872; δH(400 MHz; CDCl3) 8.69 (d, 1 H, J 4.5, H-2′), 7.97 (d, 1 H, J 9.2, H-8′), 7.49 (dd, 1 H, J 15.2 and 11.6,H-12), 7.38–7.35 (m, 2 H, H-7′, H-5′), 7.32 (d, 1 H, J 4.5, H-3′), 6.74 (d, 1 H, J 6.6, H-9), 6.26 (dd, 1 H, J 11.6 and 9.2, H-11), 6.14 (d, 1 H, J 9.2, H-10), 5.95 (d, 1 H, J 15.2, H-13), 3.94 (s, 3 H, H-11′), 3.91 (d, 2 H, J 6.7, H-15), 3.69–3.61 (m, 1 H, H-8), 3.42–3.23 (m, 2 H, H-2, H-2), 2.99–2.82 (m, 3 H, H-6, H-6, H-3), 2.14 (s, 3 H, H-20), 2.02–1.93 (m, 1 H, H-4), 1.83–1.79 (m, 1 H, H-7), 1.69–1.57 (m, 3 H, H-7, H-5, H-5), 1.45–1.37 (m, 1 H, H-16) and 0.97–0.89 (m, 6 H, H-17, H-18); δC(100 MHz; CDCl3) 169.42 (C, C-19), 167.51 (C, C-14), 158.09 (C, C-6′), 147.34 (CH, C-2′), 144.84 (C, C-10′), 143.39 (C, C-4′), 143.02 (CH, C-12), 138.71 (CH, C-13), 131.82 (CH, C-8′), 127.78 (CH, C-11), 126.55 (C, C-9′), 122.76 (CH, C-10), 122.15 (CH, C-7′), 118.32 (CH, C-3′), 101.29 (CH, C-5′), 72.69 (CH, C-9), 70.61 (CH2, C-15), 58.81 (CH, C-8), 55.61 (CH3, C-11′), 49.82 (CH2, C-2), 49.27 (CH2, C-6), 34.07 (CH, C-3), 27.78 (CH, C-4), 25.68 (CH2, C-7), 24.30 (CH2, C-5), 21.13 (CH3, C-20), 19.12 (CH, C-16) and 13.76 (CH3, C-17, C-18); m/z (FAB) (EI) 493 (M+ + H, 17), 242 (100), 184 (8) and 142 (9).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(3-oxopropylidene)-6′-methoxycinchonan 35d.
(Z
)-9-Acetoxy-11-(3-oxopropylidene)-6′-methoxycinchonan 35d.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and acrolein (66 μl, 0.99 mmol, 4.0 eq.) to afford (Z,E
)-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and acrolein (66 μl, 0.99 mmol, 4.0 eq.) to afford (Z,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene 35d (81%, 84 mg, 0.20 mmol); νmax(CHCl3)/cm−1 3000, 2944, 2876, 1744, 1676, 1624, 1588, 1508, 1472, 1456, 1432, 1364, 1304, 1228, 1172, 1136, 1088, 1032, 988 and 844; δH(400 MHz; CDCl3) 9.61 (d, 1 H, J 7.5, H-14), 8.70 (d, 1 H, J 4.6, H-2′), 7.98 (d, 1 H, J 9.2, H-8′), 7.40–7.30 (m, 4 H, H-7′, H-5′, H-12, H-3′), 6.55 (m, 1 H, H-9), 6.40–6.25 (m, 2 H, H-10, H-11), 6.20–6.10 (m, 1 H, H-13), 3.92 (s, 3 H, H-11′), 3.38–3.24 (m, 3 H, H-8, H-2, H-2), 3.05–2.75 (m, 3 H, H-6, H-6, H-3), 2.13 (s, 3 H, H-16), 1.96–1.85 (m, 1 H, H-4) and 1.71–1.35 (m, 4 H, H-7, H-7, H-5, H-5); δC(100 MHz; CDCl3) 193.79 (CH, C-14), 169.88 (C, C-15), 157.91 (C, C-6′), 147.36 (CH, C-2′), 146.27 (CH, C-12), 144.95 (C, C-10′), 144.80 (C, C-4′), 143.59 (CH, C-13), 132.40 (CH, C-11), 131.77 (CH, C-8′), 127.41 (CH, C-10), 126.98 (C, C-9′), 121.85 (CH, C-7′), 118.31 (CH, C-3′), 101.42 (CH, C-5′), 73.31 (CH, C-9), 58.76 (CH, C-8), 55.53 (CH3, C-11′), 49.89 (CH2, C-2), 49.42 (CH2, C-6), 34.97 (CH, C-3), 28.08 (CH, C-4), 25.11 (CH2, C-7), 23.47 (CH2, C-5) and 21.13 (CH3, C-16); m/z (MAT, 90 °C) (EI) 420.2048 (M+, C25H28N2O4 requires 420.2049), 392 (8%), 378 (10), 361 (14), 349 (2), 325 (16), 294 (2), 265 (6), 242 (100), 231 (32), 211 (28), 190 (31), 173 (11), 155 (10), 142 (60), 128 (24), 99 (41) and 91 (41).
