Marine natural products
D. John
Faulkner
Scripps Institution of Oceanography, University of California at San Diego, La Jolla, CA 92093-0212, USA
First published on 9th January 2001
Abstract
Covering: 1999. Previous review: 2000, 17, 7.
 D. John Faulkner D. John Faulkner | D. John Faulkner, born in England in 1942, received his BSc and PhD degrees from Imperial College, London, where he studied synthetic organic chemistry under the guidance of Sir Derek Barton. He received postdoctoral training from R. B. Woodward at Harvard University and W. S. Johnson at Stanford University before joining the faculty of the Scripps Institution of Oceanography, University of California at San Diego, in 1968. Recognizing the need to ‘do something more marine’, he began a new career in marine natural products chemistry. He is currently Professor of Marine Chemistry |
1 Introduction
This report is a review of the marine natural products literature for 1999. Earlier reports published in this journal cover the period from 1977 to December 1998. In comparison with 1998,1 the last year was relatively quiet although steady progress was reported on most fronts. Once again, sponges were the favourite organisms for natural product studies, with tunicates and coelenterates following close behind. The accelerating advances in marine biotechnology have made their mark on marine natural products chemistry in the form of a steady increase in papers emphasizing marine microorganisms and symbiotic relationships involving marine microbes.2 Studies of the biosynthesis of marine natural products, which also bear the mark of the latest genetic techniques, are reviewed elsewhere in this journal.3 Marine natural products continue to be prime targets for synthesis although papers reporting partial and formal syntheses, which are not included in this review, far outnumber the more useful papers that record total syntheses and, in particular, synthetic studies that redefine the structures of marine natural products. This report continues to emphasize the bioactivity of new marine natural products while specifically excluding biochemical and pharmacological studies of known marine natural products. The patent literature, which is not covered in detail in this report, was the subject of a review entitled “Marine natural products as therapeutic agents”.4 The format for this review is identical to its predecessor.A number of reviews of specific topics appeared during 1999. An excellent review of “Marine opisthobranch molluscs: Chemistry and ecology in sacoglossans and dorids”![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 covered the period 1988–1998 while the mini review “Chemical defence and evolutionary trends in biosynthetic capacity among dorid nudibranchs (Mollusca: Gastropoda: Opisthobranchia)”
5 covered the period 1988–1998 while the mini review “Chemical defence and evolutionary trends in biosynthetic capacity among dorid nudibranchs (Mollusca: Gastropoda: Opisthobranchia)”![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 explores the complex relationships between taxonomy and natural products chemistry. Different families of marine natural products are highlighted in “Pyrroloquinoline and pyridoacridine alkaloids from marine sources”,7
“The dolastatins, a family of promising antineoplastic agents”,8
“Biogenesis and biological function of marine algal oxylipins”
6 explores the complex relationships between taxonomy and natural products chemistry. Different families of marine natural products are highlighted in “Pyrroloquinoline and pyridoacridine alkaloids from marine sources”,7
“The dolastatins, a family of promising antineoplastic agents”,8
“Biogenesis and biological function of marine algal oxylipins”![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9 and “Total synthesis and chemical biology of the sarcodictyins”.10 Topics of more general interest include “Toxins from sea cucumbers (Holothuroids): chemical structures, properties, taxonomic distribution, biosynthesis and evolution”,11
“Emerging harmful algal blooms and human health: Pfiesteria and related organisms”,12
“Steroids from sponges: Recent reports”,13 and “Discovery and development of antineoplastic agents from natural sources”.14 Broader-based reviews that include many marine natural products include “The diversity of naturally occurring organobromine compounds”
9 and “Total synthesis and chemical biology of the sarcodictyins”.10 Topics of more general interest include “Toxins from sea cucumbers (Holothuroids): chemical structures, properties, taxonomic distribution, biosynthesis and evolution”,11
“Emerging harmful algal blooms and human health: Pfiesteria and related organisms”,12
“Steroids from sponges: Recent reports”,13 and “Discovery and development of antineoplastic agents from natural sources”.14 Broader-based reviews that include many marine natural products include “The diversity of naturally occurring organobromine compounds”![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15 and “Peroxy natural products”.16 Three reviews that are intended for general audiences are “Testing the water”,17
“Products of chemistry – Exploring the ocean – Stating the case for chemistry”
15 and “Peroxy natural products”.16 Three reviews that are intended for general audiences are “Testing the water”,17
“Products of chemistry – Exploring the ocean – Stating the case for chemistry”![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 18 and “Chemistry of marine natural products: yesterday, today and tomorrow”.19 Forty-two articles on various aspects of marine biotechnology, some of which review aspects of marine natural products chemistry, were featured in a special issue of the Journal of Biotechnology.20
18 and “Chemistry of marine natural products: yesterday, today and tomorrow”.19 Forty-two articles on various aspects of marine biotechnology, some of which review aspects of marine natural products chemistry, were featured in a special issue of the Journal of Biotechnology.20
2 Marine microorganisms and phytoplankton
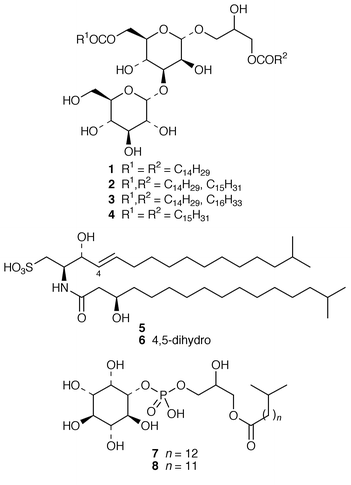
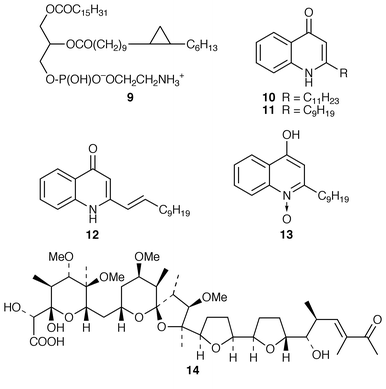
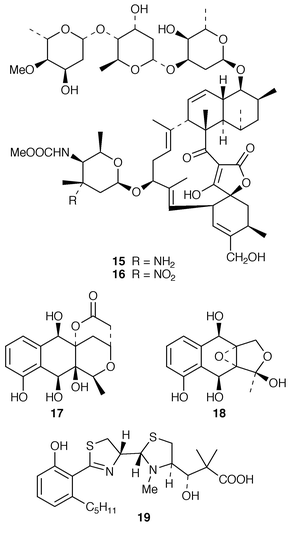
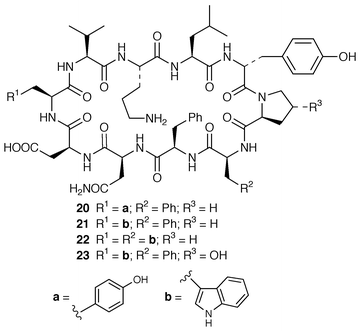
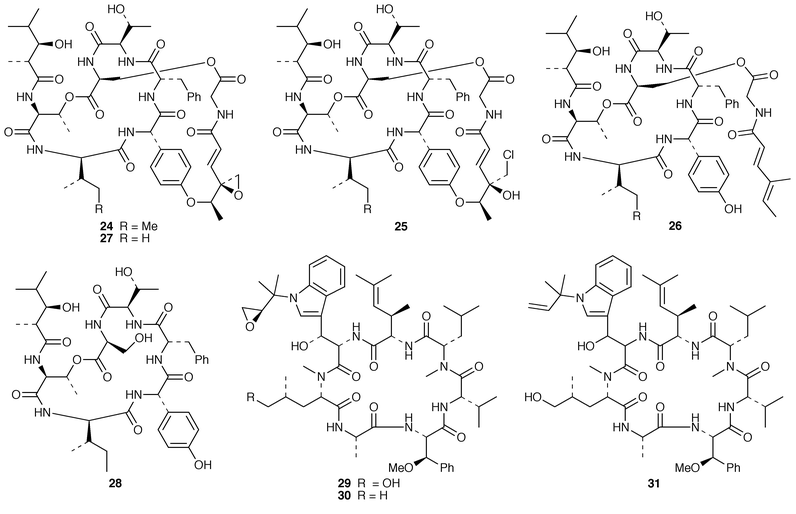
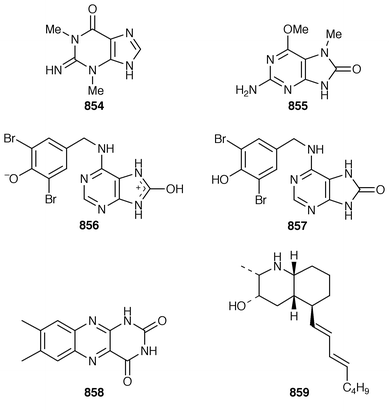
