TFA-catalyzed ring transformation of 4-hydroxycyclobutenone: A simple and general route for preparation of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) )-ones
)-ones![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) †
†
Received (in Cambridge, UK) 12th June 2000, Accepted 30th October 2000
First published on 11th December 2000
Abstract
Following a convenient sequence of alkylation and amination of 3,4-diisopropoxycyclobutene-1,2-dione 11, three typical substituted groups (H, n-Bu, and Ph) and three typical amino groups (unsubstituted, primary and secondary) are easily introduced onto its C-3 and C-4 positions, respectively, to yield 4-substituted 3-aminocyclobutene-1,2-diones 14. Reduction of compounds 14 with NaBH4 yield 2-substituted 3-amino-4-hydroxycyclobutenones 15 in high yields. By ring transformation of products 15 catalyzed by TFA, a simple and general route for the preparation of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones 4 is developed. A two-step mechanism is proposed to describe the ring transformation of enones 15. The experimental results show that the process in refluxing p-xylene is essential for the initial thermal electrocyclic opening of 15 to yield intermediate hydroxy ketenes 16a and 16b. They are then lactonized in the presence of TFA to give furanones 4 in 51–94% yield.
)-ones 4 is developed. A two-step mechanism is proposed to describe the ring transformation of enones 15. The experimental results show that the process in refluxing p-xylene is essential for the initial thermal electrocyclic opening of 15 to yield intermediate hydroxy ketenes 16a and 16b. They are then lactonized in the presence of TFA to give furanones 4 in 51–94% yield.
Introduction
Although very few derivatives of 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one occur in nature, their synthetic analogues are widely used in chemical, pharmaceutical and agrochemical research. Some 4-aminofuran-2(5H
)-one occur in nature, their synthetic analogues are widely used in chemical, pharmaceutical and agrochemical research. Some 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones have been intermediates in the synthesis of natural products.1 Many have been patented as prodrugs or insecticides and herbicides
)-ones have been intermediates in the synthesis of natural products.1 Many have been patented as prodrugs or insecticides and herbicides![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 (for example, see: 1–3 in Chart 1).
2 (for example, see: 1–3 in Chart 1).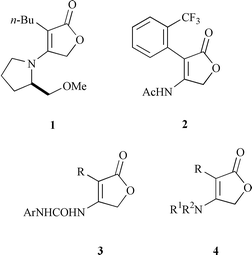 |
| | Chart 1 | |
Many methods have been developed for the preparation of 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones.3–7 Using cyanohydrin derivatives as starting materials, primary 4-aminofuran-2(5H
)-ones.3–7 Using cyanohydrin derivatives as starting materials, primary 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones, being mono- or bis-substituted at C-5 to meet the requirements of the reaction mechanism, were obtained.4 The sequence of aminoaddition on acetylenecarboxylates followed by intramolecular cyclizations afforded 3-unsubstituted primary or secondary 4-aminofuran-2(5H
)-ones, being mono- or bis-substituted at C-5 to meet the requirements of the reaction mechanism, were obtained.4 The sequence of aminoaddition on acetylenecarboxylates followed by intramolecular cyclizations afforded 3-unsubstituted primary or secondary 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones smoothly.5 Although 4-halogeno- or 4-hydroxyfuran-2(5H
)-ones smoothly.5 Although 4-halogeno- or 4-hydroxyfuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones can be aminated to yield the corresponding unsubstituted, primary and secondary 4-aminofuran-2(5H
)-ones can be aminated to yield the corresponding unsubstituted, primary and secondary 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones conveniently,1c,1d,6 the method has been limited by inaccessible precursors.8 Very few procedures can be employed for direct alkylation of 4-aminofuran-2(5H
)-ones conveniently,1c,1d,6 the method has been limited by inaccessible precursors.8 Very few procedures can be employed for direct alkylation of 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones at C-3.6a,7
)-ones at C-3.6a,7
To continue our agrochemical research project, a series of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (4 in Chart 1) were designed as targets for synthesis. Herein, we report a novel and practical route for the synthesis of 3-substituted 4-aminofuran-2(5H
)-ones (4 in Chart 1) were designed as targets for synthesis. Herein, we report a novel and practical route for the synthesis of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones that overcomes some of the limitations to published procedures.
)-ones that overcomes some of the limitations to published procedures.
Results and discussion
Strategy
4-Hydroxycyclobutenones with an unsaturated substituent at C-4 (5a–c in Chart 2) undergo thermal electrocyclic ring openings to give a variety of ring-expansion products including highly substituted phenols (6),9 quinones (7)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 10 and heteroaromatic compounds (8) (Chart 2).11
10 and heteroaromatic compounds (8) (Chart 2).11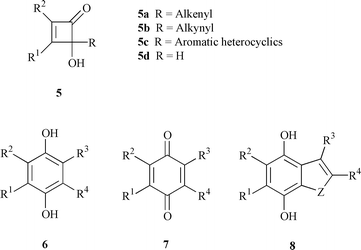 |
| | Chart 2 | |
The effect of substituents on the direction of conrotation in electrocyclic reactions of 4-hydroxycyclobutenones has been explained and predicted by Houk’s work. The hydroxy group as an electron-donating substituent favors intermediate 9 by outward rotation (Chart 3).12 For this reason, furan-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones formed by intermediate 10 in thermal electrocyclic reactions were believed to be unusual by-products,13 even though they were obtained as sole products in photochemistry or in metal-catalyzed reactions.14
)-ones formed by intermediate 10 in thermal electrocyclic reactions were believed to be unusual by-products,13 even though they were obtained as sole products in photochemistry or in metal-catalyzed reactions.14
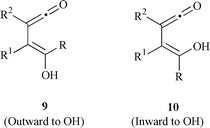 |
| | Chart 3 | |
The thermal electrocyclic opening of 4-substituted 4-hydroxybutenones 5a–c is reversible; hence the final product might arise from trapping intermediate 9 in an equilibrating mixture of 9 and 10. When the reactivity of R in 9 was inhibited by steric hindrance or a hydrogen bond, the corresponding furan-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one was obtained by attack of the hydroxy group on the ketene involved in the intermediate 10.13,15 It clearly implies that the thermolysis of 5d (R = H) should yield a furan-2(5H
)-one was obtained by attack of the hydroxy group on the ketene involved in the intermediate 10.13,15 It clearly implies that the thermolysis of 5d (R = H) should yield a furan-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one because only the hydroxy group as trapping group is present in the molecule. As shown in Scheme 1, this transformation has been developed as a key step for the preparation of 3-substituted 4-aminofuran-2(5H
)-one because only the hydroxy group as trapping group is present in the molecule. As shown in Scheme 1, this transformation has been developed as a key step for the preparation of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones 4 in our synthetic strategy.
)-ones 4 in our synthetic strategy.
 |
| | Scheme 1 | |
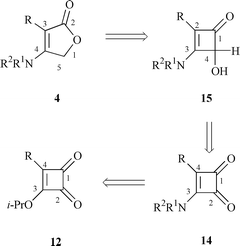
Synthesis of 4-substituted 3-aminocyclobutene-1,2-diones 14
Three typical 4-substituted 3-isopropoxycyclobutene-1,2-diones (12a–c) were chosen as starting materials, which were routinely prepared in 65–90% yield by known procedures![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 16 from 3,4-diisopropoxycyclobutene-1,2-dione 11. The vinylogous ester at C-3 in the structures 12a–c made the substitution of the isopropoxy group by various amines very easy.17 Thus, a mixture of 3-isopropoxy-4-phenylcyclobutene-1,2-dione 12a and ammonium hydroxide (28% solution in water, 13a) in methanol was stirred for 30 min at room temperature, and 3-amino-4-phenylcyclobutene-1,2-dione 14a was separated in 96% yield. To explore the scope of the reaction, allylic, benzyl and cyclic amines (13b–g) were tested and all of them gave excellent yields (14b–g, 88–96%) (Scheme 2).
16 from 3,4-diisopropoxycyclobutene-1,2-dione 11. The vinylogous ester at C-3 in the structures 12a–c made the substitution of the isopropoxy group by various amines very easy.17 Thus, a mixture of 3-isopropoxy-4-phenylcyclobutene-1,2-dione 12a and ammonium hydroxide (28% solution in water, 13a) in methanol was stirred for 30 min at room temperature, and 3-amino-4-phenylcyclobutene-1,2-dione 14a was separated in 96% yield. To explore the scope of the reaction, allylic, benzyl and cyclic amines (13b–g) were tested and all of them gave excellent yields (14b–g, 88–96%) (Scheme 2).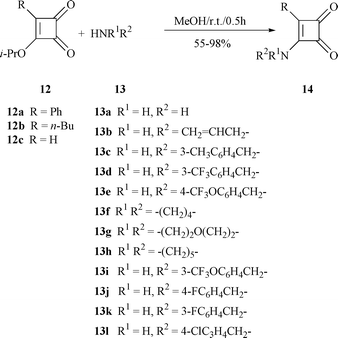 |
| | Scheme 2 | |
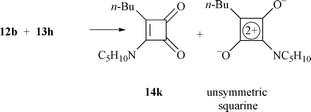
Similarly, diones 12b,c were treated with amines 13 to give 14h–o in moderate to high yields (55–98%) (Scheme 2 and Table 1). It was noted that the amination of 4-n-butyl-substituted compound 12b usually gave 4-n-butyl-3-aminocyclobutene-1,2-diones 14h–k in 55–87% yield together with the unsymmetrical squarine by-product in around 10–15% yield.17a Unfortunately, more than 20% of squarine by-product was produced in the reaction between 12b and 13h (Scheme 3), with the main product being 14k.