)-diene 35d (81%, 84 mg, 0.20 mmol); νmax(CHCl3)/cm−1 3000, 2944, 2876, 1744, 1676, 1624, 1588, 1508, 1472, 1456, 1432, 1364, 1304, 1228, 1172, 1136, 1088, 1032, 988 and 844; δH(400 MHz; CDCl3) 9.61 (d, 1 H, J 7.5, H-14), 8.70 (d, 1 H, J 4.6, H-2′), 7.98 (d, 1 H, J 9.2, H-8′), 7.40–7.30 (m, 4 H, H-7′, H-5′, H-12, H-3′), 6.55 (m, 1 H, H-9), 6.40–6.25 (m, 2 H, H-10, H-11), 6.20–6.10 (m, 1 H, H-13), 3.92 (s, 3 H, H-11′), 3.38–3.24 (m, 3 H, H-8, H-2, H-2), 3.05–2.75 (m, 3 H, H-6, H-6, H-3), 2.13 (s, 3 H, H-16), 1.96–1.85 (m, 1 H, H-4) and 1.71–1.35 (m, 4 H, H-7, H-7, H-5, H-5); δC(100 MHz; CDCl3) 193.79 (CH, C-14), 169.88 (C, C-15), 157.91 (C, C-6′), 147.36 (CH, C-2′), 146.27 (CH, C-12), 144.95 (C, C-10′), 144.80 (C, C-4′), 143.59 (CH, C-13), 132.40 (CH, C-11), 131.77 (CH, C-8′), 127.41 (CH, C-10), 126.98 (C, C-9′), 121.85 (CH, C-7′), 118.31 (CH, C-3′), 101.42 (CH, C-5′), 73.31 (CH, C-9), 58.76 (CH, C-8), 55.53 (CH3, C-11′), 49.89 (CH2, C-2), 49.42 (CH2, C-6), 34.97 (CH, C-3), 28.08 (CH, C-4), 25.11 (CH2, C-7), 23.47 (CH2, C-5) and 21.13 (CH3, C-16); m/z (MAT, 90 °C) (EI) 420.2048 (M+, C25H28N2O4 requires 420.2049), 392 (8%), 378 (10), 361 (14), 349 (2), 325 (16), 294 (2), 265 (6), 242 (100), 231 (32), 211 (28), 190 (31), 173 (11), 155 (10), 142 (60), 128 (24), 99 (41) and 91 (41).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-9-Acetoxy-11-(2-carbamoylethylidene)-6′-methoxycinchonan 35e.
(Z
)-9-Acetoxy-11-(2-carbamoylethylidene)-6′-methoxycinchonan 35e.