Marine microorganisms continue to be the subject of vigorous chemical investigation although studies of marine bacteria may be decreasing in comparison with those of other microorganisms. A mixture of four monoacyldiglycosyl-monoacylglycerols 1–4 were obtained from Flavobacterium marinotypicum (ATCC 19260) but the positions of the different ester groups were not well defined.21 Flavocristamides A 5 and B 6, which are sulfonolipids from a Chryseobacterium (= Flavobacterium) sp.,22 have been synthesized from L-cysteine.23 Two lysophosphatidyl inositols 7 and 8 were obtained as antifungal agents from a Streptomyces sp. (strain # M428) isolated from a marine sediment.24 A phosphatidyl glyceride 9 and four quinolone derivatives 10–13 were isolated from a Pseudomonas sp. cultured from the sponge Homophymia sp. from New Caledonia.25 Arenaric acid 14 is a pentacyclic polyether, isolated as its sodium salt from an unidentified marine Streptomyces sp. (isolate # CNH-248) from a San Diego estuarine sediment sample.26 The antiinflammatory macrolides lobophorins A 15 and B 16 were obtained from cultures of a tropical marine actinomycete (strain # CNB-837) that was isolated from the surface of the brown alga Lobophora variegata from Belize.27 Two aromatic tetraols, luisols A 17 and B 18, were produced by an unidentified Streptomyces sp. (isolate # CHN-370) cultured from a sediment sample from an estuary near San Diego.28 The cytotoxic thiazole alkaloid agrochelin 19, which was obtained from a marine Agrobacterium sp., formed a complex with Zn2+ ions.29,30 Cultures of an unidentified marine bacterium MK-PNG-276A obtained from the reefs off Loloata Island, Papua New Guinea, have yielded a series of cyclic decapeptide antibiotics, loloatins A–D 20–23, that inhibit methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and drug-resistant Streptococcus pneumoniae.31 Salinamides A–E 24–28 are minor antiinflammatory bicyclic depsipeptides from a marine Streptomyces sp. (isolate # CNB-091) isolated from the surface of the jellyfish Cassiopeia xamachana from the Florida Keys.32 The absolute configurations of the previously-reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 33 salinamides A 24 and B 25 have been revised using chiral capillary electrophoresis of the derivatized hydrolysates. The prenylated cyclic peptides, cyclomarins A–C 29–31 were isolated as cytotoxins from an unidentified Streptomyces sp. (isolate # CNB-982) from a Mission Bay, San Diego, sediment sample but cyclomarin A 30 was later found to possess significant antiinflammatory activity.34 Holyrines A 32 and B 33 are possible intermediates in staurosporine biosynthesis from an actinomycete (strain # N96C-47) from a sediment core sample from Newfoundland.35 The absolute stereochemistry of korormicin 34, which is an antibiotic from a Pseudomonas sp. F-420,36 has been determined by total synthesis.37
33 salinamides A 24 and B 25 have been revised using chiral capillary electrophoresis of the derivatized hydrolysates. The prenylated cyclic peptides, cyclomarins A–C 29–31 were isolated as cytotoxins from an unidentified Streptomyces sp. (isolate # CNB-982) from a Mission Bay, San Diego, sediment sample but cyclomarin A 30 was later found to possess significant antiinflammatory activity.34 Holyrines A 32 and B 33 are possible intermediates in staurosporine biosynthesis from an actinomycete (strain # N96C-47) from a sediment core sample from Newfoundland.35 The absolute stereochemistry of korormicin 34, which is an antibiotic from a Pseudomonas sp. F-420,36 has been determined by total synthesis.37
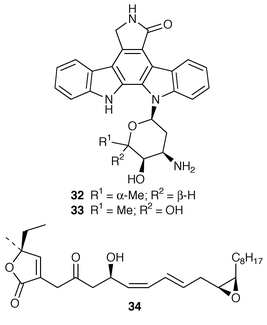
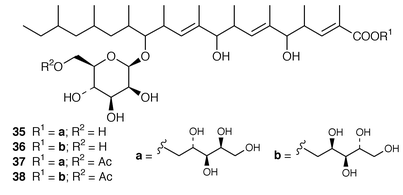
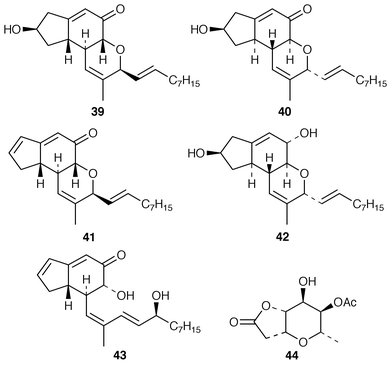
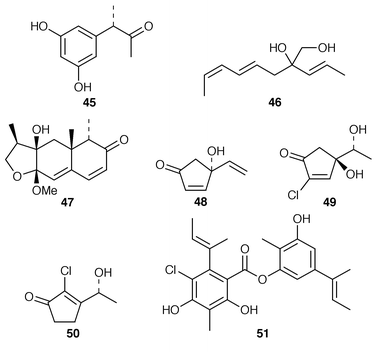
Studies of marine fungi appear to be expanding at a much faster rate than those of other unicellular organisms. Roselipins 1A 35, 1B 36, 2A 37 and 2B 38, from the marine fungus Gliocladium roseum KF-1040, are highly methylated glycolipids, possibly polypropionates, that inhibit the enzyme diacylglycerol acyl transferase (DGAT).38–40 The absolute stereochemistries of penostatins A–E 39–43, earlier reported from a Penicillium sp. isolated from the alga Enteromorpha intestinalis,41 have been determined using the modified Mosher’s method and CD measurements.42 A marine Penicillium sp. (isolate # 386) from the South China Sea contained the pyranolactone 44.43 A Coniothyrium sp. isolated from the sponge Ectyoplasia ferox contained (3S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-(3,5-dihydroxyphenyl)butan-2-one 45 and 2-(1(E
)-(3,5-dihydroxyphenyl)butan-2-one 45 and 2-(1(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-propenyl)-octa-4(E
)-propenyl)-octa-4(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ),6(Z
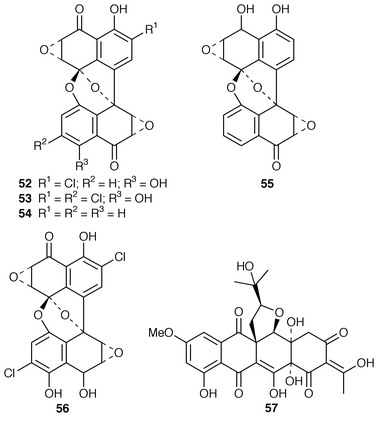
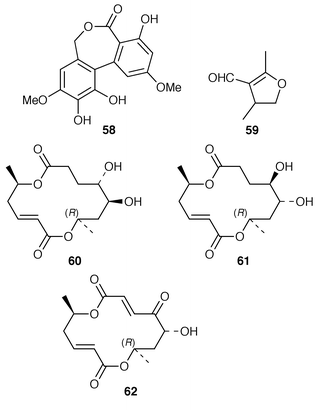
),6(Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diene-1,2-diol 46 while a Microsphaeropsis sp. isolated from the sponge Myxilla incrustans produced microsphaeropsin 47.44 The absolute stereochemistries of trichodenones A–C 48–50, which are metabolites of Trichoderma harzianum (OUPS-N115) from the sponge Halichondria okadai,45 have been determined by total synthesis.46 A marine derived strain of Emericella unguis, obtained from both a mollusc and a medusa, produced the chlorinated depside guisinol 51, which exhibited mild antibacterial activity.47 Five DNA cleaving antitumour antibiotics, spiroxins A–E 52–56, were obtained from a marine fungal strain LL-37H248 that had been isolated from an orange soft coral from Vancouver Island.48 An unidentified fungus cultured from the Okinawan red alga Ceratodictyon spongiosum produced seragakinone A 57, which possessed mild antimicrobial activity.49 The Callyspongia vaginalis derived fungus Ulocladium botrytis produced the tyrosine kinase (p56lck) inhibitor ulocladol 58 and the known antifungal agent 1-hydroxy-6-methyl-8-(hydroxymethyl)xanthone.50 The culture medium of Asteromyces cruciatus contained (+)-2,4-dimethyl-4,5-dihydrofuran-3-carboxaldehyde 59, while Varicosporina ramulosa produced two new macrodiolides, (6R,11S,12S,14R)-colletodiol 60 and (6R,11R,12R,14R)-colletodiol 61.50 The structure of colletoketol 62, which is a metabolite of the same obligate marine mitosporic fungus Varicosporina ramulosa, obtained from an unidentified alga of the genus Cystoseira from Tenerife, Spain, was determined by X-ray crystallography.51
)-diene-1,2-diol 46 while a Microsphaeropsis sp. isolated from the sponge Myxilla incrustans produced microsphaeropsin 47.44 The absolute stereochemistries of trichodenones A–C 48–50, which are metabolites of Trichoderma harzianum (OUPS-N115) from the sponge Halichondria okadai,45 have been determined by total synthesis.46 A marine derived strain of Emericella unguis, obtained from both a mollusc and a medusa, produced the chlorinated depside guisinol 51, which exhibited mild antibacterial activity.47 Five DNA cleaving antitumour antibiotics, spiroxins A–E 52–56, were obtained from a marine fungal strain LL-37H248 that had been isolated from an orange soft coral from Vancouver Island.48 An unidentified fungus cultured from the Okinawan red alga Ceratodictyon spongiosum produced seragakinone A 57, which possessed mild antimicrobial activity.49 The Callyspongia vaginalis derived fungus Ulocladium botrytis produced the tyrosine kinase (p56lck) inhibitor ulocladol 58 and the known antifungal agent 1-hydroxy-6-methyl-8-(hydroxymethyl)xanthone.50 The culture medium of Asteromyces cruciatus contained (+)-2,4-dimethyl-4,5-dihydrofuran-3-carboxaldehyde 59, while Varicosporina ramulosa produced two new macrodiolides, (6R,11S,12S,14R)-colletodiol 60 and (6R,11R,12R,14R)-colletodiol 61.50 The structure of colletoketol 62, which is a metabolite of the same obligate marine mitosporic fungus Varicosporina ramulosa, obtained from an unidentified alga of the genus Cystoseira from Tenerife, Spain, was determined by X-ray crystallography.51
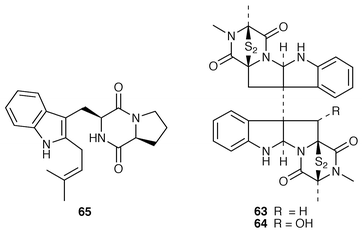
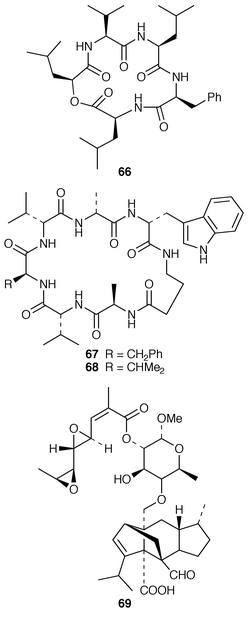
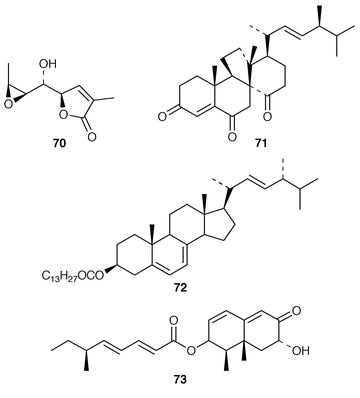
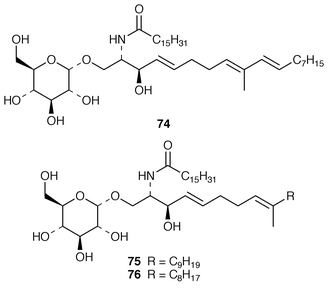