Table 1 Substituents and yields of compounds 14, 15, and 4
| No. | R | R2 (R1 = H) or R1R2 | 14 (%) | 15 (%) | 4 (%) | Time (t/h)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
|---|
| Reaction time for 4. |
|---|
| a | Ph | H | 96 | 94 | 84 | 4.0 |
| b | Ph | H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHCH2 CHCH2 | 95 | 91 | 72 | 3.0 |
| c | Ph | 3-CH3C6H4CH2 | 95 | 93 | 82 | 2.0 |
| d | Ph | 3-CF3C6H4CH2 | 91 | 94 | 80 | 2.5 |
| e | Ph | 4-CF3OC6H4CH2 | 96 | 93 | 70 | 3.0 |
| f | Ph | (CH2)4 | 93 | 89 | 68 | 2.5 |
| g | Ph | (CH2)2O(CH2)2 | 88 | 82 | 62 | 3.0 |
| h | n-Bu | 3-CF3OC6H4CH2 | 87 | 97 | 51 | 3.0 |
| i | n-Bu | 4-FC6H4CH2 | 82 | 95 | 69 | 2.5 |
| j | n-Bu | 3-CF3C6H4CH2 | 82 | 89 | 57 | 3.0 |
| k | n-Bu | (CH2)5 | 55 | 89 | 61 | 4.0 |
| l | H | 4-ClC6H4CH2 | 90 | 96 | 94 | 0.5 |
| m | H | 3-FC6H4CH2 | 96 | 94 | 72 | 0.25 |
| n | H | (CH2)4 | 98 | 92 | 74 | 2.5 |
| o | H | (CH2)2O(CH2)2 | 91 | 89 | 69 | 1.0 |
 |
| | Scheme 3 | |
Synthesis of 2-substituted 3-amino-4-hydroxycyclobutenones 15
In the published procedure, 3-(dibenzylamino)-4-methylcyclobutene-1,2-dione was reduced to 3-(dibenzylamino)-4-hydroxy-2-methylcyclobutenone by Li(t-BuO)3AlH in 85% yield.9a Realizing that C-2 ketone in compounds 14 is a vinylogous ketone, we used NaBH4 to replace Li(t-BuO)3AlH to furnish this conversion. Typically, when a solution of 3-amino-4-phenylcyclobutene-1,2-dione 14a in methanol was treated with NaBH4 at 0 °C for 30 min, 3-amino-4-hydroxy-2-phenylcyclobutenone 15a was obtained in 94% yield. In the same way, ketones 14b–o were reduced to the corresponding acyloins 15b–o in 82–97% yield. This new procedure featured short reaction time, low cost and high efficiency (Scheme 4, Table 1).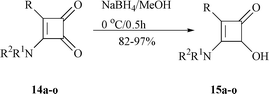 |
| | Scheme 4 | |
Synthesis of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-ones 4
)-ones 4
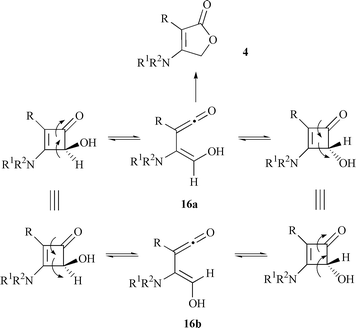
Following published procedures,13c,15 4-hydroxy-2-phenyl-3-pyrrolidinocyclobutenone 15f was refluxed in p-xylene until it disappeared completely by TLC. To our disappointment, this process took 46 h and the desired product, 3-phenyl-4-pyrrolidinofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 4f, was separated in 24% yield by column chromatography (Scheme 5). The low yield may reflect the fact that the electrocyclic opening of 15f favors ‘unsuitable’ intermediate 16b by outward rotation obeying Houk’s theory. Then 16b was converted into ‘suitable’ intermediate 16a though a reversible equilibration illustrated in Scheme 6. Finally, an intramolecular cyclization occurred to give target compound 4 by an attack of the hydroxy group on the ketene in 16a.
)-one 4f, was separated in 24% yield by column chromatography (Scheme 5). The low yield may reflect the fact that the electrocyclic opening of 15f favors ‘unsuitable’ intermediate 16b by outward rotation obeying Houk’s theory. Then 16b was converted into ‘suitable’ intermediate 16a though a reversible equilibration illustrated in Scheme 6. Finally, an intramolecular cyclization occurred to give target compound 4 by an attack of the hydroxy group on the ketene in 16a.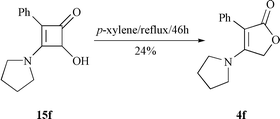 |
| | Scheme 5 | |
 |
| | Scheme 6 | |
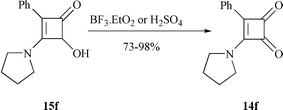
Since the intermediates 16b and 16a were hypothesized, the intramolecular cyclization between ketene and hydroxy group should be the key step to affect the reaction time and yield. It has been well known that the reaction between a ketene and a hydroxy group could be promoted significantly by acids and Lewis acids with variable results.18 Herein, several typical acids and Lewis acids were employed to test their effects on this conversion. As shown in Table 2, the lactonization can be accelerated by most Lewis acids, but without improvement in the yield. Reagents BF3–Et2O and H2SO4 induced an alternative result to give the oxidized product 3-phenyl-4-pyrrolidinocyclobutene-1,2-dione 14f (73 and 98% yield respectively) (Scheme 7).
Table 2 Effects of catalysts on the reaction of 15f
| Catalysts | mol% | Time (t/h) | 4f (%) |
|---|
| An oxidized product was obtained. Compound 15f disappeared completely on TLC. |
|---|
| AlCl3 | 5 | 18 | 28![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| ZnCl2 | 5 | 36 | 40![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| SnCl2 | 5 | 3 | 30![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| BF3–Et2O | 5 | 2 | 75![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
| H2SO4 | 100 | 0.5 | 98![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| HOAc | 100 | 46 | 34![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| TsOH | 100 | 10 | 42![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| TFA | 110 | 2.5 | 68![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| TFA | 200 | 2.0 | b |
 |
| | Scheme 7 | |
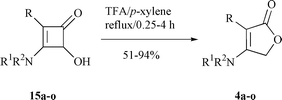
Acetic acid did not benefit either the reaction time or the yield of the reaction. However, the reaction catalyzed by TsOH was completed within 10 h in 42% yield. The best results were obtained in the presence of 1.1 mole equivalents of trifluoroacetic acid (TFA). Thus, a mixture of 15f (1.0 eq.) and TFA (1.1 eq.) in p-xylene was refluxed for 2.5 h gave 4f in 68% yield (Scheme 8). An attempt to decrease the reaction temperature failed. For example, 15f was recovered completely after being refluxed in THF for 12 h, even though 2.0 equivalents of TFA were used. This result strongly supported the two-step mechanism proposed in Scheme 6. Similarly, 14a–o were converted to the corresponding lactones 4a–o in 51–94% yield under the same conditions (Table 1).
 |
| | Scheme 8 | |
In conclusion, we have developed a simple and general route for preparation of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones. The typical substituted groups, such as H, n-Bu, and Ph on C-3 were introduced easily. The derivatives with unsubstituted, primary and secondary amines substituted on C-4 can all be obtained in satisfactory yields.
)-ones. The typical substituted groups, such as H, n-Bu, and Ph on C-3 were introduced easily. The derivatives with unsubstituted, primary and secondary amines substituted on C-4 can all be obtained in satisfactory yields.