(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and acrylamide (70 mg, 0.99 mmol, 4.0 eq.) to afford (Z,E
)-11-(2-Iodovinyl)quinidine 11b (121 mg, 0.25 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (3 mg, 0.01 mmol, 0.05 eq.), K2CO3 (85 mg, 0.62 mmol, 2.5 eq.), TBAI (91 mg, 0.25 mmol, 1 eq.) and acrylamide (70 mg, 0.99 mmol, 4.0 eq.) to afford (Z,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene 35d (90%, 96 mg, 0.22 mmol); νmax(CHCl3)/cm−1 2940, 2876, 1744, 1676, 1624, 1592, 1508, 1472, 1456, 1432, 1372, 1304, 1228, 1156, 1120, 1084, 1032, 988 and 956; δH(400 MHz; CDCl3) 8.72 (d, 1 H, J 4.5, H-2′), 8.01 (d, 1 H, J 9.9, H-8′), 7.51 (dd, 1 H, J 14.9 and 11.2, H-12), 7.36–7.32 (m, 3 H, H-7′, H-5′, H-3′), 6.53 (d, 1 H, J 6.7, H-9), 6.20–6.08 (m, 2 H, H-10, H-11), 5.99 (d, 1 H, J 14.8, H-13), 3.92 (s, 3 H, H-11′), 3.32–3.23 (m, 3 H, H-8, H-2, H-2), 3.00–2.71 (m, 3 H, H-6, H-6, H-3), 2.13 (s, 3 H, H-16), 1.91–1.85 (m, 1 H, H-7), 1.76–1.72 (m, 1 H, H-4) and 1.67–1.38 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 170.02 (C, C-14), 168.23 (C, C-15), 157.85 (C, C-6′), 147.35 (CH, C-2′), 144.59 (C, C-10′), 143.47 (C, C-4′), 141.62 (CH, C-12), 136.76 (CH, C-13), 132.05 (CH, C-11), 131.69 (CH, C-8′), 126.88 (C, C-9′), 123.93 (CH, C-10), 121.86 (CH, C-7′), 118.56 (CH, C-3′), 101.44 (CH, C-5′), 73.51 (CH, C-9), 58.84 (CH, C-8), 55.63 (CH3, C-11′), 49.92 (CH2, C-2), 49.35 (CH2, C-6), 34.53 (CH, C-3), 27.98 (CH, C-4), 25.31 (CH2, C-7), 24.09 (CH2, C-5) and 21.19 (CH3, C-16); m/z (MAT, 70 °C) (EI) 436.2224 (M+, C25H30N3O4 requires 436.2236), 420 (2%), 392 (2), 376 (11), 349 (1), 242 (2), 231 (2), 205 (100), 189 (17), 173 (10), 154 (10), 142 (25), 132 (8), 95 (4) and 79 (8).
)-diene 35d (90%, 96 mg, 0.22 mmol); νmax(CHCl3)/cm−1 2940, 2876, 1744, 1676, 1624, 1592, 1508, 1472, 1456, 1432, 1372, 1304, 1228, 1156, 1120, 1084, 1032, 988 and 956; δH(400 MHz; CDCl3) 8.72 (d, 1 H, J 4.5, H-2′), 8.01 (d, 1 H, J 9.9, H-8′), 7.51 (dd, 1 H, J 14.9 and 11.2, H-12), 7.36–7.32 (m, 3 H, H-7′, H-5′, H-3′), 6.53 (d, 1 H, J 6.7, H-9), 6.20–6.08 (m, 2 H, H-10, H-11), 5.99 (d, 1 H, J 14.8, H-13), 3.92 (s, 3 H, H-11′), 3.32–3.23 (m, 3 H, H-8, H-2, H-2), 3.00–2.71 (m, 3 H, H-6, H-6, H-3), 2.13 (s, 3 H, H-16), 1.91–1.85 (m, 1 H, H-7), 1.76–1.72 (m, 1 H, H-4) and 1.67–1.38 (m, 3 H, H-7, H-5, H-5); δC(100 MHz; CDCl3) 170.02 (C, C-14), 168.23 (C, C-15), 157.85 (C, C-6′), 147.35 (CH, C-2′), 144.59 (C, C-10′), 143.47 (C, C-4′), 141.62 (CH, C-12), 136.76 (CH, C-13), 132.05 (CH, C-11), 131.69 (CH, C-8′), 126.88 (C, C-9′), 123.93 (CH, C-10), 121.86 (CH, C-7′), 118.56 (CH, C-3′), 101.44 (CH, C-5′), 73.51 (CH, C-9), 58.84 (CH, C-8), 55.63 (CH3, C-11′), 49.92 (CH2, C-2), 49.35 (CH2, C-6), 34.53 (CH, C-3), 27.98 (CH, C-4), 25.31 (CH2, C-7), 24.09 (CH2, C-5) and 21.19 (CH3, C-16); m/z (MAT, 70 °C) (EI) 436.2224 (M+, C25H30N3O4 requires 436.2236), 420 (2%), 392 (2), 376 (11), 349 (1), 242 (2), 231 (2), 205 (100), 189 (17), 173 (10), 154 (10), 142 (25), 132 (8), 95 (4) and 79 (8).