A Penicillium sp. (strain # CNC-350) from the surface of the Caribbean green alga Avrainvillea longicaulis produced two diketopiperazine dimers, 11,11′-dideoxyverticillin A 63 and 11′-deoxyverticillin A 64, both of which exhibited potent in vitro cytotoxicity against the HCT-116 cell line.52 A full account of the synthesis of tryprostatin B 65, which is a metabolite of Aspergillus fumigatus (strain # BM939),53 has been presented.54 The cyclic pentadepsipeptide sansalvamide 66 was produced by a Fusarium sp. (isolate # CNL-292) collected from the surface of the seagrass Halodule wrightii from Little San Salvador Island, Bahamas.55 Sansalvamide 66 was initially reported as having selective cytotoxicity against the COLO 205 and SK-MEL-2 cell lines but was later shown to inhibit molluscum contagiosum virus (MCV) topoisomerase.56 Unguisins A 67 and B 68 are GABA-containing cyclic heptapeptides from the same culture of Emericella unguis described above.57 The fermentation broth of the facultative marine fungus Hypoxylon croceum, which was isolated from driftwood in a mangrove estuary in the Everglades, Florida, produced hypoxysordarin 69, which is a potent antifungal metabolite, and hypoxylactone 70.58 Nine compounds were isolated from the marine fungus Hypoxylon sp. but some of these appear to be possible contaminants.59 The rearranged sterol dankasterone 71, which exhibits significant cytotoxicity, was obtained from a Gymnascella dankaliensis (strain # OUPS-N134) isolated from the sponge Halichondria japonica.60 Ergosteryl myristate 72 was isolated from an unidentified fungus cultured from the alga Sargassum thunbergii from Korea.61 Dendryphiellin C 73, which is a trinor-sesquiterpene from the deuteromycete Dendryphiella salina,62 has been synthesized in a convergent manner.63 The marine protist Thraustochytrium globosum, collected from the surface of the seagrass Thalassia testudinum in the Bahamas, contained the glycosphingolipids thraustochytrosides A–C 74–76.64
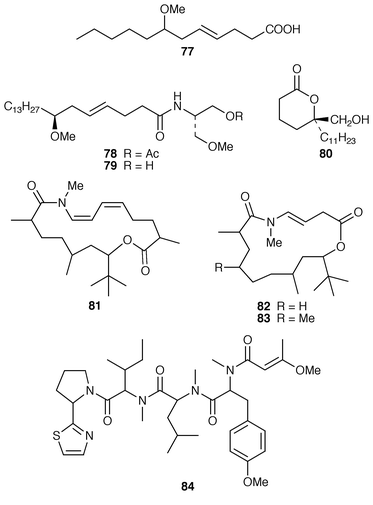
Reports of metabolites from marine cyanobacteria were restricted to filamentous species. Lyngbya majuscula from the French Mediterranean coast yielded (−)-7-methoxydodec-4(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-enoic acid 77, the structure of which was confirmed by synthesis.65 Two serinol-derived malyngamides 78 and 79 were isolated from an unidentified estuarine cyanobacterium from the King George River in Northwestern Australia.66 Tanikolide 80 is a toxic and antifungal lactone from a Madagascan collection of L. majuscula.67 Two nitrogenous macrolides, madangolide 81 and laingolide A 82, a lower homolog of laingolide 83,68 were isolated from L. bouillonii collected in Papua New Guinea.69 The same specimen of L. bouillonii also contained the modified tetrapeptide lyngbyapeptin A 84, the stereochemistry of which was not determined.70
)-enoic acid 77, the structure of which was confirmed by synthesis.65 Two serinol-derived malyngamides 78 and 79 were isolated from an unidentified estuarine cyanobacterium from the King George River in Northwestern Australia.66 Tanikolide 80 is a toxic and antifungal lactone from a Madagascan collection of L. majuscula.67 Two nitrogenous macrolides, madangolide 81 and laingolide A 82, a lower homolog of laingolide 83,68 were isolated from L. bouillonii collected in Papua New Guinea.69 The same specimen of L. bouillonii also contained the modified tetrapeptide lyngbyapeptin A 84, the stereochemistry of which was not determined.70
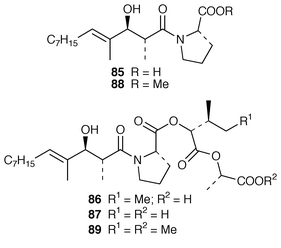
A mixed assemblage of L. majuscula and Schizothrix calcicola from Tumon Bay, Guam, contained five acyl depsipeptides, tumonic acids A–C 85–87 and methyl tumonoates A 88 and B 89.71
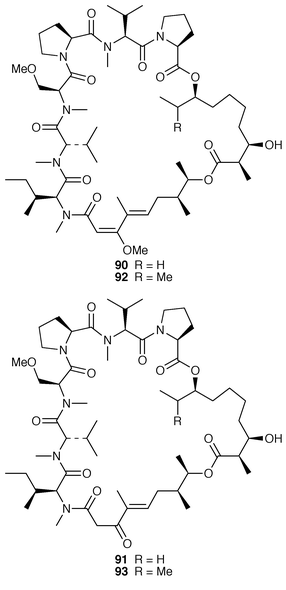
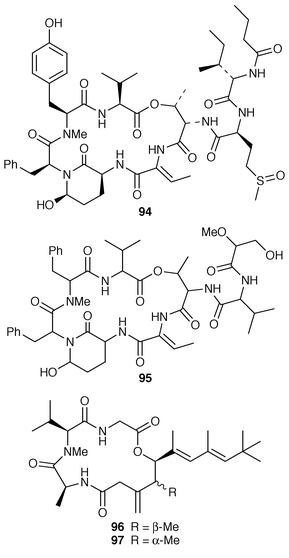
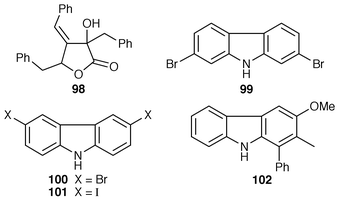
The cytotoxic cyclic depsipeptides lyngbyastatin 2 90 and norlyngbyastatin 2 91, which are analogs of the sea hare cytotoxins dolastatin G 92 and nordolastatin G 93,72 were isolated from L. majuscula from Guam.73 Symplostatin 2 94, which is somewhat similar in structure to dolastatin 13 95, a metabolite of the sea hare Dolabella auricularia,74 was isolated from Symploca hydnoides from Guam.75 The structure of antillatoxin, which is an ichthyotoxic metabolite of L. majuscula from Curacao,76 has been revised from 96 to 97 as a result of total syntheses of both the reported and corrected structures.77,78 The epilithic encrusting cyanobacterium Kyrtuthrix maculans from the shores of Hong Kong contained maculactone L 98 and three halogenated carbazoles 99–101, which had not been reported previously as natural products.79 Hyellazole 102, which is a carbazole alkaloid from Hyella caespitosa,80 has been synthesized using an organometallic approach.81
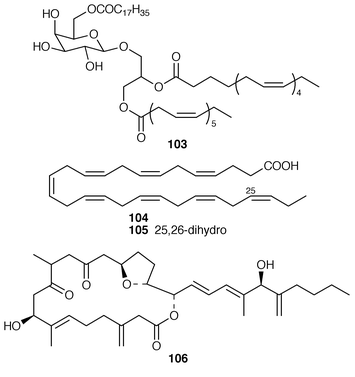
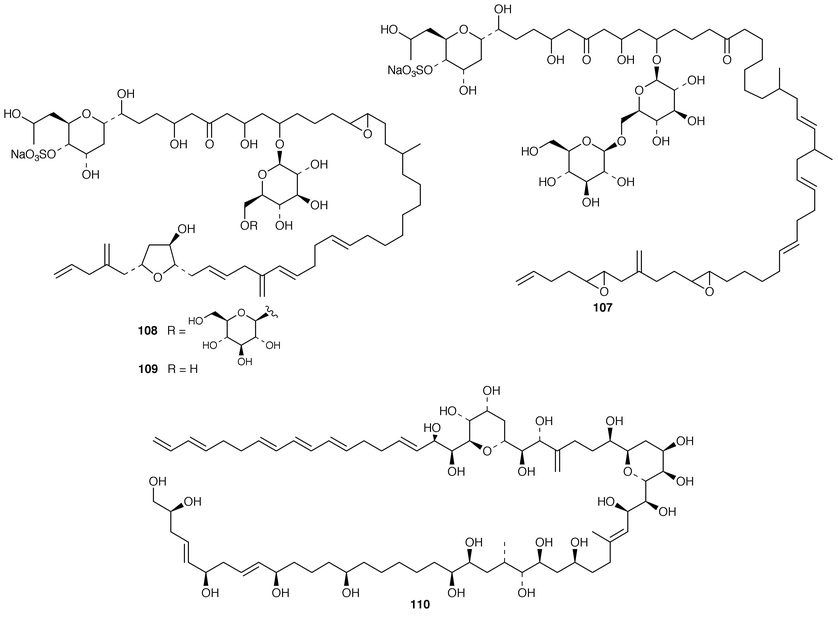
The cultured marine dinoflagellate Amphidinium sp., collected from the coast of NW Spain, contained a monogalactosyl triacylglycerol 103.82 Two very long, highly unsaturated fatty acids 104 and 105 were isolated from seven marine dinoflagellate species.83 Amphidinolide U 106 is a 20-membered macrolide from a cultured Amphidinium sp. (Y-56) isolated from the flatworm Amphiscolops sp. from Okinawa.84 A different strain of Amphidinium sp. (Y-5) produced colopsinols A–C 107–109, of which colopsinol A 107 inhibited DNA polymerases α and β and colopsinol C 109 exhibited cytotoxicity.85,86 The absolute configuration of amphidinol 3 110, which is a metabolite of A. klebsii,87 was determined by analysis of carbon–hydrogen spin-coupling constants.88
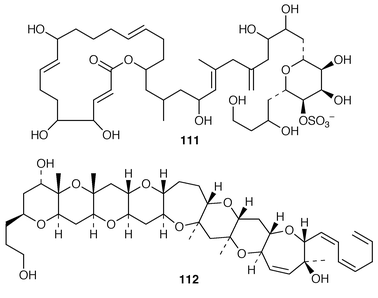
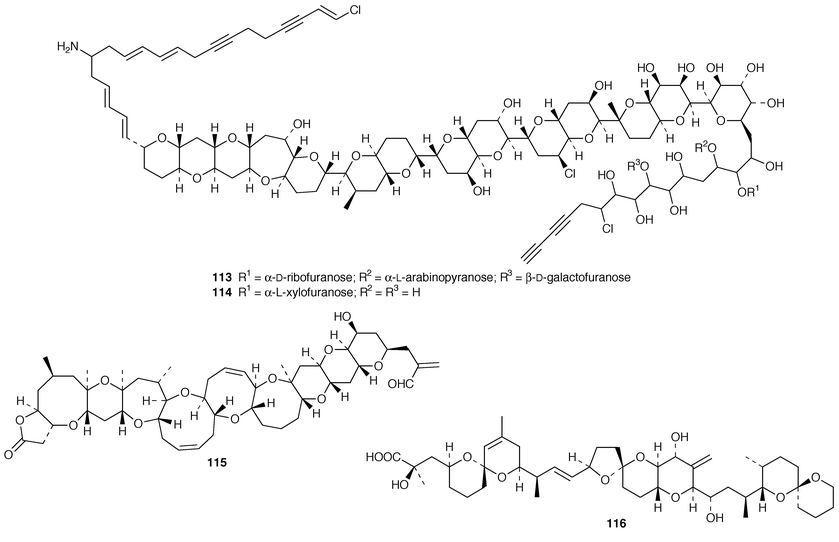
Cultures of the toxin-producing dinoflagellate Prorocentrum hoffamnnianum contained the non-cytotoxic macrolide, hoffmanniolide 111.89 The absolute configuration of gambierol 112, a toxic polyether from Gambierdiscus toxicus,90 was determined by application of Mosher’s method.91 The structures and partial stereochemical assignments of prymnesins-1 113 and -2 114, which are hemolytic and icthyotoxic glycosides from Prymnesium parvum,92 have been determined.93 The total synthesis of brevetoxin A 115 from the dinoflagellate Gymnodinium breve![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 94 has been described in detail.95 The structure of 7-deoxyokadaic acid 116, which is a potent serine/threonine-specific protein phosphatase inhibitor from Prorocentrum lima, has been confirmed by total synthesis.96
94 has been described in detail.95 The structure of 7-deoxyokadaic acid 116, which is a potent serine/threonine-specific protein phosphatase inhibitor from Prorocentrum lima, has been confirmed by total synthesis.96
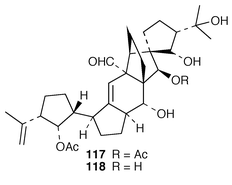
Vannusals A 117 and B 118 were isolated from an Indonesian specimen of the ciliate Euplotes vannus.97
3 Green algae
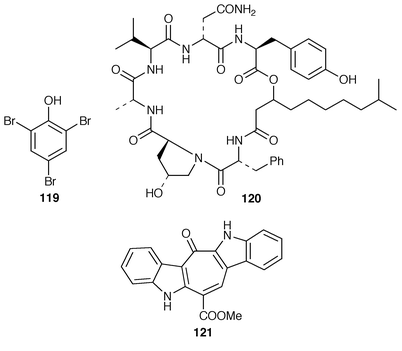
The green macro alga Ulvella lens contains a bromoperoxidase that produces dibromomethane and bromoform.98 A survey of the metabolites of Ulva lactata led to the proposal that 4-hydroxybenzoic acid is the most likely biosynthetic precursor of 2,4,6-tribromophenol 119.99 Kahalalide K 120 is an additional cyclic depsipeptide from a Hawaiian species of Bryopsis.100 The structure of caulersin 121, a metabolite of Caulerpa serrulata,101 has been confirmed by total synthesis.1024 Brown Algae
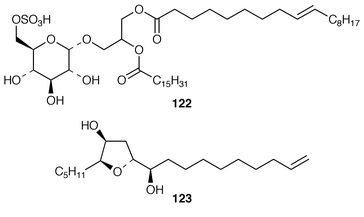
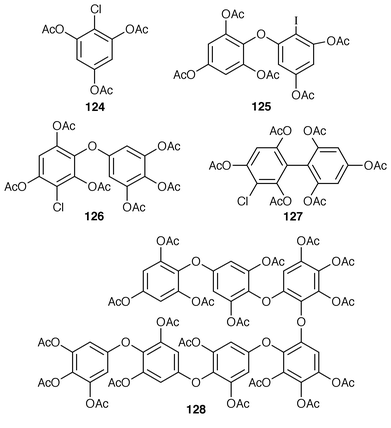
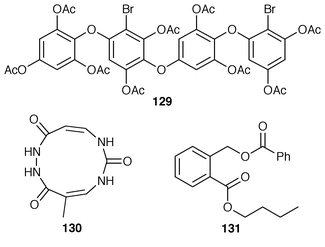