Experimental
All mps were determined on a Yanaco melting point apparatus and are uncorrected. IR spectra were recorded on a Nicolet FT-IR 5DX spectrometer for samples as KBr pellets. 1H NMR spectra were recorded on a Bruker MD500 or MD300 spectrometer with TMS as internal reference in CDCl3 unless otherwise specified. J-Values are given in Hz. MS spectra were obtained on a VG-ZAB-HS mass spectrometer with 70 eV. Elemental analyses were performed on a Perkin-Elmer 240C instrument and satisfactory results obtained (C ± 0.29, H ± 0.0.24, N ± 0.0.27%) for all new compounds 14a–m, 15a–o, 4a–m and 4o. Light petroleum refers to the fraction with distillation range 60–90 °C.General procedure for preparation of 4-substituted 3-aminocyclobutene-1,2-diones 14
A mixture of a 4-substituted 3-isopropoxycyclobutene-1,2-dione 12 (10 mmol) and an amine 13 (10 mmol) in methanol (30 cm3) was stirred for 30 min (monitored by TLC). Then it was poured into water (40 cm3) and the resultant mixture was extracted with EtOAc (3 × 40 cm3). The combined organic layers were washed successively with water (40 cm3) and brine (40 cm3) and dried over MgSO4. The solvent was removed to give the crude product 14, which was purified by chromatography or recrystallization.3-Amino-4-phenylcyclobutene-1,2-dione 14a. Mp 257 °C (from EtOH–light petroleum) (Found: C, 69.20; H, 4.18; N, 8.09. C10H7NO2 requires C, 69.36; H, 4.07; N, 8.09%); νmax/cm−1 3316, 3119, 1791, 1721, 1661, 1600; δH (CD3OD) 7.99–7.97 (m, 2H), 7.53–7.47 (m, 3H); m/z 173 (M+, 13%), 145 (20), 117 (100), 104 (15), 89 (34), 77 (4), 63 (13), 51 (6).
3-Allylamino-4-phenylcyclobutene-1,2-dione 14b. Mp 189–191 °C (from EtOH–light petroleum); νmax/cm−1 1779, 1724; δH (acetone-d6) 8.02–8.00 (m, 2H), 7.54–7.46 (m, 3H), 6.08–6.02 (m, 1H), 5.33 (d, J 17.1, 1H), 5.20 (d, J 9.9, 1H), 4.48 (d, J 4.9, 2H); m/z 213 (M+, 27%), 89 (100).
3-(3-Methylbenzylamino)-4-phenylcyclobutene-1,2-dione 14c. Mp 235–237 °C (from EtOH–light petroleum); νmax/cm−1 1772, 1718; δH (acetone-d6) 8.01–8.00 (m, 2H), 7.51–7.45 (m, 3H), 7.27–7.23 (m, 3H), 7.14–7.13 (m, 1H), 5.04 (s, 2H), 2.32 (s, 3H); m/z 277 (M+, 59%), 105 (100).
3-Phenyl-4-[3-(trifluoromethyl)benzylamino]cyclobutene-1,2-dione 14d. Mp 222–223 °C (from EtOH–light petroleum); νmax/cm−1 1773, 1719; δH (acetone-d6) 8.00–7.99 (m, 2H), 7.83–7.79 (m, 2H), 7.69–7.62 (m, 2H), 7.52–7.46 (m, 3H), 5.20 (s, 2H); m/z 331 (M+, 20%), 89 (100).
3-Phenyl-4-[4-(trifluoromethoxy)benzylamino]cyclobutene-1,2-dione 14e. Mp 216–218 °C (from EtOH); νmax/cm−1 1781, 1722; δH (CD3OD) 7.97–7.96 (m, 2H), 7.53–7.48 (m, 5H), 7.31–7.30 (m, 2H), 5.03 (s, 2H); m/z 347 (M+, 21%), 175 (100).
3-Phenyl-4-pyrrolidinocyclobutene-1,2-dione 14f. Mp 132–133 °C (from EtOH–light petroleum); νmax/cm−1 1752, 1716; δH (acetone-d6) 7.76–7.75 (m, 2H), 7.51–7.48 (m, 2H), 7.44–7.41 (m, 1H), 4.04 (t, J 5.4, 2H), 3.69 (d, J 5.9, 2H), 2.07–2.05 (m, 4H); m/z 227 (M+, 4%), 171 (100).
3-Morpholino-4-phenylcyclobutene-1,2-dione 14g. Mp 146–147 °C (from EtOH–light petroleum); νmax/cm−1 1778, 1731; δH (acetone-d6) 7.63–7.44 (m, 5H), 4.11 (s, 2H), 3.85 (s, 4H), 3.70 (s, 2H); m/z 243 (M+, 5%), 187 (100).
3-Butyl-4-[3-(trifluoromethoxy)benzylamino]cyclobutene-1,2-dione 14h. Mp 68–71 °C (from C6H6–light petroleum); νmax/cm−1 1780, 1722; δH 7.47–7.14 (m, 4H), 4.89 (d, J 6.3, 1H), 4.67 (d, J 5.9, 1H), 2.54 (t, J 7.5, 2H), 1.63–1.50 (m, 2H), 1.37–1.26 (m, 2H), 0.88 (t, J 7.3, 3H); m/z 327 (M+, 9%), 195 (100).
3-Butyl-4-(4-fluorobenzylamino)cyclobutene-1,2-dione 14i. Mp 104–105 °C (from benzene–light petroleum); νmax/cm−1 1779, 1715; δH 7.32–7.25 (m, 2H), 7.12–7.00 (m, 2H), 4.83 (d, J 6.3, 1H), 4.60 (d, J 5.8, 1H), 2.53 (t, J 7.5, 2H), 1.64–1.53 (m, 2H), 1.38–1.29 (m, 2H), 0.90 (t, J 7.3, 3H); m/z 261 (M+, 3%), 109 (100).
3-Butyl-4-[3-(trifluoromethyl)benzylamino]cyclobutene-1,2-dione 14j. Mp 85–88 °C (from benzene–light petroleum); νmax/cm−1 1782, 1723; δH 7.57–7.43 (m, 4H), 4.91 (s, 2H), 2.52 (t, J 7.5, 2H), 1.62–1.55 (m, 2H), 1.35–1.26 (m, 2H), 0.86 (t, J 7.3, 3H); m/z 311 (M+, 4%), 159 (100).
3-Butyl-4-piperidinocyclobutene-1,2-dione 14k. An oil; νmax/cm−1 1778, 1731; δH 3.94 (s, 2H), 3.52 (s, 2H), 2.65 (t, J 7.6, 2H), 1.74 (s, 6H), 1.67–1.59 (m, 2H), 1.51–1.35 (m, 2H), 0.93 (t, J 7.3, 3H); m/z 221 (M+, 4%), 164 (100).
3-(4-Chlorobenzylamino)cyclobutene-1,2-dione 14l. Mp 118–119 °C (from benzene); νmax/cm−1 1777, 1741; δH 7.93 (s, 1H), 7.39–7.36 (m, 2H), 7.27–7.24 (m, 2H), 4.50 (s, 2H); m/z 221 (M+, 3%), 68 (100).
3-(3-Fluorobenzylamino)cyclobutene-1,2-dione 14m. Mp 69–70 °C (from benzene); νmax/cm−1 1779, 1751; δH 7.93 (s, 1H), 7.40–7.00 (m, 4H), 4.87 (s, 1H), 4.52 (s, 2H); m/z 205 (M+, 4%), 68 (100).
3-Pyrrolidinocyclobutene-1,2-dione 14n. Mp 104–105 °C (from benzene–light petroleum) (lit.,19 99 °C). Although 14n had a quite different mp from that of the reference material, it had exactly the same spectral data: νmax/cm−1 1775, 1745; δH 7.90 (s, 1H), 3.90–3.86 (m, 2H), 3.54–3.48 (m, 2H), 2.07–2.05 (m, 4H). 3-Morpholinocyclobutene-1,2-dione 14o. Mp 151–152 °C (from n-PrOH) (lit.,19 141 °C). Although 14o had a quite different mp from that of the reference material, it had exactly the same spectral data: νmax/cm−1 1779, 1739; δH 7.91 (s, 1H), 3.90–3.84 (m, 6H), 3.46–3.40 (m, 2H). General procedure for preparation of 2-substituted 3-amino-4-hydroxycyclobutenones (15)
To a stirred solution of a 4-substituted 3-aminocyclobutene-1,2-dione 14 (15 mmol) in methanol (50 cm3) was added partly powdered NaBH4 (1.0 g, 26 mmol) at 0 °C. After the reaction mixture had been kept for another 30 min (monitored by TLC) it was poured into ice–water (60 cm3). The resultant mixture was extracted with EtOAc (3 × 40 cm3) and the combined organic layers were washed successively with water (40 cm3) and brine (40 cm3) and dried over MgSO4. The solvent was removed to give the crude product 15, which was purified by chromatography or recrystallization.3-Amino-4-hydroxy-2-phenylcyclobutenone 15a. Mp 232–233 °C (decomp.) (from EtOH–light petroleum) (Found: C, 68.46; H, 5.13; N, 7.93. C10H9NO2 requires C, 68.56; H, 5.18; N, 8.00%); νmax/cm−1 3285, 3237, 3102, 1734, 1661, 1600; δH (CD3OD) 7.62–7.61 (m, 2H), 7.34–7.31 (m, 2H), 7.19–7.16 (m, 1H), 4.96 (s, 1H); m/z 175 (M+, 100%), 147 (66), 130 (56), 118 (96), 102 (94), 89 (86), 77 (35), 63 (43), 51 (39), 44 (37).