![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-5-oxohexa-1,3-dienyl]-1-azabicyclo[2.2.2]octane 36.
(E
)-5-oxohexa-1,3-dienyl]-1-azabicyclo[2.2.2]octane 36.
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-11-(2-Iodovinyl)quincorine 17 (68 mg, 0.17 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (2 mg, 0.01 mmol, 0.05 eq.), K2CO3 (57 mg, 0.42 mmol, 2.5 eq.), TBAI (62 mg, 0.17 mmol, 1 eq.) and methyl vinyl ketone (47 mg, 0.67 mmol, 4.0 eq.) to afford (Z,E
)-11-(2-Iodovinyl)quincorine 17 (68 mg, 0.17 mmol, 1 eq.) was allowed to react according to the general procedure with Pd(OAc)2 (2 mg, 0.01 mmol, 0.05 eq.), K2CO3 (57 mg, 0.42 mmol, 2.5 eq.), TBAI (62 mg, 0.17 mmol, 1 eq.) and methyl vinyl ketone (47 mg, 0.67 mmol, 4.0 eq.) to afford (Z,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene 36 (84%, 49 mg, 0.14 mmol); νmax(CHCl3)/cm−1 2960, 2936, 2876, 1672, 1632, 1468, 1408, 1384, 1360, 1316, 1256, 1232, 1112, 1092, 1064, 996 and 836; δH(400 MHz; CDCl3) 7.13–7.03 (dd, 1 H, J 15.6 and 9.7, H-12), 6.32–6.20 (m, 2 H, H-10, H-11), 6.08–6.03 (d, 1 H, J 15.7, H-13), 3.74–3.68 (m, 2 H, H-9, H-9), 3.65–3.42 (m, 3 H, H-6, H-6, H-2), 3.20–2.95 (m, 2 H, H-7, H-7), 2.35–2.32 (m, 1 H, H-5), 2.22 (s, 3 H, H-15), 2.12–2.04 (m, 2 H, H-4, H-3), 1.95–1.74 (m, 3 H, H-8, H-8, H-3), 0.89 (s, 9 H, SiC(CH3)3), 0.07 (s, 3 H, SiCH3) and 0.05 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 198.73 (C, C-14), 142.82 (CH, C-12), 130.62 (CH, C-13), 130.58 (CH, C-11), 130.42 (CH, C-10), 62.64 (CH2, C-9), 58.14 (CH, C-2), 54.01 (CH2, C-6), 42.79 (CH2, C-7), 27.60 (CH, C-5), 26.81 (CH, C-4), 25.97 (CH3, SiC(C
)-diene 36 (84%, 49 mg, 0.14 mmol); νmax(CHCl3)/cm−1 2960, 2936, 2876, 1672, 1632, 1468, 1408, 1384, 1360, 1316, 1256, 1232, 1112, 1092, 1064, 996 and 836; δH(400 MHz; CDCl3) 7.13–7.03 (dd, 1 H, J 15.6 and 9.7, H-12), 6.32–6.20 (m, 2 H, H-10, H-11), 6.08–6.03 (d, 1 H, J 15.7, H-13), 3.74–3.68 (m, 2 H, H-9, H-9), 3.65–3.42 (m, 3 H, H-6, H-6, H-2), 3.20–2.95 (m, 2 H, H-7, H-7), 2.35–2.32 (m, 1 H, H-5), 2.22 (s, 3 H, H-15), 2.12–2.04 (m, 2 H, H-4, H-3), 1.95–1.74 (m, 3 H, H-8, H-8, H-3), 0.89 (s, 9 H, SiC(CH3)3), 0.07 (s, 3 H, SiCH3) and 0.05 (s, 3 H, SiCH3); δC(100 MHz; CDCl3) 198.73 (C, C-14), 142.82 (CH, C-12), 130.62 (CH, C-13), 130.58 (CH, C-11), 130.42 (CH, C-10), 62.64 (CH2, C-9), 58.14 (CH, C-2), 54.01 (CH2, C-6), 42.79 (CH2, C-7), 27.60 (CH, C-5), 26.81 (CH, C-4), 25.97 (CH3, SiC(C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3)3), 24.69 (CH2, C-8), 24.31 (CH2, C-3), 22.15 (CH3, C-15), 18.28 (C, SiC(CH3)3), −5.20 (CH3, SiC