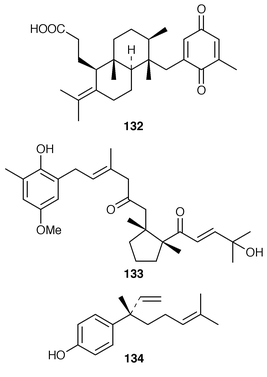
The sulfonoglycolipid 122 from the Australian brown alga Dictyota ciliolata was active in the constitutive nitric oxide synthetase assay.103Iyengaria stelleta from Pakistan was reported to contain a very interesting cyclopropyl ether but the spectral data do not support the proposed structure.104 An additional synthesis of (6S,7S,9R,10R)-6,9-epoxynonadec-18-ene-7,10-diol 123, which is a metabolite of Notheia anomola,105 has been accomplished using an oxiranyl anion strategy.106 After peracetylation, forty-five phloroglucinol derivatives, including 2,4,6-triacetoxychlorobenzene 124, 2[D′]-iododiphlorethol pentaacetate 125, 3[A]-chlorobifuhalol hexaacetate 126, 3[A4]-chlorodifucol hexaacetate 127, and larger compounds such as trihydroxyheptaphlorethol-A octadecaacetate 128 were isolated from Carpophyllum angustifolium from New Zealand.107,108 An additional three phlorethols, ten fucophlorethols, ten halogenated phlorethols, exemplified by 129, and two chlorinated fucophlorethols were isolated as their peracetates from Cystophora retroflexa.109,110 An unusual eleven-membered heterocyclic metabolite 130 was isolated from Sargassum vachellianum![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 111 from the South China Sea.112 A specimen of Spatoglossum variabile from Karachi, Pakistan contained spatazoate 131.113 Stypoquinonic acid 132 is a tyrosine kinase inhibitor from Stypopodium zonale from the Canary Islands.114 Methoxybifurcarenone 133 is an antifungal and antibacterial meroditerpenoid from a Moroccan specimen of Cystoseira tamariscifolia.115 (−)-Sporochnol A 134, which is the enantiomer of a fish feeding deterrent from Sporochnus bolleanus,116 has been synthesized using an enantiospecific route from (R)-(+)-malonic acid benzyl ester.117
111 from the South China Sea.112 A specimen of Spatoglossum variabile from Karachi, Pakistan contained spatazoate 131.113 Stypoquinonic acid 132 is a tyrosine kinase inhibitor from Stypopodium zonale from the Canary Islands.114 Methoxybifurcarenone 133 is an antifungal and antibacterial meroditerpenoid from a Moroccan specimen of Cystoseira tamariscifolia.115 (−)-Sporochnol A 134, which is the enantiomer of a fish feeding deterrent from Sporochnus bolleanus,116 has been synthesized using an enantiospecific route from (R)-(+)-malonic acid benzyl ester.117
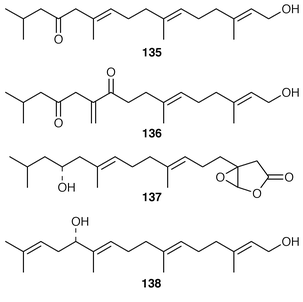
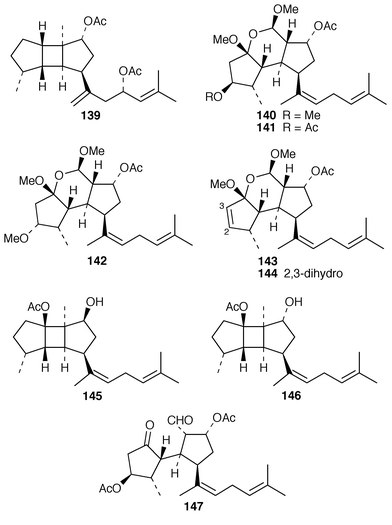
Three additional diterpenes 135, 136, isolated as their corresponding acetates, and 137 were obtained from specimens of Bifurcaria bifurcata from two locations in Brittany.118 An enantioselective synthesis of the cytotoxic diterpenoid (−)-bifurcadiol 138, obtained from Bifurcaria bifurcata,119 has been reported.120 A specimen of Stoechospermum marginatum contained 5(R),16(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-diacetoxyspata-13,17-diene 139, the absolute stereochemistry of which was established using Mosher’s method.121 Secospatacetals A–E 140–144 were obtained from Dilophus okamurai from Japan and the structure of a spatane derivative previously reported from an Australian specimen of S. marginatum
)-diacetoxyspata-13,17-diene 139, the absolute stereochemistry of which was established using Mosher’s method.121 Secospatacetals A–E 140–144 were obtained from Dilophus okamurai from Japan and the structure of a spatane derivative previously reported from an Australian specimen of S. marginatum![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 122 was revised from 145 to 146.123 A different specimen of D. okamurai
122 was revised from 145 to 146.123 A different specimen of D. okamurai![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 124 from Japan contained dilkamural 147, which may be a hydrolysis product of secospatacetal B 141.125
124 from Japan contained dilkamural 147, which may be a hydrolysis product of secospatacetal B 141.125
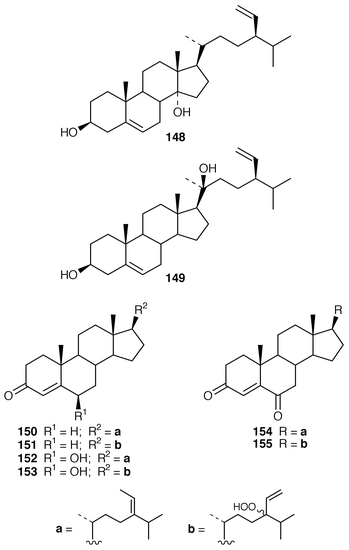
Spatosterol 148 and varninasterol 149 are new fucosterol derivatives from Spatoglossum variabile from Pakistan.126,113 Six additional fucosterol derivatives, 150–155, were obtained from Turbinaria conoides from Taiwan.127
5 Red algae
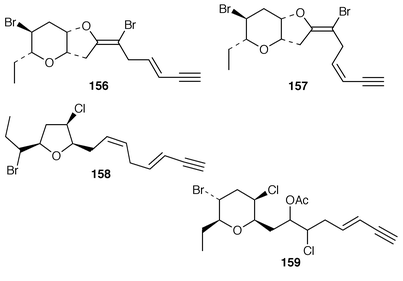
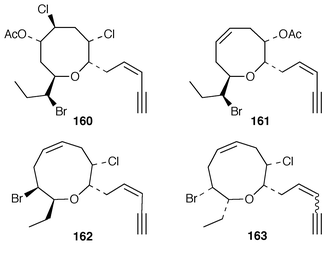
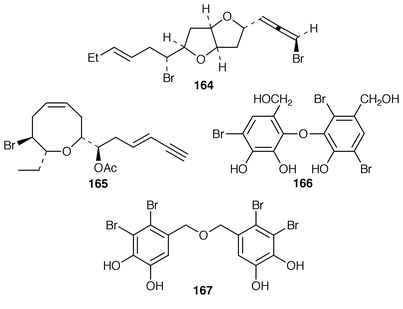
Interest in red algae continues to decline slowly, probably due to the paucity of bioactivity reported for the halogenated metabolites that make up the bulk of their metabolites. As part of a chemotaxonomic study, two C15 acetogenins, japonenynes A 156 and B 157, were isolated from Laurencia japonensis from Japan,128 while an undescribed Laurencia sp. from Japan contained bisezakynes A 158 and B 159.129 Laurencienyne B 160 is an additional halogenated acetogenin from a Greek specimen of L. obtusa.130L. obtusa from Turkey contained the brominated acetogenin acetate 161.131 The structure of (+)-obtusenyne 162, which is a metabolite of L. obtusa,132,133 has been confirmed by total synthesis.134 The absolute configuration of Norte’s obtusenynes 163 (a mixture of 3Z and 3E isomers) from L. pinnatifida![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 135 has also been determined by total synthesis.136 (−)-Kumausallene 164, a metabolite of L. nipponica,137 has been synthesized in an enantioselective manner.138 A total synthesis of (+)-laurencin 165, isolated from L. glandulifera,139 was accomplished using an olefin metathesis to form the 8-membered ring.140 A brominated diphenyl ether 166 and a brominated dibenzyl ether 167 that were isolated from Odonthalia corymbifera were shown to inactivate α-glucosidase.141
135 has also been determined by total synthesis.136 (−)-Kumausallene 164, a metabolite of L. nipponica,137 has been synthesized in an enantioselective manner.138 A total synthesis of (+)-laurencin 165, isolated from L. glandulifera,139 was accomplished using an olefin metathesis to form the 8-membered ring.140 A brominated diphenyl ether 166 and a brominated dibenzyl ether 167 that were isolated from Odonthalia corymbifera were shown to inactivate α-glucosidase.141
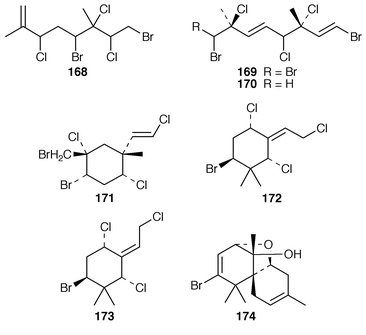
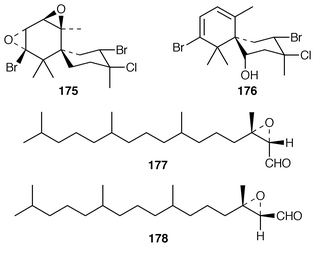
Plocamium costatum from Tasmania contained an additional halogenated monoterpene 168 in addition to the known tribromotrichloromonoterpene 169 from P. cartilagineum,142 the ![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13C NMR spectrum of which was recorded for the first time.143 Eleven known compounds, one of which 170 was obtained pure for the first time, were isolated from a Great Barrier Reef specimen of P. hamatum: the structure of the anti-algal metabolite 171 was confirmed by X-ray analysis.144 Apakaochtodenes A 172, the structure of which was determined by X-ray analysis, and B 173 were obtained from a specimen of Portieria hornemannii from Guam.145 Claviol 174 is a tricyclic brominated sesquiterpene that was isolated along with five known metabolites from Laurencia claviformis from Easter Island.146 A total of fourteen chamigrane derivatives, only two of which, 175 and 176, were previously undescribed, were isolated from L. nidifica from Oahu.147 The cultivated edible seaweed Porphyra yezoensis from Japan contained two isomeric diterpenes 177 and 178, which did not appear to be photooxidation products of phytol.148 Aplysin 179, debromoaplysin 180, aplysinol 181, debromoaplysinol 182, isoaplysin 183, isolaurenterol 184 and debromoisolaurinterol 185, all of which are metabolites of Laurencia spp.,149–151 were synthesized using a diastereoselective radical to polar crossover sequence,152 while aplysin 179 and debromoaplysin 180 were constructed using a similar sulfur mediated cyclization strategy.153 A “symmetrical” total synthesis of teurilene 186, which is a polyether metabolite of L. obtusa,154 required only ten steps from a commercially available compound.155 The structure of (−)-polycavernosamide A 187, a toxin isolated from Polycavernosa tsudai,156 was confirmed by two total syntheses.157,158 An additional total synthesis of (−)-kainic acid 188, which is an anthelmintic metabolite from Digenea simplex,159 utilizes a short, efficient and highly stereoselective approach.160