3-Allylamino-4-hydroxy-2-phenylcyclobutenone 15b. Mp 187–189 °C (from EtOH–light petroleum); νmax/cm−1 3302, 1725; δH (acetone-d6) 7.69–7.67 (m, 2H), 7.32–7.29 (m, 2H), 7.16–7.13 (m, 1H), 6.10–6.06 (m, 1H), 5.32 (d, J 17.1, 1H), 5.17 (d, J 10.1, 1H), 5.11 (s, 1H), 4.29–4.24 (m, 1H), 4.19–4.16 (m, 1H); m/z 215 (M+, 61%), 174 (100).
4-Hydroxy-3-(3-methylbenzylamino)-2-phenylcyclobutenone 15c. Mp 202–203 °C (from EtOH–light petroleum); νmax/cm−1 3330, 1722; δH (acetone-d6) 7.69–7.68 (m, 2H), 7.26–7.14 (m, 7H), 5.20 (s, 1H), 4.76 (s, 2H), 2.32 (s, 3H); m/z 279 (M+, 21%), 105 (100).
4-Hydroxy-2-phenyl-3-[3-(trifluoromethyl)benzylamino]cyclobutenone 15d. Mp 200–201 °C (from EtOH–light petroleum); νmax/cm−1 3325, 1727; δH (acetone-d6) 7.85–7.81 (m, 2H), 7.69–7.62 (m, 4H), 7.31–7.28 (m, 2H), 7.16–7.13 (m, 1H), 5.28 (s, 1H), 4.94 (s, 2H); m/z 333 (M+, 53%), 159 (100).
4-Hydroxy-2-phenyl-3-[4-(trifluoromethoxy)benzylamino]cyclobutenone 15e. Mp 192–194 °C (from EtOH–light petroleum); νmax/cm−1 3316, 1724; δH (CD3OD) 7.63–7.62 (m, 2H), 7.55–7.53 (m, 2H), 7.35–7.27 (m, 4H), 7.20–7.17 (m, 1H), 5.15 (s, 1H), 4.75 (s, 2H); m/z 349 (M+, 19%), 175 (100).
4-Hydroxy-2-phenyl-3-pyrrolidinocyclobutenone 15f. Mp 199–201 °C (from EtOH–light petroleum); νmax/cm−1 1725; δH (acetone-d6) 7.49–7.47 (m, 2H), 7.32–7.27 (m, 2H), 7.18–7.15 (m, 1H), 5.05 (s, 1H), 4.06–4.02 (m, 1H), 3.71–3.68 (m, 1H), 3.63–3.58 (m, 1H), 3.40–3.36 (m, 1H), 2.05–1.99 (m, 4H); m/z 229 (M+, 43%), 70 (100).
4-Hydroxy-3-morpholino-2-phenylcyclobutenone 15g. Mp 195–197 °C (from EtOH–light petroleum); νmax/cm−1 3216, 1729; δH (acetone-d6) 7.40–7.19 (m, 5H), 5.11 (s, 1H), 3.80 (br s, 6H), 3.56 (br s, 2H); m/z 245 (M+, 48%), 87 (100).
2-Butyl-4-hydroxy-3-[3-(trifluoromethoxy)benzylamino]cyclobutenone 15h. Mp 92 °C (from benzene); νmax/cm−1 3225, 1731; δH 7.39–7.12 (m, 4H), 6.15 (br s, 1H), 4.99 (s, 1H), 4.55 (d, J 5.5, 2H), 1.90 (t, J 7.8, 2H), 1.25–1.23 (m, 2H), 1.17–1.13 (m, 2H), 0.78 (t, J 7.2, 3H); m/z 329 (M+, 15%), 175 (100).
2-Butyl-4-hydroxy-3-(4-fluorobenzylamino)cyclobutenone 15i. Mp 113–114 °C (from benzene); νmax/cm−1 3239, 1735; δH 7.29–7.25 (m, 2H), 7.04–6.94 (m, 2H), 6.16 (br s, 1H), 4.95 (s, 1H), 4.50 (d, J 5.6, 2H), 1.91 (t, J 7.5, 2H), 1.28–1.14 (m, 4H), 0.80 (t, J 7.3, 3H); m/z 263 (M+, 6%), 109 (100).
2-Butyl-4-hydroxy-3-[3-(trifluoromethyl)benzylamino]cyclobutenone 15j. Mp 133 °C (from benzene); νmax/cm−1 3234, 1731; δH (CD3OD) 7.69–7.54 (m, 4H), 5.00 (s, 1H), 4.67 (s, 2H), 2.04 (t, J 7.6, 2H), 1.49–1.44 (m, 2H), 1.36–1.29 (m, 2H), 0.90 (t, J 7.3, 3H); m/z 313 (M+, 12%), 159 (100).
2-Butyl-4-hydroxy-3-piperidinocyclobutenone 15k. Mp 84–86 °C (from benzene); νmax/cm−1 3244, 1737; δH 5.05 (s, 1H), 3.65 (s, 2H), 3.48 (s, 2H), 2.11 (t, J 7.6, 2H), 1.70 (s, 6H), 1.50–1.40 (m, 2H), 1.37–1.26 (m, 2H), 0.89 (t, J 7.3, 3H); m/z 223 (M+, 0.6%), 57 (100).
3-(4-Chlorobenzylamino)-4-hydroxycyclobutenone 15l. Mp 148–150 °C (from MeOH–water); νmax/cm−1 3230, 1728; δH (CDCl3–CD3OD) 7.35–7.33 (m, 2H), 7.30–7.25 (m, 2H), 4.93 (s, 1H), 4.43–4.35 (m, 1H), 4.27 (s, 2H); m/z 223 (M+, 16%), 125 (100).
3-(3-Fluorobenzylamino)-4-hydroxycyclobutenone 15m. Mp 108–111 °C (from benzene); νmax/cm−1 3201, 1718; δH (CDCl3–CD3OD) 7.40–7.32 (m, 1H), 7.13–7.09 (m, 1H), 7.06–7.00 (m, 2H), 4.95 (s, 1H), 4.45–4.37 (m, 1H), 4.10 (s, 2H); m/z 207 (M+, 14%), 109 (100).
4-Hydroxy-3-pyrrolidinocyclobutenone 15n. Mp 133–136 °C (from benzene–light petroleum); νmax/cm−1 3178, 1729; δH 6.04 (br s, 1H), 5.12 (s, 1H), 4.82 (s, 1H), 3.98–3.93 (m, 1H), 3.50–3.45 (m, 1H), 3.39–3.37 (m, 2H), 2.05–2.00 (m, 4H); m/z 153 (M+, 67%), 95 (100).
4-Hydroxy-3-morpholinocyclobutenone 15o. Mp 117–119 °C (from benzene–light petroleum); νmax/cm−1 3232, 1725; δH 6.15 (br s, 1H), 5.17 (s, 1H), 4.93 (s, 1H), 3.86–3.81 (m, 5H), 3.64–3.60 (m, 1H), 3.48–3.41 (m, 2H); m/z 169 (M+, 68%), 55 (100).
General procedure for preparation of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-ones 4
)-ones 4
A mixture of a 2-substituted 3-amino-4-hydroxycyclobutenone 15 (15 mmol) and TFA (1.88 g, 16.5 mmol) in anhydrous p-xylene (35 cm3) was refluxed until compound 15 had disappeared completely on TLC. Then it was washed with water (35 cm3) and the organic layer was dried over MgSO4. The solvent was removed to give the crude product 4, which was purified by chromatography or recrystallization.4-Amino-3-phenylfuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4a. Mp 198–200 °C (from EtOH–water) (Found: C, 68.66; H, 5.26; N, 8.09. C10H9NO2 requires C, 68.56; H, 5.18; N, 8.00%); νmax/cm−1 3463, 3299, 3177, 1696, 1641, 1609, 1592; δH (acetone-d6) 7.55–7.52 (m, 2H), 7.38–7.35 (m, 2H), 7.22–7.20 (m, 1H), 6.11 (br s, 2H), 4.74 (s, 2H); m/z 175 (M+, 100%), 146 (56), 130 (17), 118 (51), 91 (24), 77 (8), 63 (9).
)-one 4a. Mp 198–200 °C (from EtOH–water) (Found: C, 68.66; H, 5.26; N, 8.09. C10H9NO2 requires C, 68.56; H, 5.18; N, 8.00%); νmax/cm−1 3463, 3299, 3177, 1696, 1641, 1609, 1592; δH (acetone-d6) 7.55–7.52 (m, 2H), 7.38–7.35 (m, 2H), 7.22–7.20 (m, 1H), 6.11 (br s, 2H), 4.74 (s, 2H); m/z 175 (M+, 100%), 146 (56), 130 (17), 118 (51), 91 (24), 77 (8), 63 (9). 4-Allylamino-3-phenylfuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4b. Mp 84–85 °C (from benzene–light petroleum); νmax/cm−1 1708; δH 7.48–7.26 (m, 5H), 5.90–5.82 (m, 1H), 5.52 (br s, 1H), 5.29 (s, 1H), 5.25 (d, J 9.6, 1H), 4.77 (s, 2H), 3.78 (t, J 5.2, 2H); m/z 215 (M+, 100%).