H3)3), 24.69 (CH2, C-8), 24.31 (CH2, C-3), 22.15 (CH3, C-15), 18.28 (C, SiC(CH3)3), −5.20 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3) and −5.38 (CH3, SiC
H3) and −5.38 (CH3, SiC![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) H3); m/z (FAB) (EI) 350 (M+ + H, 23), 242 (100), 184 (10) and 142 (17).
H3); m/z (FAB) (EI) 350 (M+ + H, 23), 242 (100), 184 (10) and 142 (17).
Acknowledgements
We thank the Fonds der Chemischen Industrie (J. F.) and the Friedrich Naumann Stiftung (W. M. B.) for PhD fellowships, Degussa AG Hanau for a generous gift of palladium salts, Buchler GmbH-Chininfabrik Braunschweig for a generous gift of Cinchona alkaloids and Ulrike Eggert for her help.References
- P. J. Harrington , Transition Metals in Total Synthesis, Wiley & Sons, New York, 1990; Search PubMed; F. Diederich and P. J. Stang , Metal-catalyzed Cross-coupling Reactions, Wiley-VCH, Weinheim, New York, 1998, pp. 110–229; Search PubMed; A. J. Pearson, Synlett, 1990, 10 Search PubMed; D. Seebach, Angew. Chem., Int. Ed. Engl., 1990, 29, 30 Search PubMed.
- K. Sonogashira , Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, New York, 1991, 3, p. 521. Search PubMed.
- A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379 CrossRef; K. Fuji, Chem. Rev., 1993, 93, 2037 CrossRef CAS.
- H. J. Wadsworth, S. M. Jenkins, B. S. Orlek, F. Cassidy, M. S. G. Clark, F. Brown, G. J. Riley, D. Graves, J. Hawkins and C. B. Naylor, J. Med. Chem., 1992, 35, 1280 CrossRef CAS; R. Baker, L. J. Street, A. J. Reeve and J. Saunders, J. Chem. Soc., Chem. Commun., 1991, 760 RSC.
- J. Saunders, M. Cassidy, S. B. Freedman, E. A. Harley, L. L. Iversen, C. Kneen, A. M. Macleod, K. J. Merchant, R. J. Snow and R. Baker, J. Med. Chem., 1990, 33, 1128 CrossRef CAS; G. Nordvall, S. Sundquist, L. Nilvebrant and U. Hacksell, Bioorg. Med. Chem. Lett., 1994, 4, 2837 CrossRef CAS; L. A. Flippin, D. S. Carter, J. Berger, R. D. Clark, D. W. Bonhaus, E. Leung and R. M. Eglen, Bioorg. Med. Chem. Lett., 1996, 6, 477 CrossRef CAS; R. D. Clark, K. K. Weinhardt, J. Berger, C.-H. Lee, E. Leung, E. H. F. Wong, W. L. Smith and R. M. Eglen, Bioorg. Med. Chem. Lett., 1993, 3, 1375 CrossRef CAS; M. Langlois, J. L. Soulier, M. Allainmat, S. Shen and C. Gallais, Bioorg. Med. Chem. Lett., 1993, 3, 1555 CrossRef CAS.