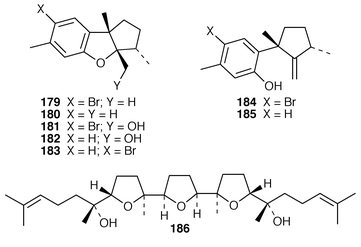
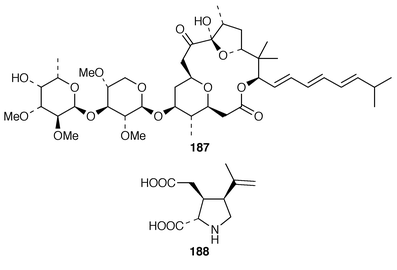
13C NMR spectrum of which was recorded for the first time.143 Eleven known compounds, one of which 170 was obtained pure for the first time, were isolated from a Great Barrier Reef specimen of P. hamatum: the structure of the anti-algal metabolite 171 was confirmed by X-ray analysis.144 Apakaochtodenes A 172, the structure of which was determined by X-ray analysis, and B 173 were obtained from a specimen of Portieria hornemannii from Guam.145 Claviol 174 is a tricyclic brominated sesquiterpene that was isolated along with five known metabolites from Laurencia claviformis from Easter Island.146 A total of fourteen chamigrane derivatives, only two of which, 175 and 176, were previously undescribed, were isolated from L. nidifica from Oahu.147 The cultivated edible seaweed Porphyra yezoensis from Japan contained two isomeric diterpenes 177 and 178, which did not appear to be photooxidation products of phytol.148 Aplysin 179, debromoaplysin 180, aplysinol 181, debromoaplysinol 182, isoaplysin 183, isolaurenterol 184 and debromoisolaurinterol 185, all of which are metabolites of Laurencia spp.,149–151 were synthesized using a diastereoselective radical to polar crossover sequence,152 while aplysin 179 and debromoaplysin 180 were constructed using a similar sulfur mediated cyclization strategy.153 A “symmetrical” total synthesis of teurilene 186, which is a polyether metabolite of L. obtusa,154 required only ten steps from a commercially available compound.155 The structure of (−)-polycavernosamide A 187, a toxin isolated from Polycavernosa tsudai,156 was confirmed by two total syntheses.157,158 An additional total synthesis of (−)-kainic acid 188, which is an anthelmintic metabolite from Digenea simplex,159 utilizes a short, efficient and highly stereoselective approach.160
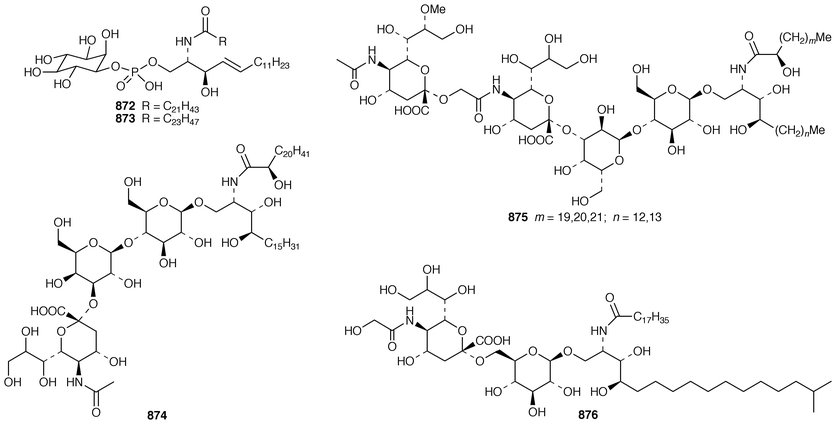
6 Sponges
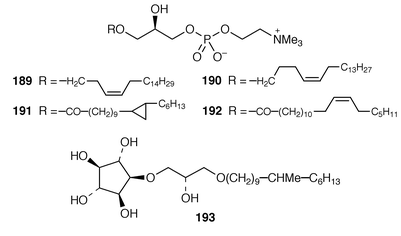
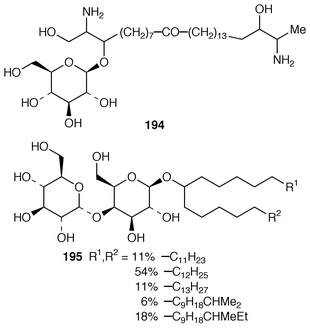
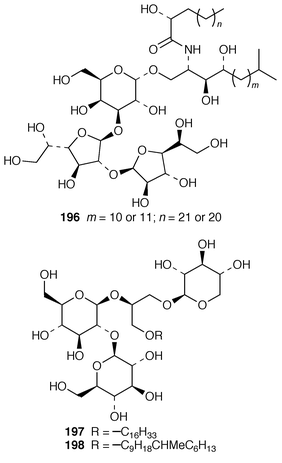
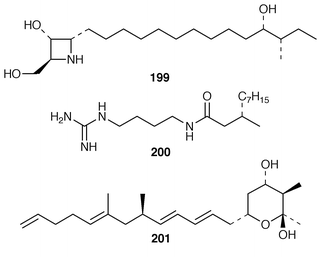
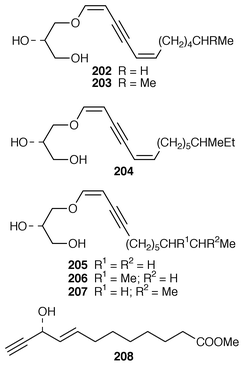
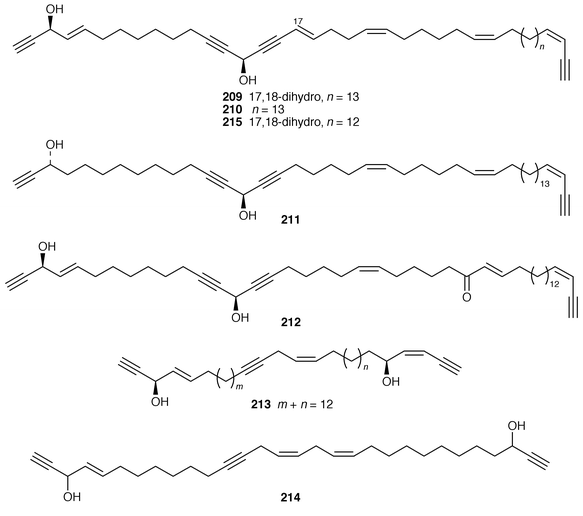
Once again, sponges have provided the largest number of new marine natural products, many of which have interesting biomedical potential. A series of phospholipids that included four previously undescribed representatives, 189–192, were obtained as inhibitors of cholesterol biosynthesis from a Korean specimen of Spirastrella abata.161 A Petrosia sp. from Korea contained a cyclitol derivative 193 that inhibited in vitro SV40 DNA replication.162 Oceanapiside 194 is an antifungal bis-aminohydroxylipid glycoside from a Southern Australian specimen of Oceanapia phillipensis.163 A Caribbean specimen of Plakortis simplex contained the simplexides 195, a group of immunosuppressive glycolipids that inhibit proliferation of activated T-cells by a non-cytotoxic mechanism.164 Agelagalastatin 196 is a cytotoxic glycosphingolipid from an Agelas sp. from Papua New Guinea.165 Myrmekiosides A 197 and B 198 from a Japanese Myrmekioderma sp. are mono-O-alkyl-diglycosylglycerols that reverse the phenotype of melanoma H-ras transformed NIH3T3 cells.166 Penaresidin A 199, which is a potent actomyosin ATPase inhibitor from an Okinawan Penares sp.,167,168 was synthesized in an enantioselective manner.169 The asymmetric synthesis of (−)-aplysillamide B 200, which is an antimicrobial agent from Psammaplysilla purpurea,170 illustrated the use of a ‘superquat’ reagent as a chiral auxiliary.171 The (2S,3R,4S,6R,12R) configuration of (+)-rottnestol 201, which is a metabolite of a Haliclona sp. from Rottnest Island, Western Australia,172 has been proposed on the basis of total synthesis.173Six additional acetylenic enol ethers, petroraspailynes A1 202, A2 203, A3 204, B1 205, B2 206 and B3 207, and the alcohol 208, the absolute configurations of which were determined by Mosher’s method, have been isolated from a Korean species of Petrosia.174 Seven additional cytotoxic polyacetylenes, (3S,14S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-petrocortyne A 209, petrotetrayndiols A 210, B 211 and C 212, dideoxypetrosynols E 213 and F 214, and nor-3S,14S-petrocortyne A 215, were also isolated from a Petrosia sp. from Korea.175,176 The isolation of pellynols E–H 216–219, together with swinholides, from an undescribed Theonella sp. from Chuuk Atoll, Micronesia, was unexpected from a chemotaxonomic viewpoint.177 The xestosterol esters 220 and 221 of two brominated acetylenic acids were obtained from an Australian specimen of Xestospongia testudinaria.178 A chemoenzymatic synthesis of both the (4E,15Z
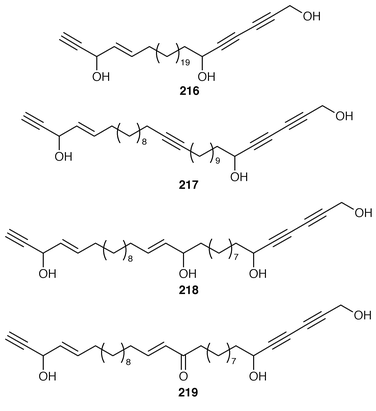
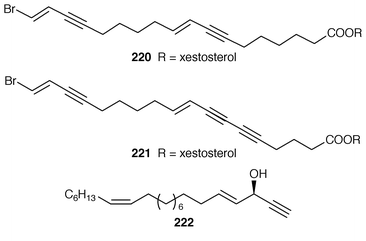
)-petrocortyne A 209, petrotetrayndiols A 210, B 211 and C 212, dideoxypetrosynols E 213 and F 214, and nor-3S,14S-petrocortyne A 215, were also isolated from a Petrosia sp. from Korea.175,176 The isolation of pellynols E–H 216–219, together with swinholides, from an undescribed Theonella sp. from Chuuk Atoll, Micronesia, was unexpected from a chemotaxonomic viewpoint.177 The xestosterol esters 220 and 221 of two brominated acetylenic acids were obtained from an Australian specimen of Xestospongia testudinaria.178 A chemoenzymatic synthesis of both the (4E,15Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) and (4E,15E
) and (4E,15E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) isomers
) isomers![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 179 resulted in assignment of the (3S,4E,15Z
179 resulted in assignment of the (3S,4E,15Z![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) stereochemistry to (+)-docosa-4,15-dien-1-yn-3-ol 222, which is a cytotoxic metabolite of Cribrochalina vasculum.180–182 The proposed stereochemistry at C-3 for both (3S,4E
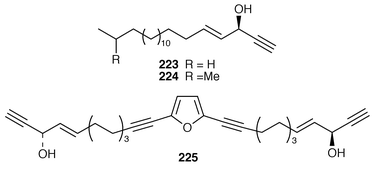
) stereochemistry to (+)-docosa-4,15-dien-1-yn-3-ol 222, which is a cytotoxic metabolite of Cribrochalina vasculum.180–182 The proposed stereochemistry at C-3 for both (3S,4E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-eicos-4-en-1-yn-3-ol 223 and (3S,4E
)-eicos-4-en-1-yn-3-ol 223 and (3S,4E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-19-methylicos-4-en-1-yn-3-ol 224, which are also metabolites of C. vasculum,180 has been confirmed by two independent total syntheses.183,184 Petrofuran 225, from an Okinawan Petrosia sp.,185 (aka adociaacetylene B from an Okinawan Adocia sp.)