)-one 4b. Mp 84–85 °C (from benzene–light petroleum); νmax/cm−1 1708; δH 7.48–7.26 (m, 5H), 5.90–5.82 (m, 1H), 5.52 (br s, 1H), 5.29 (s, 1H), 5.25 (d, J 9.6, 1H), 4.77 (s, 2H), 3.78 (t, J 5.2, 2H); m/z 215 (M+, 100%). 4-(3-Methylbenzylamino)-3-phenylfuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4c. Mp 98–99 °C (from EtOAc–light petroleum); νmax/cm−1 1713; δH 7.48–7.07 (m, 9H), 5.71 (br s, 1H), 4.76 (s, 2H), 4.31 (d, J 6.1, 2H), 2.36 (s, 3H); m/z 279 (M+, 18%), 106 (100).
)-one 4c. Mp 98–99 °C (from EtOAc–light petroleum); νmax/cm−1 1713; δH 7.48–7.07 (m, 9H), 5.71 (br s, 1H), 4.76 (s, 2H), 4.31 (d, J 6.1, 2H), 2.36 (s, 3H); m/z 279 (M+, 18%), 106 (100). 3-Phenyl-4-[3-(trifluoromethyl)benzylamino]furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4d. Mp 125.5–127 °C (from EtOAc–light petroleum); νmax/cm−1 1714; δH 7.61–7.24 (m, 9H), 5.80 (s, 1H), 4.74 (s, 2H), 4.40 (s, 2H); m/z 333 (M+, 40%), 159 (100).
)-one 4d. Mp 125.5–127 °C (from EtOAc–light petroleum); νmax/cm−1 1714; δH 7.61–7.24 (m, 9H), 5.80 (s, 1H), 4.74 (s, 2H), 4.40 (s, 2H); m/z 333 (M+, 40%), 159 (100). 3-Phenyl-4-[4-(trifluoromethoxy)benzylamino]furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4e. Mp 126–127 °C (from benzene–light petroleum); νmax/cm−1 1714; δH 7.46–7.21 (m, 9H), 5.87 (s, 1H), 4.72 (s, 2H), 4.34 (s, 2H); m/z 349 (M+, 72%), 175 (100).
)-one 4e. Mp 126–127 °C (from benzene–light petroleum); νmax/cm−1 1714; δH 7.46–7.21 (m, 9H), 5.87 (s, 1H), 4.72 (s, 2H), 4.34 (s, 2H); m/z 349 (M+, 72%), 175 (100). 3-Phenyl-4-pyrrolidinofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4f. Mp 129–131 °C (from EtOAc–light petroleum); νmax/cm−1 1710; δH 7.34–7.24 (m, 5H), 4.74 (s, 2H), 3.17 (br s, 4H), 1.88 (br s, 4H); m/z 229 (M+, 67%), 70 (100).
)-one 4f. Mp 129–131 °C (from EtOAc–light petroleum); νmax/cm−1 1710; δH 7.34–7.24 (m, 5H), 4.74 (s, 2H), 3.17 (br s, 4H), 1.88 (br s, 4H); m/z 229 (M+, 67%), 70 (100). 4-Morpholino-3-phenylfuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4g. Mp 189–190 °C (from benzene–light petroleum); νmax/cm−1 1713; δH 7.38–7.27 (m, 5H), 4.78 (s, 2H), 3.66 (t, J 4.8, 4H), 3.18 (t, J 4.8, 4H); m/z 245 (M+, 56%), 40 (100).
)-one 4g. Mp 189–190 °C (from benzene–light petroleum); νmax/cm−1 1713; δH 7.38–7.27 (m, 5H), 4.78 (s, 2H), 3.66 (t, J 4.8, 4H), 3.18 (t, J 4.8, 4H); m/z 245 (M+, 56%), 40 (100). 3-Butyl-4-[3-(trifluoromethoxy)benzylamino]furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4h. An oil; νmax/cm−1 1725; δH 7.41–7.14 (m, 4H), 6.13 (br s, 1H), 4.53 (s, 2H), 4.38 (d, J 6.3, 2H), 2.15 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.29–1.20 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 329 (M+, 5%), 175 (100).
)-one 4h. An oil; νmax/cm−1 1725; δH 7.41–7.14 (m, 4H), 6.13 (br s, 1H), 4.53 (s, 2H), 4.38 (d, J 6.3, 2H), 2.15 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.29–1.20 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 329 (M+, 5%), 175 (100). 3-Butyl-4-(4-fluorobenzylamino)furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4i. An oil; νmax/cm−1 1723; δH 7.29–7.23 (m, 2H), 7.06–6.96 (m, 2H), 5.97 (br s, 1H), 4.53 (s, 2H), 4.32 (d, J 6.1, 2H), 2.14 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.26 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 263 (M+, 7%), 109 (100).
)-one 4i. An oil; νmax/cm−1 1723; δH 7.29–7.23 (m, 2H), 7.06–6.96 (m, 2H), 5.97 (br s, 1H), 4.53 (s, 2H), 4.32 (d, J 6.1, 2H), 2.14 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.26 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 263 (M+, 7%), 109 (100). 3-Butyl-4-[3-(trifluoromethyl)benzylamino]furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4j. An oil; νmax/cm−1 1724; δH 7.57–7.48 (m, 4H), 5.94 (s, 1H), 4.54 (s, 2H), 4.41 (s, 2H), 2.16 (t, J 7.7, 2H), 1.46–1.39 (m, 2H), 1.32–1.25 (m, 2H), 0.87 (t, J 7.2, 3H); m/z 313 (M+, 9%), 159 (100).
)-one 4j. An oil; νmax/cm−1 1724; δH 7.57–7.48 (m, 4H), 5.94 (s, 1H), 4.54 (s, 2H), 4.41 (s, 2H), 2.16 (t, J 7.7, 2H), 1.46–1.39 (m, 2H), 1.32–1.25 (m, 2H), 0.87 (t, J 7.2, 3H); m/z 313 (M+, 9%), 159 (100). 3-Butyl-4-piperidinofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4k. An oil; νmax/cm−1 1730; δH 4.56 (s, 2H), 3.94 (s, 1H), 3.52 (s, 1H), 3.33 (s, 2H), 2.65 (t, J 7.6, 1H), 2.31 (t, J 7.3, 1H), 1.74 (s, 6H), 1.47–1.32 (m, 4H), 0.93 (t, J 7.3, 3H); m/z 223 (M+, 13%), 180 (100).
)-one 4k. An oil; νmax/cm−1 1730; δH 4.56 (s, 2H), 3.94 (s, 1H), 3.52 (s, 1H), 3.33 (s, 2H), 2.65 (t, J 7.6, 1H), 2.31 (t, J 7.3, 1H), 1.74 (s, 6H), 1.47–1.32 (m, 4H), 0.93 (t, J 7.3, 3H); m/z 223 (M+, 13%), 180 (100). 4-(4-Chlorobenzylamino)furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4l. Mp 161–162 °C (from CHCl3–light petroleum); νmax/cm−1 1698; δH (CDCl3–CD3OD) 7.37–7.32 (m, 2H), 7.25–7.23 (m, 2H), 4.70 (s, 2H), 4.26 (s, 2H), 3.97 (s, 1H); m/z 223 (M+, 15%), 125 (100).
)-one 4l. Mp 161–162 °C (from CHCl3–light petroleum); νmax/cm−1 1698; δH (CDCl3–CD3OD) 7.37–7.32 (m, 2H), 7.25–7.23 (m, 2H), 4.70 (s, 2H), 4.26 (s, 2H), 3.97 (s, 1H); m/z 223 (M+, 15%), 125 (100). 4-(3-Fluorobenzylamino)furan-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4m. Mp 165–166.5 °C (from EtOAc–light petroleum); νmax/cm−1 1701; δH (CDCl3–CD3OD) 7.47–7.01 (m, 4H), 4.62 (s, 1H), 4.42 (s, 2H), 4.31 (s, 2H); m/z 207 (M+, 20%), 109 (100).
)-one 4m. Mp 165–166.5 °C (from EtOAc–light petroleum); νmax/cm−1 1701; δH (CDCl3–CD3OD) 7.47–7.01 (m, 4H), 4.62 (s, 1H), 4.42 (s, 2H), 4.31 (s, 2H); m/z 207 (M+, 20%), 109 (100). 4-Pyrrolidinofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4n. Mp 119–121 °C (from benzene) (lit.,6b 120–121 °C).
)-one 4n. Mp 119–121 °C (from benzene) (lit.,6b 120–121 °C). 4-Morpholinofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4o. Mp 107–109 °C (from benzene); νmax/cm−1 1714; δH 4.74 (s, 2H), 4.72 (s, 1H), 3.79 (t, J 4.7, 4H), 3.23 (t, J 4.7, 4H); m/z 169 (M+, 100%).