- T. M. Fong, M. A. Cascieri, H. Yu, A. Bansai, C. Swain and C. D. Strader, Nature, 1993, 362, 350 CrossRef CAS; R. M. Snider, J. W. Constantine, J. A. Lowe III, K. P. Longo, W. S. Lebel, H. A. Woody, S. E. Drozda, M. C. Desai, F. J. Vinick and R. W. Spencer, Science, 1991, 251, 435 CAS; S. McLean, A. H. Ganong, T. F. Seeger, D. K. Bryce, K. G. Pratt, L. S. Reynolds, C. J. Siok, J. A. Lowe III and J. Heym, Science, 1991, 251, 437 CAS; C. J. Swain, T. M. Fong, K. Haworth, S. N. Owen, E. M. Seeward and C. D. Strader, Bioorg. Med. Chem. Lett., 1995, 5, 1261 CrossRef CAS.
- G. R. Brown, A. J. Foubister, S. Freeman, F. McTaggart, D. J. Mirrlees, A. C. Reid, G. J. Smith, M. J. Taylor, D. A. Thomason and P. R. O. Whittamore, Bioorg. Med. Chem. Lett., 1997, 7, 597 CrossRef CAS; G. R. Brown, D. S. Clarke, A. J. Foubister, S. Freeman, P. J. Harrison, M. C. Johnson, K. B. Mallion, J. McCormick, F. McTaggart, A. C. Reid, G. J. Smith and M. J. Taylor, J. Med. Chem., 1996, 39, 2971 CrossRef CAS.
- G. Johansson, S. Sundquist, G. Nordvall, B. M. Nilsson, M. Brisander, L. Nilvebrant and U. Hacksell, J. Med. Chem., 1997, 40, 3804 CrossRef CAS.
- W. C. Bornmann and M. E, Kuehne, J. Org. Chem., 1992, 57, 1752 CrossRef CAS; M. E. Kuehne, P. A. Matson and W. G. Bornmann, J. Org. Chem., 1991, 56, 513 CrossRef CAS.
- H. C. Kolb, M. S. VanNieuwenhze and K. B. Sharpless, Chem. Rev., 1994, 94, 2483 CrossRef CAS; W. Amberg, Y. L. Bennani, R. K. Chadha, G. A. Crispino, W. D. Davis, J. Hartung, K. S. Jeong, Y. Ogino, T. Shibata and K. B. Sharpless, J. Org. Chem., 1993, 58, 844 CrossRef CAS.
- A. F. Cowman, L. W. Deady, E. Deharo, J. Desneves and L. Tilley, Aust. J. Chem., 1997, 50, 1091 CrossRef CAS.
- K. Raynes, D. Galatis, A. F. Cowman, L. Tilley and L. W. Deady, J. Med. Chem., 1995, 38, 204 CrossRef CAS; K. Raynes, M. Foley, L. Tilley and L. W. Deady, Biochem. Pharmacol., 1996, 52, 551 CrossRef CAS.
- C. Reichardt and J. Stein, Eur. J. Org. Chem., 1999, 2899 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef.
- K. C. Nicolaou and W.-M. Dai, Angew. Chem., 1991, 103, 1453 CrossRef CAS; K. C. Nicolaou and W.-M. Dai, Angew. Chem., Int Ed. Engl., 1991, 30, 1387 CrossRef; K. C. Nicolaou, A. L. Smith and E. W. Yue, Proc. Natl. Acad. Sci. U.S.A., 1993, 90, 5881 CAS; J. A. Proco Jr., F. J. Schoenen, T. J. Stout, J. Clardy and S. L. Schreiber, J. Am. Chem. Soc., 1990, 112, 7410 CrossRef.