)-19-methylicos-4-en-1-yn-3-ol 224, which are also metabolites of C. vasculum,180 has been confirmed by two independent total syntheses.183,184 Petrofuran 225, from an Okinawan Petrosia sp.,185 (aka adociaacetylene B from an Okinawan Adocia sp.)![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 186 has been synthesized using an enantioselective reduction to produce the two chiral alcohols.187
186 has been synthesized using an enantioselective reduction to produce the two chiral alcohols.187
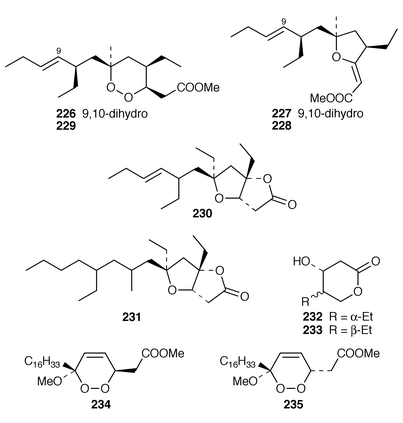
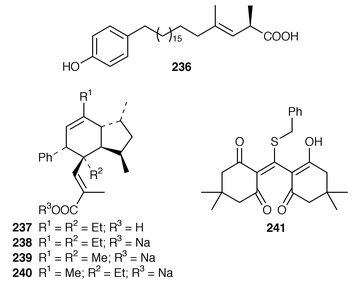
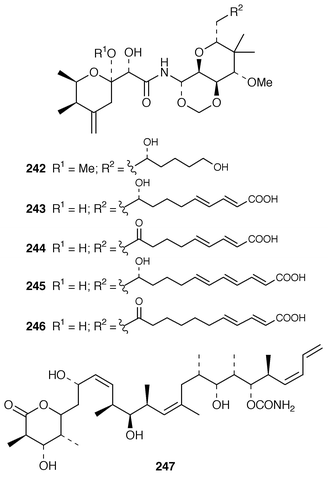
Studies of Plakortis simplex from the Caribbean resulted in the isolation of the cyclic peroxide dihydroplakortin 226 and two related furans 227 and 228, as well as providing the absolute stereochemistry of plakortin 229.188 A subsequent report of metabolites from P. simplex described four additional lactones, plakortones E 230 and F 231 and simplactones A 232 and B 233.189 The structures and absolute stereochemistries of the cyclic peroxides chondrillin 234 from a Great Barrier Reef Chondrilla sp.190 and plakorin 235 from Plakortis spp.191,192 have been confirmed by syntheses of (+)- and (−)-chondrillin and (+)- and (−)-plakorin.193 Elenic acid 236, which is a topoisomerase II inhibitor from an Indonesian Plakinastrella sp.,194 was synthesized by a short efficient route.195Homo-plakotenin 237 and its sodium salt 238, nor-plakotenin sodium salt 239 and plakotenin sodium salt 240, some of which significantly reduced proliferation of rheumatoid synovial fibroblasts, are additional members of the plakotenin family from a Palauan specimen of Plakortis lita.196 Benzylthiocrellidone 241, the structure of which was confirmed by synthesis, was isolated from Crella spinulata: it should be noted that the Australian group has previously studied the use of synthetic dimedone derivatives as sunblocks.197 Five additional antifungal and cytotoxic metabolites, theopederins F–J 242–246, were isolated from a Japanese specimen of Theonella swinhoei.198 (+)-Discodermolide 247, a potentially important antimitotic agent from the Caribbean deep-water sponge Discodermia dissoluta,199 has been synthesized on a scale that will enable future development as an antimitotic agent.200
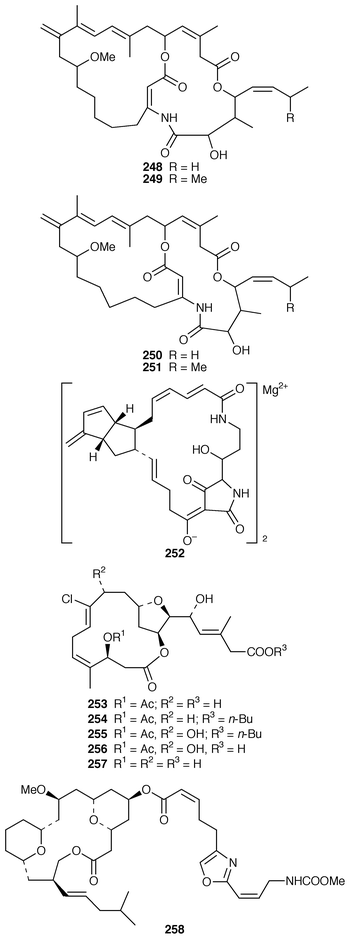
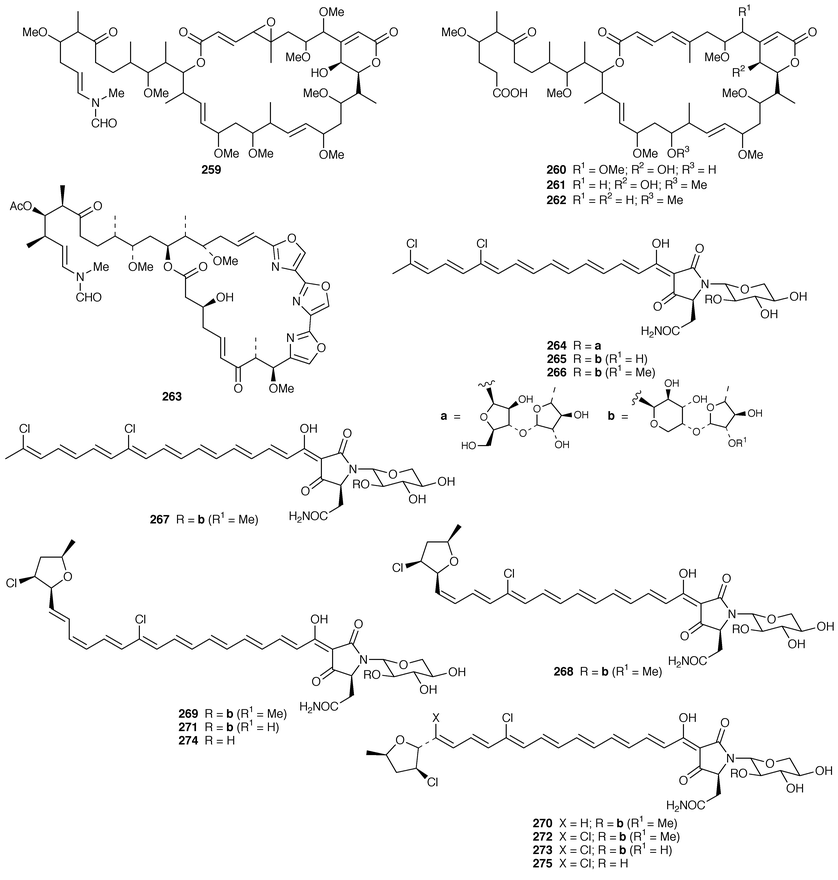
The bicyclic macrolides, amphilactams A–D 248–251, are nematocides from the Southern Australian Amphimedon sp.201 A Geodia sp. from Southern Australia contained geodin A magnesium salt 252, which also has nematocidal activity.202 Haterumalides NA 253, NB 254, NC 255, ND 256 and NE 257 are cytotoxic chlorinated macrolides from an Okinawan species of Ircinia.203 The calcareous sponge Leucascandra caveolata from New Caledonia contained an additional macrolide, leucascandrolide B 258.204 Four additional cytotoxic macrolides, sphinxolides E–G 259–261 and reidispongiolide C 262, were isolated from the New Caledonian sponges Neosiphonia superstes and Reidispongia coerulea, respectively.205 The relative and absolute stereochemistry of the mycalolides, exemplified by mycalolide A 263 from a Japanese Mycale sp.,206 were determined by chemical synthesis, degradation and spectroscopic analyses.207 An additional tetramic acid glycoside, aurantoside C 264, was isolated from the lithistid sponge Homophymia conferta from the Philippines![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 208 while aurantosides D–F 265–267, which possessed antifungal activity, were isolated from the Japanese lithistid Siliquariaspongia japonica.209S. japonica also contained a related series of tetramic acid glycosides, rubrosides A–H 268–275, that induced large intracellular vacuoles in 3Y1 rat fibroblasts.210
208 while aurantosides D–F 265–267, which possessed antifungal activity, were isolated from the Japanese lithistid Siliquariaspongia japonica.209S. japonica also contained a related series of tetramic acid glycosides, rubrosides A–H 268–275, that induced large intracellular vacuoles in 3Y1 rat fibroblasts.210
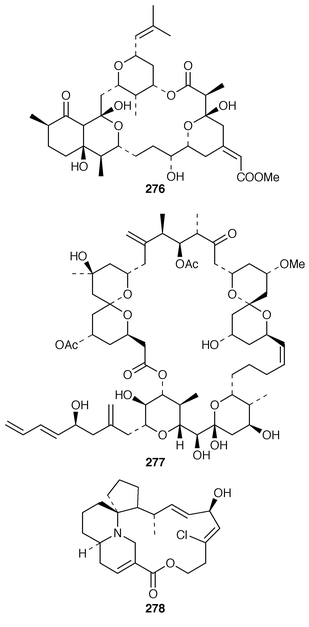
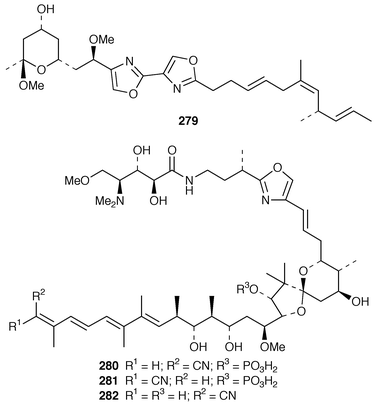
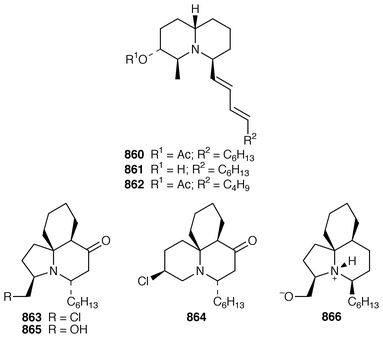
The synthesis of macrolides and related complex molecules from sponges has attracted much attention. The absolute configuration of (+)-miyakolide 276, which is a weakly cytotoxic tetracyclic macrolide from a Japanese Polyfibrospongia sp.,211 was established by a very efficient synthesis of its enantiomer.212 The potent cytotoxin altohyrtin C 277 from Hyrtios altum,213 also known as spongistatin 2 from Spongia sp.,214 has been synthesized by an enantioselective route.215 A stereocontrolled total synthesis of (+)-halichlorine 278, which is an inhibitor of VCAM-1 expression from Halichondria okadai from Japan,216 has been accomplished.217 (−)-Hennoxazole A 279, which is an antiviral agent from a Polyfibrospongia species,218 has been synthesized using a convergent enantio-controlled strategy.219 A convergent total synthesis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 220 led to the antipodes of calyculin A 280 and calyculin B 281, which are serine-threonine phosphatase inhibitors from a Japanese specimen of Discodermia calyx.221,222 The solution structures of calyculin A 280 and dephosphonocalyculin A 282 have been determined using NMR methods.223
220 led to the antipodes of calyculin A 280 and calyculin B 281, which are serine-threonine phosphatase inhibitors from a Japanese specimen of Discodermia calyx.221,222 The solution structures of calyculin A 280 and dephosphonocalyculin A 282 have been determined using NMR methods.223
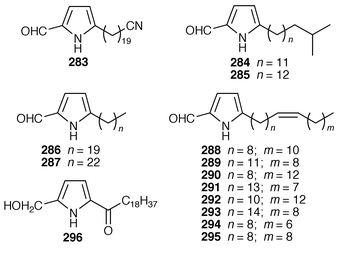
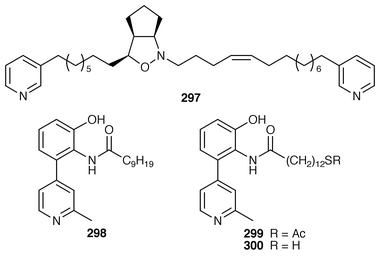
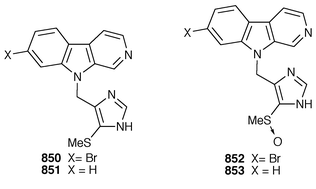