)-one 4o. Mp 107–109 °C (from benzene); νmax/cm−1 1714; δH 4.74 (s, 2H), 4.72 (s, 1H), 3.79 (t, J 4.7, 4H), 3.23 (t, J 4.7, 4H); m/z 169 (M+, 100%). Acknowledgements
We are grateful to the Education Ministry of China and the Education Department of Jiangsu Province for financial support.References
- For example, see:
(a) R. H. Schlessinger, A. M. M. Mjalli and A. D. Adams, J. Org. Chem., 1992, 57, 2992 CrossRef CAS;
(b) R. H. Schlessinger and T. R. T. Pettus, J. Org. Chem., 1994, 59, 3246 CrossRef CAS;
(c) K. Nishide, A. Aramata, T. Kamanaka, T. Inoue and M. Node, Tetrahedron, 1994, 50, 8337 CrossRef CAS;
(d) R. H. Schlessinger and Y. Li, J. Am. Chem. Soc., 1996, 118, 3301 CrossRef CAS;
(e) S. M Dankwardt, J. W. Dankwardt and R. H. Schlessinger, Tetrahedron Lett., 1998, 39, 4971 CrossRef CAS.
- For example, see:
(a) T. Takeshi and
K. Masumoto,
Jpn. Kokai, Tokkyo Koho JP 63 93,774 (Chem. Abstr.,
1988, 109, 110244j).
Search PubMed;
(b) T. Nakai,
K. Masumoto,
M. Mizutani and
A. Yoshida,
Jpn. Kokai, Tokkyo Koho JP 63 174, 983 (Chem. Abstr.,
1988, 110, 2909s).
Search PubMed;
(c) W. KraemerG. KleefeldJ. Bachmann,
P. Babczinski,
H. J. Santel,
K. Luerssen and
R. R. Schmidt,
Ger. Offen. DE 4, 014, 420 (Chem. Abstr.,
1991, 115, 71376f).
Search PubMed;
(d) H. Ohishi,
T. Iihama,
K. Ishimitsu and
T. Yamada,
PCT Int. Appl. WO 92 00, 964 (Chem. Abstr.,
1992, 117, 7806k).
Search PubMed.
- K. J. Boosen, Helv. Chim. Acta, 1977, 60, 1256 CrossRef CAS.
-
(a) T. Hiyama, H. Oishi, Y. Suetsugu, K. Nishide and H. Saimoto, Bull. Chem. Soc. Jpn., 1987, 60, 2139 CAS;
(b) J. J. Duffield and A. C. Regan, Tetrahedron: Asymmetry, 1996, 7, 663 CrossRef CAS.
-
(a) J. V. Greenhill, M. Ramli and T. Tomassini, J. Chem. Soc., Perkin Trans. 1, 1975, 588 RSC;
(b) R. R. Schmidt and J. Talbiersky, Angew. Chem., Int. Ed. Engl., 1978, 17, 204 CrossRef;
(c) R. H. Schlessinger, E. J. Iwanowicz and J. P. Springer, Tetrahedron Lett., 1988, 29, 1489 CrossRef CAS.
-
(a) F. Farina, M. V. Martin, F. Sanchez, M. C. Maestro and M. R. Martin, Synthesis, 1983, 397 CrossRef CAS;
(b) T. Momose, N. Toyooka, T. Nishi and Y. Takeuchi, Heterocycles, 1988, 27, 1907 Search PubMed;
(c) M. R. Martin and A. I. Mateo, Tetrahedron: Asymmetry, 1994, 5, 1385 CrossRef CAS;
(d) Y. Hitotsuyanagi, M. Kobayashi, M. Fukuyo, K. Takeya and H. Hokawa, Tetrahedron Lett., 1997, 38, 8295 CrossRef CAS.
- B. de Ancos, M. C. Maestro, M. R. Martin and A. I. Mateo, Tetrahedron, 1994, 50, 13857 CrossRef CAS.
-
(a) J. R. Arderson, R. L. Edwards and A. J. S. Whalley, J. Chem. Soc., Perkin Trans. 1, 1982, 215 RSC;
(b) R. Ramage, G. J. Griffiths and F. E. Shutt, J. Chem. Soc., Perkin Trans. 1, 1984, 1539 RSC;
(c) L. R. Krepski, L. E. Lynch, S. M. Heilmann and J. K. Rasmussen, Tetrahedron Lett., 1985, 26, 981 CrossRef CAS;
(d) J. Syed, S. Forster and F. Effenberger, Tetrahedron: Asymmetry, 1998, 9, 805 CrossRef CAS.
-
(a) D. J. Krysan, A. Gurski and L. S. Liebeskind, J. Am. Chem. Soc., 1992, 114, 1412 CrossRef CAS;
(b) A. Gurski and L. S. Liebeskind, J. Am. Chem. Soc., 1993, 115, 6101 CrossRef CAS;
(c) N. A. Petasis and D. K. Fu, Synlett, 1996, 155 CrossRef CAS.
-
(a) L. D. Foland, J. O. Karlsson, S. T. Perri, R. Schwabe, S. L. Xu, S. Patil and H. W. Moore, J. Am. Chem. Soc., 1989, 111, 975 CrossRef CAS;
(b) S. T. Perri and H. W. Moore, J. Am. Chem. Soc., 1990, 112, 1897 CrossRef CAS;
(c) S. L. Xu, M. Taing and H. W. Moore, J. Org. Chem., 1991, 56, 6104 CrossRef CAS;
(d) H. Xia and H. W. Moore, J. Org. Chem., 1992, 57, 3765 CrossRef CAS;
(e) R. Tiedmann, M. J. Heileman and H. W. Moore, J. Org. Chem., 1999, 64, 2170 CrossRef;
(f) R. Tiedemann, P. Turnbull and H. W. Moore, J. Org. Chem., 1999, 64, 4030 CrossRef CAS;
(g) P. Wipf and C. R. Hopkins, J. Org. Chem., 1999, 64, 6879 CrossRef.
-
(a) L. S. Liebeskind and J. Wang, J. Org. Chem., 1993, 58, 3550 CrossRef CAS;
(b) P. Mingo, S. Zhang and L. S. Liebeskind, J. Org. Chem., 1999, 64, 2145 CrossRef CAS;
(c) S. Zhang and L. S. Liebeskind, J. Org. Chem., 1999, 64, 4042 CrossRef CAS;
(d) A. R. Hergueta and H. W. Moore, J. Org. Chem., 1999, 64, 5979 CrossRef.
-
(a) W. Kirmse, N. G. Rondan and K. N. Houk, J. Am. Chem. Soc., 1984, 106, 7989 CrossRef CAS;
(b) S. Niwayama, E. Adam Kallel, C. Sheu and K. N. Houk, J. Org. Chem., 1996, 61, 2517 CrossRef CAS.
-
(a) H. W. Moore and S. T. Perri, J. Org. Chem., 1988, 53, 996 CrossRef CAS;
(b) D. J. Pollart and H. W. Moore, J. Org. Chem., 1989, 54, 5444 CrossRef;
(c) A. G. Birchler, F. Liu and L. S. Liebeskind, J. Org. Chem., 1994, 59, 7737 CrossRef CAS;
(d) P. Turnbull, M. J. Heileman and H. W. Moore, J. Org. Chem., 1996, 61, 2584 CrossRef CAS;
(e) F. Liu and L. S. Liebeskind, J. Org. Chem., 1998, 63, 2835 CrossRef CAS.
-
(a) S. T. Perri, L. D. Foland and H. W. Moore, Tetrahedron Lett., 1988, 29, 3529 CrossRef CAS;
(b) Y. Yamamoto, M. Ohno and S. Eguchi, J. Org. Chem., 1994, 59, 4707 CrossRef CAS;
(c) Y. Yamamoto, M. Ohno and S. Eguchi, J. Am. Chem. Soc., 1995, 117, 9653 CrossRef CAS.
- Y. Yamamoto, M. Ohno and S. Eguchi, Tetrahedron, 1994, 50, 7783 CrossRef CAS.
-
(a) E. V. Dehmlow and H. G. Schell, Chem. Ber., 1980, 113, 1 Search PubMed;
(b) J. L. Kraus, Tetrahedron Lett., 1985, 26, 1867 CrossRef;
(c) L. S. Liebeskind, R. W. Fengl, K. R. Wirtz and T. T. Shawe, J. Org. Chem., 1988, 53, 2482 CrossRef CAS.
-
(a) A. H. Schmidt, Synthesis, 1980, 961 CrossRef CAS;
(b) L. F. Tietze, M. Arlt, M. Beller, K. H. Glusenkamp, E. Jahde and M. F. Rajewsky, Chem. Ber., 1991, 124, 1215 CrossRef CAS;
(c) O. Blixt and T. Norberg, Carbohydr. Res., 1999, 319, 80 CrossRef CAS.
-
(a) R. N. Lacey, Adv. Org. Chem., 1960, 2, 213 Search PubMed;
(b) H. Pracejus and R. Samtleben, Z. Chem., 1972, 12, 153 Search PubMed;
(c) N. L. Poon and D. P. N. Satchell, J. Chem. Soc., Perkin Trans. 2, 1984, 1083 RSC;
(d) N. L. Poon and D. P. N. Satchell, J. Chem. Soc., Perkin Trans. 2, 1985, 1551 RSC.