- O. Schrake, W. M. Braje, H. M. R. Hoffmann and R. Wartchow, Tetrahedron: Asymmetry, 1998, 9, 3717 CrossRef CAS; W. M. Braje, J. Frackenpohl, O. Schrake, R. Wartchow, W. Beil and H. M. R. Hoffmann, Helv. Chim. Acta, 2000, 83, 777 CrossRef CAS; R. Wartchow, O. Schrake, W. M. Braje and H. M. R. Hoffmann, Z. Kristallogr. New Cryst. Struct., 1999, 214, 285 Search PubMed; J. Frackenpohl, S. Röper, H. M. R. Hoffmann and R. Wartchow, Z. Kristallogr. New Cryst. Struct., 2000, 215, 437 Search PubMed; S. Röper, J. Frackenpohl, O. Schrake, R. Wartchow and H. M. R. Hoffmann, Org. Lett., 2000, 2, 1661 CrossRef CAS X-Ray crystal structure of 10,11-didehydro QCI (18a) and 10,11-didehydro QCD (20a) see R. Wartchow , J. Frackenpohl and H. M. R. Hoffmann , Z. Kristallogr. New Cryst. Struct., Search PubMedin press and references therein.
- K. Takai, K. Nitta and K. Utimoto, J. Am. Chem. Soc., 1986, 108, 7408 CrossRef CAS; K. Takai, K. Nitta, O. Fujimura and K. Utimoto, J. Org. Chem., 1989, 54, 4732 CrossRef CAS.
- J. Frackenpohl and H. M. R. Hoffmann, J. Org. Chem., 2000, 65, 3982 CrossRef CAS.
- K. R. Buszek and J. Yeong, Synth. Commun., 1994, 24, 2461 CAS; M. W. Miller and C. R. Johnson, J. Org. Chem., 1997, 62, 1582 CrossRef CAS; I. Saito, K. Yamaguchi, R. Nagata and E. Murahashi, Tetrahedron Lett., 1990, 31, 7469 CrossRef CAS.
- S. Thorand and N. Krause, J. Org. Chem., 1998, 63, 8551 CrossRef CAS; J. Li, A. W.-H. Mau and C. R. Strauss, Chem. Commun., 1997, 1275 RSC.
- S. Torii, L. H. Xu and H. Okumoto, Synlett, 1992, 515 CrossRef CAS.
- R. C. Larock and E. K. Yum, J. Am. Chem. Soc., 1991, 113, 6689 CrossRef CAS; L. Xu, I. R. Lewis, S. K. Davidsen and J. B. Summers, Tetrahedron Lett., 1998, 39, 5159 CrossRef CAS.
- V. Ratovelomana and G. Linstrumelle, Synth. Commun., 1981, 11, 917 CAS; M. Alami and G. Linstrumelle, Tetrahedron Lett., 1991, 32, 6109 CrossRef CAS.
- H. L. Anderson and J. K. M. Sanders, J. Chem. Soc., Perkin Trans. 1, 1995, 2223 RSC.
- Q. Liu and D. J. Burton, Tetrahedron Lett., 1997, 38, 4371 CrossRef CAS.
- L. E. Overman, Pure Appl. Chem., 1994, 66, 1423 CrossRef CAS.
- T. Jeffery, Tetrahedron, 1996, 52, 10113 CrossRef; T. Jeffery, Tetrahedron Lett., 1985, 26, 2667 CrossRef CAS.
- S. J. Rowan, D. J. Reynolds and J. K. M. Saunders, J. Org. Chem., 1999, 64, 5804 CrossRef CAS.
- J. Frackenpohl , PhD Thesis, University of Hannover, 2000. Search PubMedFor the preparation of quinidine-based C10 aldehydes see also W. M. Braje, J. Frackenpohl, P. Langer and H. M. R. Hoffmann, Tetrahedron, 1998, 54, 3495 Search PubMed.
Footnotes |
| † Current address: J. Frackenpohl, ETH, Laboratorium für Organische Chemie, Universitätsstrasse 16, CH-8092 Zürich, Switzerland. |
| ‡ Current address: W. Braje, BASF AG, Hauptlaboratorium, D-67056 Ludwigshafen, Germany. |
| This journal is © The Royal Society of Chemistry 2001 |