Thirteen additional 5-alkylpyrrole-2-carboxaldehydes 283–295 were isolated from the Caribbean sponges Mycale microsigmatosa and Desmapsamma anchorata from Venezuela.224 A general method has been reported for the synthesis of mycalazol 11 296 from Mycale micracanthoxea![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 225 and related pyrroles.226 Pyrinodemin A 297, the structure of which was elucidated largely on the basis of its EIMS fragmentation pattern, is a cytotoxic pyridine alkaloid from a Japanese species of Amphimedon.227 Echinoclathrines A–C 298–300, which show weak immunosuppressive activity, are members of a new class of pyridine alkaloids from an Okinawan species of Echinoclathria.228 Bengamides Y 301 and Z 302 and bengazole Z 303, which were isolated from an Australian Jaspis sp., showed striking differential cytotoxicity patterns against a panel of human tumour cell lines.229 Bengamide L 304 and six additional bengazoles 305–310 were isolated from a Pachastrissa sp. from the Djibouti coast.230 The first total synthesis of bengazole A 311, which is an antifungal agent from a Jaspis sp.,231 employed consecutive regiocontrolled metalation–addition reactions.232 Neofolitispates 1–3 312–314 are additional pentacyclic guanidine alkaloids from Neofolitispa dianchora from the Andaman Islands, India, that inhibited the hepatitis B virus.233 13,14,15-Isocrambescidin 800 315, a rare metabolite of Crambe crambe,234 has been synthesized in an enantioselective manner.235 The ring junction stereochemistry of the left-hand portion of batzelladine F, a metabolite of a Caribbean Batzella sp. that induces p56
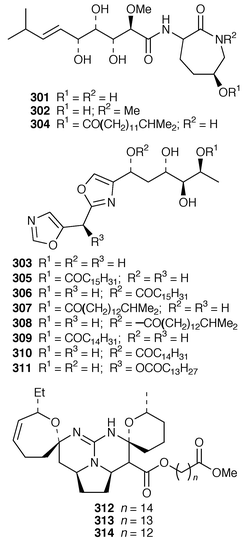
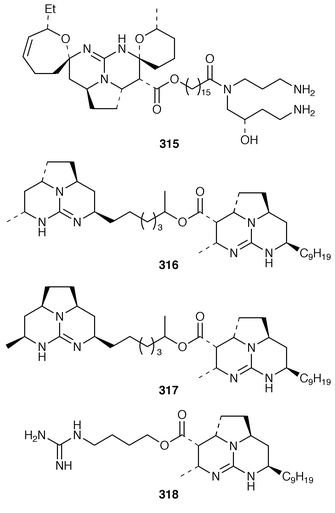
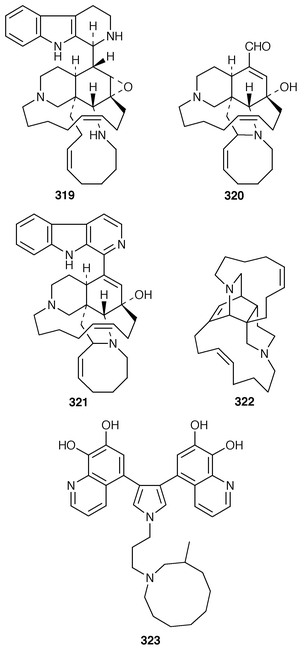
225 and related pyrroles.226 Pyrinodemin A 297, the structure of which was elucidated largely on the basis of its EIMS fragmentation pattern, is a cytotoxic pyridine alkaloid from a Japanese species of Amphimedon.227 Echinoclathrines A–C 298–300, which show weak immunosuppressive activity, are members of a new class of pyridine alkaloids from an Okinawan species of Echinoclathria.228 Bengamides Y 301 and Z 302 and bengazole Z 303, which were isolated from an Australian Jaspis sp., showed striking differential cytotoxicity patterns against a panel of human tumour cell lines.229 Bengamide L 304 and six additional bengazoles 305–310 were isolated from a Pachastrissa sp. from the Djibouti coast.230 The first total synthesis of bengazole A 311, which is an antifungal agent from a Jaspis sp.,231 employed consecutive regiocontrolled metalation–addition reactions.232 Neofolitispates 1–3 312–314 are additional pentacyclic guanidine alkaloids from Neofolitispa dianchora from the Andaman Islands, India, that inhibited the hepatitis B virus.233 13,14,15-Isocrambescidin 800 315, a rare metabolite of Crambe crambe,234 has been synthesized in an enantioselective manner.235 The ring junction stereochemistry of the left-hand portion of batzelladine F, a metabolite of a Caribbean Batzella sp. that induces p56![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) lck-CD4 dissociation,236 has been revised from anti316 to syn317 as the result of partial synthesis.237,238 An enantioselective total synthesis of batzelladine D 318, from the same Batzella sp.,239 has been described.240 A new manzamine alkaloid, 1,2,3,4-tetrahydromanzamine B 319, was obtained from a Japanese species of Amphimedon.241 An enantioselective total synthesis of ircinal A 320, which is a metabolite of a Japanese Ircinia sp.,242 also completes a formal synthesis of manzamine A 321.243 Studies on the biomimetic synthesis of the manzamine alkaloids resulted in the total synthesis of keramaphidin B 322,244 which was obtained from an Okinawan species of Amphimedon.
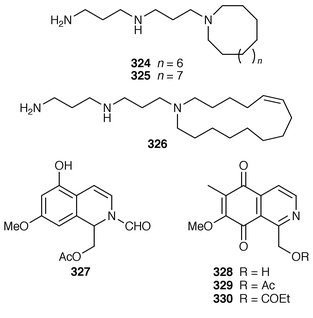
lck-CD4 dissociation,236 has been revised from anti316 to syn317 as the result of partial synthesis.237,238 An enantioselective total synthesis of batzelladine D 318, from the same Batzella sp.,239 has been described.240 A new manzamine alkaloid, 1,2,3,4-tetrahydromanzamine B 319, was obtained from a Japanese species of Amphimedon.241 An enantioselective total synthesis of ircinal A 320, which is a metabolite of a Japanese Ircinia sp.,242 also completes a formal synthesis of manzamine A 321.243 Studies on the biomimetic synthesis of the manzamine alkaloids resulted in the total synthesis of keramaphidin B 322,244 which was obtained from an Okinawan species of Amphimedon.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 245 Halitulin 323 is an unusual cytotoxic alkaloid from Haliclona tulearensis from South Africa.246 Two groups have synthesized motuporamines A 324 and B 325,247,248 which are metabolites of Xestospongia exigua,249 and one of these groups also determined the position of the double bond in motuporamine C 326.248 A new 1,2-dihydroisoquinoline 327 was isolated from Petrosia similis from the Mandapam coast of India.250 Renierol 328, which is a metabolite of Xestospongia caycedoi,251 and the corresponding acetate and propionate esters 329 and 330 were synthesized using a thermal electrocyclic reaction as the key step.252 A deep-water Caribbean sponge of the genus Batzella contained secobatzellines A 331 and B 332, both of which were cytotoxic and inhibited calcineurin, while only secobatzelline A 331 inhibited the peptidase activity of CPP32.253 Similar bioactivity was ascribed to discorhabdin P 333, the structure of which was determined by X-ray crystallography, which was isolated from a similar deep-water Batzella sp. from the Bahamas.254 Discorhabdin Q 334 was isolated as a cytotoxic constituent from Latrunculia purpurea and at least three Zyzzya species.255 The biomimetic syntheses of discorhabdins C 335 and E 336, which are metabolites of Latrunculia species from New Zealand,256,257 employ cupric chloride-catalyzed oxidative cyclizations of suitably substituted indoloquinonimines.258 Batzellines A 337 and B 338 and isobatzellines A 339 and B 340, which are metabolites of a deep-water Caribbean Batzella sp.,259,260 have all been synthesized using a similar strategy.261 The total synthesis of the cytotoxic agent makaluvamine F 341 from Zyzzya cf. marsailis
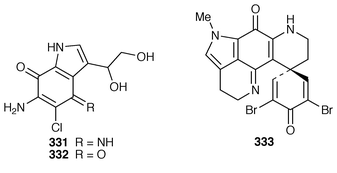
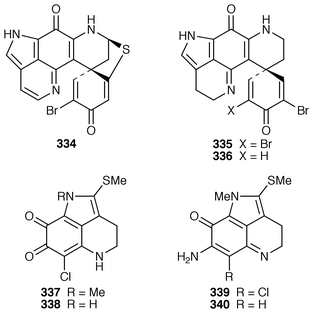
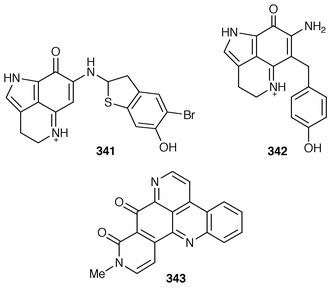