- G. Seitz, H. Morck, R. Schmidel and R. Sutrisno, Synthesis, 1979, 361 CrossRef CAS.
Footnote |
| † Elemental analyses results and detailed IR, MS spectra of new compounds 14a–o, 15a–o, 4a–m and 4o are available as supplementary data. For direct electronic access see http://www.rsc.org/suppdata/p1/b0/b004654j/ |
|
| This journal is © The Royal Society of Chemistry 2001 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) )-ones
)-ones![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) †
†![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones 4 is developed. A two-step mechanism is proposed to describe the ring transformation of enones 15. The experimental results show that the process in refluxing p-xylene is essential for the initial thermal electrocyclic opening of 15 to yield intermediate hydroxy ketenes 16a and 16b. They are then lactonized in the presence of TFA to give furanones 4 in 51–94% yield.
)-ones 4 is developed. A two-step mechanism is proposed to describe the ring transformation of enones 15. The experimental results show that the process in refluxing p-xylene is essential for the initial thermal electrocyclic opening of 15 to yield intermediate hydroxy ketenes 16a and 16b. They are then lactonized in the presence of TFA to give furanones 4 in 51–94% yield.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one occur in nature, their synthetic analogues are widely used in chemical, pharmaceutical and agrochemical research. Some 4-aminofuran-2(5H
)-one occur in nature, their synthetic analogues are widely used in chemical, pharmaceutical and agrochemical research. Some 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones have been intermediates in the synthesis of natural products.1 Many have been patented as prodrugs or insecticides and herbicides
)-ones have been intermediates in the synthesis of natural products.1 Many have been patented as prodrugs or insecticides and herbicides![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 (for example, see: 1–3 in Chart 1).
2 (for example, see: 1–3 in Chart 1).
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones.3–7 Using cyanohydrin derivatives as starting materials, primary 4-aminofuran-2(5H
)-ones.3–7 Using cyanohydrin derivatives as starting materials, primary 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones, being mono- or bis-substituted at C-5 to meet the requirements of the reaction mechanism, were obtained.4 The sequence of aminoaddition on acetylenecarboxylates followed by intramolecular cyclizations afforded 3-unsubstituted primary or secondary 4-aminofuran-2(5H
)-ones, being mono- or bis-substituted at C-5 to meet the requirements of the reaction mechanism, were obtained.4 The sequence of aminoaddition on acetylenecarboxylates followed by intramolecular cyclizations afforded 3-unsubstituted primary or secondary 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones smoothly.5 Although 4-halogeno- or 4-hydroxyfuran-2(5H
)-ones smoothly.5 Although 4-halogeno- or 4-hydroxyfuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones can be aminated to yield the corresponding unsubstituted, primary and secondary 4-aminofuran-2(5H
)-ones can be aminated to yield the corresponding unsubstituted, primary and secondary 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones conveniently,1c,1d,6 the method has been limited by inaccessible precursors.8 Very few procedures can be employed for direct alkylation of 4-aminofuran-2(5H
)-ones conveniently,1c,1d,6 the method has been limited by inaccessible precursors.8 Very few procedures can be employed for direct alkylation of 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones at C-3.6a,7
)-ones at C-3.6a,7![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (4 in Chart 1) were designed as targets for synthesis. Herein, we report a novel and practical route for the synthesis of 3-substituted 4-aminofuran-2(5H
)-ones (4 in Chart 1) were designed as targets for synthesis. Herein, we report a novel and practical route for the synthesis of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones that overcomes some of the limitations to published procedures.
)-ones that overcomes some of the limitations to published procedures.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 10 and heteroaromatic compounds (8) (Chart 2).11
10 and heteroaromatic compounds (8) (Chart 2).11
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones formed by intermediate 10 in thermal electrocyclic reactions were believed to be unusual by-products,13 even though they were obtained as sole products in photochemistry or in metal-catalyzed reactions.14
)-ones formed by intermediate 10 in thermal electrocyclic reactions were believed to be unusual by-products,13 even though they were obtained as sole products in photochemistry or in metal-catalyzed reactions.14
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one was obtained by attack of the hydroxy group on the ketene involved in the intermediate 10.13,15 It clearly implies that the thermolysis of 5d (R = H) should yield a furan-2(5H
)-one was obtained by attack of the hydroxy group on the ketene involved in the intermediate 10.13,15 It clearly implies that the thermolysis of 5d (R = H) should yield a furan-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one because only the hydroxy group as trapping group is present in the molecule. As shown in Scheme 1, this transformation has been developed as a key step for the preparation of 3-substituted 4-aminofuran-2(5H
)-one because only the hydroxy group as trapping group is present in the molecule. As shown in Scheme 1, this transformation has been developed as a key step for the preparation of 3-substituted 4-aminofuran-2(5H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones 4 in our synthetic strategy.
)-ones 4 in our synthetic strategy.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 16 from 3,4-diisopropoxycyclobutene-1,2-dione 11. The vinylogous ester at C-3 in the structures 12a–c made the substitution of the isopropoxy group by various amines very easy.17 Thus, a mixture of 3-isopropoxy-4-phenylcyclobutene-1,2-dione 12a and ammonium hydroxide (28% solution in water, 13a) in methanol was stirred for 30 min at room temperature, and 3-amino-4-phenylcyclobutene-1,2-dione 14a was separated in 96% yield. To explore the scope of the reaction, allylic, benzyl and cyclic amines (13b–g) were tested and all of them gave excellent yields (14b–g, 88–96%) (Scheme 2).
16 from 3,4-diisopropoxycyclobutene-1,2-dione 11. The vinylogous ester at C-3 in the structures 12a–c made the substitution of the isopropoxy group by various amines very easy.17 Thus, a mixture of 3-isopropoxy-4-phenylcyclobutene-1,2-dione 12a and ammonium hydroxide (28% solution in water, 13a) in methanol was stirred for 30 min at room temperature, and 3-amino-4-phenylcyclobutene-1,2-dione 14a was separated in 96% yield. To explore the scope of the reaction, allylic, benzyl and cyclic amines (13b–g) were tested and all of them gave excellent yields (14b–g, 88–96%) (Scheme 2).
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a
a![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHCH2
CHCH2
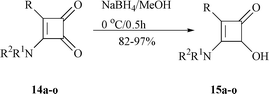
![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-ones 4
)-ones 4![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 4f, was separated in 24% yield by column chromatography (Scheme 5). The low yield may reflect the fact that the electrocyclic opening of 15f favors ‘unsuitable’ intermediate 16b by outward rotation obeying Houk’s theory. Then 16b was converted into ‘suitable’ intermediate 16a though a reversible equilibration illustrated in Scheme 6. Finally, an intramolecular cyclization occurred to give target compound 4 by an attack of the hydroxy group on the ketene in 16a.
)-one 4f, was separated in 24% yield by column chromatography (Scheme 5). The low yield may reflect the fact that the electrocyclic opening of 15f favors ‘unsuitable’ intermediate 16b by outward rotation obeying Houk’s theory. Then 16b was converted into ‘suitable’ intermediate 16a though a reversible equilibration illustrated in Scheme 6. Finally, an intramolecular cyclization occurred to give target compound 4 by an attack of the hydroxy group on the ketene in 16a.



![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones. The typical substituted groups, such as H, n-Bu, and Ph on C-3 were introduced easily. The derivatives with unsubstituted, primary and secondary amines substituted on C-4 can all be obtained in satisfactory yields.
)-ones. The typical substituted groups, such as H, n-Bu, and Ph on C-3 were introduced easily. The derivatives with unsubstituted, primary and secondary amines substituted on C-4 can all be obtained in satisfactory yields.![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-ones 4
)-ones 4![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4a. Mp 198–200 °C (from EtOH–water) (Found: C, 68.66; H, 5.26; N, 8.09. C10H9NO2 requires C, 68.56; H, 5.18; N, 8.00%); νmax/cm−1 3463, 3299, 3177, 1696, 1641, 1609, 1592; δH (acetone-d6) 7.55–7.52 (m, 2H), 7.38–7.35 (m, 2H), 7.22–7.20 (m, 1H), 6.11 (br s, 2H), 4.74 (s, 2H); m/z 175 (M+, 100%), 146 (56), 130 (17), 118 (51), 91 (24), 77 (8), 63 (9).
)-one 4a. Mp 198–200 °C (from EtOH–water) (Found: C, 68.66; H, 5.26; N, 8.09. C10H9NO2 requires C, 68.56; H, 5.18; N, 8.00%); νmax/cm−1 3463, 3299, 3177, 1696, 1641, 1609, 1592; δH (acetone-d6) 7.55–7.52 (m, 2H), 7.38–7.35 (m, 2H), 7.22–7.20 (m, 1H), 6.11 (br s, 2H), 4.74 (s, 2H); m/z 175 (M+, 100%), 146 (56), 130 (17), 118 (51), 91 (24), 77 (8), 63 (9).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4b. Mp 84–85 °C (from benzene–light petroleum); νmax/cm−1 1708; δH 7.48–7.26 (m, 5H), 5.90–5.82 (m, 1H), 5.52 (br s, 1H), 5.29 (s, 1H), 5.25 (d, J 9.6, 1H), 4.77 (s, 2H), 3.78 (t, J 5.2, 2H); m/z 215 (M+, 100%).
)-one 4b. Mp 84–85 °C (from benzene–light petroleum); νmax/cm−1 1708; δH 7.48–7.26 (m, 5H), 5.90–5.82 (m, 1H), 5.52 (br s, 1H), 5.29 (s, 1H), 5.25 (d, J 9.6, 1H), 4.77 (s, 2H), 3.78 (t, J 5.2, 2H); m/z 215 (M+, 100%).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4c. Mp 98–99 °C (from EtOAc–light petroleum); νmax/cm−1 1713; δH 7.48–7.07 (m, 9H), 5.71 (br s, 1H), 4.76 (s, 2H), 4.31 (d, J 6.1, 2H), 2.36 (s, 3H); m/z 279 (M+, 18%), 106 (100).
)-one 4c. Mp 98–99 °C (from EtOAc–light petroleum); νmax/cm−1 1713; δH 7.48–7.07 (m, 9H), 5.71 (br s, 1H), 4.76 (s, 2H), 4.31 (d, J 6.1, 2H), 2.36 (s, 3H); m/z 279 (M+, 18%), 106 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4d. Mp 125.5–127 °C (from EtOAc–light petroleum); νmax/cm−1 1714; δH 7.61–7.24 (m, 9H), 5.80 (s, 1H), 4.74 (s, 2H), 4.40 (s, 2H); m/z 333 (M+, 40%), 159 (100).
)-one 4d. Mp 125.5–127 °C (from EtOAc–light petroleum); νmax/cm−1 1714; δH 7.61–7.24 (m, 9H), 5.80 (s, 1H), 4.74 (s, 2H), 4.40 (s, 2H); m/z 333 (M+, 40%), 159 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4e. Mp 126–127 °C (from benzene–light petroleum); νmax/cm−1 1714; δH 7.46–7.21 (m, 9H), 5.87 (s, 1H), 4.72 (s, 2H), 4.34 (s, 2H); m/z 349 (M+, 72%), 175 (100).
)-one 4e. Mp 126–127 °C (from benzene–light petroleum); νmax/cm−1 1714; δH 7.46–7.21 (m, 9H), 5.87 (s, 1H), 4.72 (s, 2H), 4.34 (s, 2H); m/z 349 (M+, 72%), 175 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4f. Mp 129–131 °C (from EtOAc–light petroleum); νmax/cm−1 1710; δH 7.34–7.24 (m, 5H), 4.74 (s, 2H), 3.17 (br s, 4H), 1.88 (br s, 4H); m/z 229 (M+, 67%), 70 (100).
)-one 4f. Mp 129–131 °C (from EtOAc–light petroleum); νmax/cm−1 1710; δH 7.34–7.24 (m, 5H), 4.74 (s, 2H), 3.17 (br s, 4H), 1.88 (br s, 4H); m/z 229 (M+, 67%), 70 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4g. Mp 189–190 °C (from benzene–light petroleum); νmax/cm−1 1713; δH 7.38–7.27 (m, 5H), 4.78 (s, 2H), 3.66 (t, J 4.8, 4H), 3.18 (t, J 4.8, 4H); m/z 245 (M+, 56%), 40 (100).
)-one 4g. Mp 189–190 °C (from benzene–light petroleum); νmax/cm−1 1713; δH 7.38–7.27 (m, 5H), 4.78 (s, 2H), 3.66 (t, J 4.8, 4H), 3.18 (t, J 4.8, 4H); m/z 245 (M+, 56%), 40 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4h. An oil; νmax/cm−1 1725; δH 7.41–7.14 (m, 4H), 6.13 (br s, 1H), 4.53 (s, 2H), 4.38 (d, J 6.3, 2H), 2.15 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.29–1.20 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 329 (M+, 5%), 175 (100).
)-one 4h. An oil; νmax/cm−1 1725; δH 7.41–7.14 (m, 4H), 6.13 (br s, 1H), 4.53 (s, 2H), 4.38 (d, J 6.3, 2H), 2.15 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.29–1.20 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 329 (M+, 5%), 175 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4i. An oil; νmax/cm−1 1723; δH 7.29–7.23 (m, 2H), 7.06–6.96 (m, 2H), 5.97 (br s, 1H), 4.53 (s, 2H), 4.32 (d, J 6.1, 2H), 2.14 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.26 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 263 (M+, 7%), 109 (100).
)-one 4i. An oil; νmax/cm−1 1723; δH 7.29–7.23 (m, 2H), 7.06–6.96 (m, 2H), 5.97 (br s, 1H), 4.53 (s, 2H), 4.32 (d, J 6.1, 2H), 2.14 (t, J 7.7, 2H), 1.45–1.38 (m, 2H), 1.26 (m, 2H), 0.87 (t, J 7.3, 3H); m/z 263 (M+, 7%), 109 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4j. An oil; νmax/cm−1 1724; δH 7.57–7.48 (m, 4H), 5.94 (s, 1H), 4.54 (s, 2H), 4.41 (s, 2H), 2.16 (t, J 7.7, 2H), 1.46–1.39 (m, 2H), 1.32–1.25 (m, 2H), 0.87 (t, J 7.2, 3H); m/z 313 (M+, 9%), 159 (100).
)-one 4j. An oil; νmax/cm−1 1724; δH 7.57–7.48 (m, 4H), 5.94 (s, 1H), 4.54 (s, 2H), 4.41 (s, 2H), 2.16 (t, J 7.7, 2H), 1.46–1.39 (m, 2H), 1.32–1.25 (m, 2H), 0.87 (t, J 7.2, 3H); m/z 313 (M+, 9%), 159 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4k. An oil; νmax/cm−1 1730; δH 4.56 (s, 2H), 3.94 (s, 1H), 3.52 (s, 1H), 3.33 (s, 2H), 2.65 (t, J 7.6, 1H), 2.31 (t, J 7.3, 1H), 1.74 (s, 6H), 1.47–1.32 (m, 4H), 0.93 (t, J 7.3, 3H); m/z 223 (M+, 13%), 180 (100).
)-one 4k. An oil; νmax/cm−1 1730; δH 4.56 (s, 2H), 3.94 (s, 1H), 3.52 (s, 1H), 3.33 (s, 2H), 2.65 (t, J 7.6, 1H), 2.31 (t, J 7.3, 1H), 1.74 (s, 6H), 1.47–1.32 (m, 4H), 0.93 (t, J 7.3, 3H); m/z 223 (M+, 13%), 180 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4l. Mp 161–162 °C (from CHCl3–light petroleum); νmax/cm−1 1698; δH (CDCl3–CD3OD) 7.37–7.32 (m, 2H), 7.25–7.23 (m, 2H), 4.70 (s, 2H), 4.26 (s, 2H), 3.97 (s, 1H); m/z 223 (M+, 15%), 125 (100).
)-one 4l. Mp 161–162 °C (from CHCl3–light petroleum); νmax/cm−1 1698; δH (CDCl3–CD3OD) 7.37–7.32 (m, 2H), 7.25–7.23 (m, 2H), 4.70 (s, 2H), 4.26 (s, 2H), 3.97 (s, 1H); m/z 223 (M+, 15%), 125 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4m. Mp 165–166.5 °C (from EtOAc–light petroleum); νmax/cm−1 1701; δH (CDCl3–CD3OD) 7.47–7.01 (m, 4H), 4.62 (s, 1H), 4.42 (s, 2H), 4.31 (s, 2H); m/z 207 (M+, 20%), 109 (100).
)-one 4m. Mp 165–166.5 °C (from EtOAc–light petroleum); νmax/cm−1 1701; δH (CDCl3–CD3OD) 7.47–7.01 (m, 4H), 4.62 (s, 1H), 4.42 (s, 2H), 4.31 (s, 2H); m/z 207 (M+, 20%), 109 (100).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4n. Mp 119–121 °C (from benzene) (lit.,6b 120–121 °C).
)-one 4n. Mp 119–121 °C (from benzene) (lit.,6b 120–121 °C).![[hair space]](https://www.rsc.org/images/entities/b_char_200a.gif) )-one 4o. Mp 107–109 °C (from benzene); νmax/cm−1 1714; δH 4.74 (s, 2H), 4.72 (s, 1H), 3.79 (t, J 4.7, 4H), 3.23 (t, J 4.7, 4H); m/z 169 (M+, 100%).
)-one 4o. Mp 107–109 °C (from benzene); νmax/cm−1 1714; δH 4.74 (s, 2H), 4.72 (s, 1H), 3.79 (t, J 4.7, 4H), 3.23 (t, J 4.7, 4H); m/z 169 (M+, 100%).