245 Halitulin 323 is an unusual cytotoxic alkaloid from Haliclona tulearensis from South Africa.246 Two groups have synthesized motuporamines A 324 and B 325,247,248 which are metabolites of Xestospongia exigua,249 and one of these groups also determined the position of the double bond in motuporamine C 326.248 A new 1,2-dihydroisoquinoline 327 was isolated from Petrosia similis from the Mandapam coast of India.250 Renierol 328, which is a metabolite of Xestospongia caycedoi,251 and the corresponding acetate and propionate esters 329 and 330 were synthesized using a thermal electrocyclic reaction as the key step.252 A deep-water Caribbean sponge of the genus Batzella contained secobatzellines A 331 and B 332, both of which were cytotoxic and inhibited calcineurin, while only secobatzelline A 331 inhibited the peptidase activity of CPP32.253 Similar bioactivity was ascribed to discorhabdin P 333, the structure of which was determined by X-ray crystallography, which was isolated from a similar deep-water Batzella sp. from the Bahamas.254 Discorhabdin Q 334 was isolated as a cytotoxic constituent from Latrunculia purpurea and at least three Zyzzya species.255 The biomimetic syntheses of discorhabdins C 335 and E 336, which are metabolites of Latrunculia species from New Zealand,256,257 employ cupric chloride-catalyzed oxidative cyclizations of suitably substituted indoloquinonimines.258 Batzellines A 337 and B 338 and isobatzellines A 339 and B 340, which are metabolites of a deep-water Caribbean Batzella sp.,259,260 have all been synthesized using a similar strategy.261 The total synthesis of the cytotoxic agent makaluvamine F 341 from Zyzzya cf. marsailis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 262 was accomplished using hypervalent iodine(III)-induced reactions.263,264 Veiutamine 342, which is a cytotoxic constituent of Zyzzya fuliginosa from Fiji,265 has been synthesized for the first time.266 Neoamphimedine 343, which was obtained from a Xestospongia sp. from the Philippines and from X. cf. carbonaria from Palau, is a pyridoacridine topoisomerase II inhibitor that catenates DNA.267
262 was accomplished using hypervalent iodine(III)-induced reactions.263,264 Veiutamine 342, which is a cytotoxic constituent of Zyzzya fuliginosa from Fiji,265 has been synthesized for the first time.266 Neoamphimedine 343, which was obtained from a Xestospongia sp. from the Philippines and from X. cf. carbonaria from Palau, is a pyridoacridine topoisomerase II inhibitor that catenates DNA.267
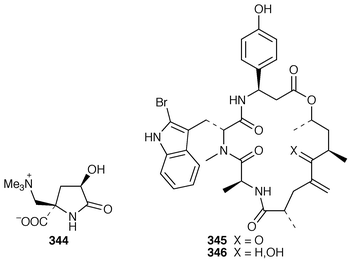
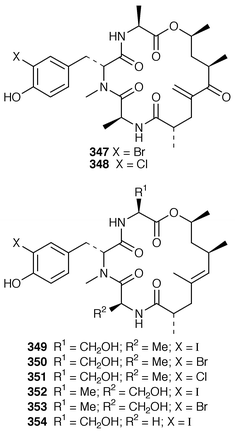
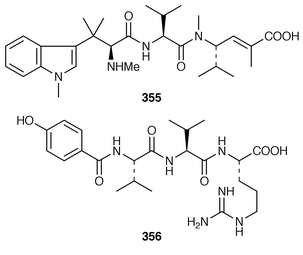
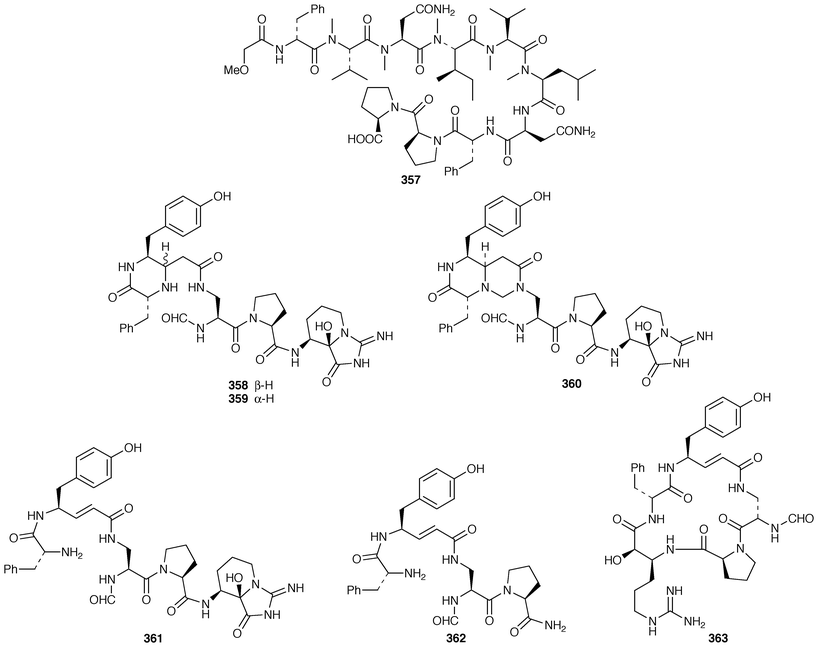
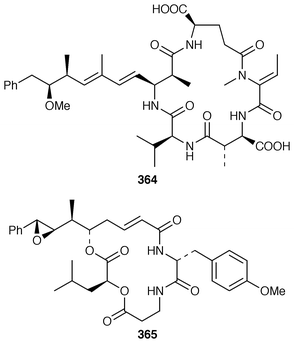
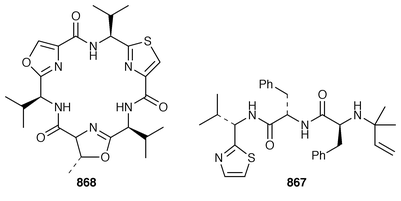
The structure of dysibetaine 344, which is an α,α-disubstituted α-amino acid derivative from Dysidea herbacea from Yap, Micronesia, was determined by X-ray crystallography.268 Two additional jaspamide derivatives, jaspamides B 345 and C 346, were obtained as moderately active cytotoxic agents from a specimen of Jaspis splendens from Vanuatu![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 269 while the related metabolites, geodiamolides J–P 347–353 and R 354, were isolated as minor metabolites of a Cymbastela sp. from Papua New Guinea.270 In a paper that compares the cytotoxicity of the hemiasterlins with other linear peptides, hemiasterlin C 355 was reported as an additional cytotoxic linear peptide from an Auletta sp. and two collections of Siphonochalina species.271 Tokaramide A 356 was isolated as a cathepsin B inhibitor with potential anticancer activity from a Japanese specimen of Theonella aff. mirabilis.272 A Japanese Theonella sp. contained the cytotoxic linear peptide koshikamide A1357.273 A mixture of linear and cyclic peptides, pseudotheonamides A1358, A2359, B2360, C 361 and D 362, and dihydrocyclotheonamide A 363, were obtained as serine protease inhibitors from a Japanese specimen of Theonella swinhoei.274 Three total syntheses of motuporin 364, which is a potent inhibitor of protein phosphatase type 1 (PP1) from a Papua New Guinea specimen of Theonella swinhoei,275 have been reported during 1999.276–278 Arenastatin A 365, which is a cytotoxic depsipeptide from Dysidea arenaria,279 has been synthesized together with related cyanobacterial cytotoxins.280
269 while the related metabolites, geodiamolides J–P 347–353 and R 354, were isolated as minor metabolites of a Cymbastela sp. from Papua New Guinea.270 In a paper that compares the cytotoxicity of the hemiasterlins with other linear peptides, hemiasterlin C 355 was reported as an additional cytotoxic linear peptide from an Auletta sp. and two collections of Siphonochalina species.271 Tokaramide A 356 was isolated as a cathepsin B inhibitor with potential anticancer activity from a Japanese specimen of Theonella aff. mirabilis.272 A Japanese Theonella sp. contained the cytotoxic linear peptide koshikamide A1357.273 A mixture of linear and cyclic peptides, pseudotheonamides A1358, A2359, B2360, C 361 and D 362, and dihydrocyclotheonamide A 363, were obtained as serine protease inhibitors from a Japanese specimen of Theonella swinhoei.274 Three total syntheses of motuporin 364, which is a potent inhibitor of protein phosphatase type 1 (PP1) from a Papua New Guinea specimen of Theonella swinhoei,275 have been reported during 1999.276–278 Arenastatin A 365, which is a cytotoxic depsipeptide from Dysidea arenaria,279 has been synthesized together with related cyanobacterial cytotoxins.280
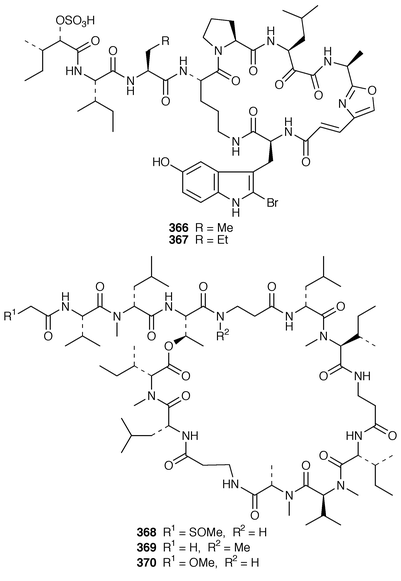
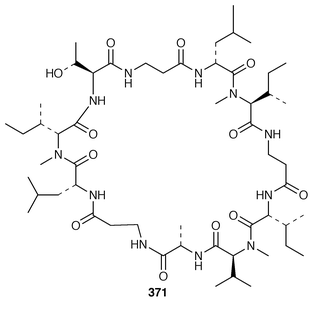
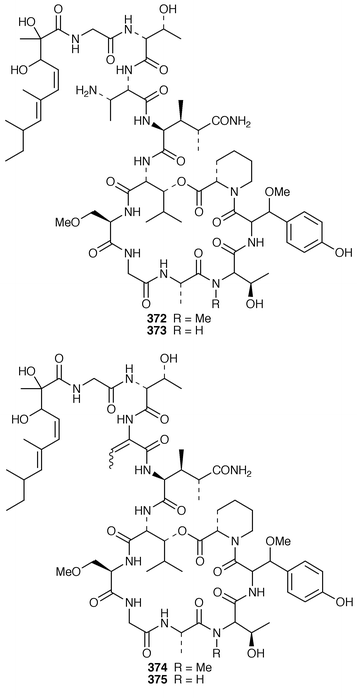
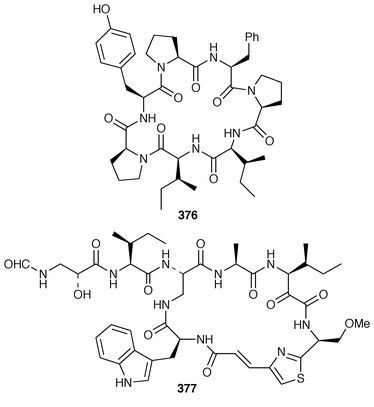
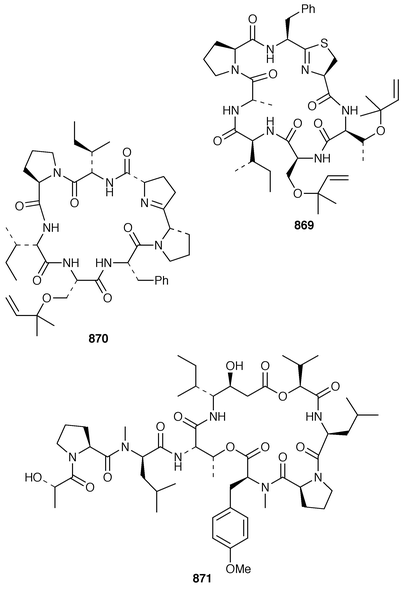
Keramamides M 366 and N 367 are additional sulfated cyclic peptides from an Okinawan Theonella species.281 A different Theonella sp. from Okinawa contained two additional theonellapeptolide congeners 368 and 369, one of which had methylsulfinylacetyl group at the N-terminus.282 An X-ray study of theonellapeptolide Id 370 from an Okinawan specimen of T. swinhoei![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 283 revealed that the crystals were highly solvated.284 An Indonesian specimen of T. swinhoei contained the cyclic peptide barangamide A 371, which is related to the theonellapeptolides.285 The Papua New Guinea sponges T. mirabilis and T. swinhoei contained the cytotoxic and HIV-inhibitory depsipeptides papuamides A 372 and B 373 and papuamides C 374 and D 375, respectively.286 The cyclic heptapeptide phakellistatin 2 376, which is a cytotoxic constituent of Phakellia carteri from the Comoros Islands,287 has been synthesized in a stepwise manner but the synthetic product did not possess the biological activity of the natural product.288 Synthesis of the proposed structure of keramamide J 377, which was isolated from an Okinawan Theonella sp.,289 indicated that the structure of the natural product should be re-examined.290
283 revealed that the crystals were highly solvated.284 An Indonesian specimen of T. swinhoei contained the cyclic peptide barangamide A 371, which is related to the theonellapeptolides.285 The Papua New Guinea sponges T. mirabilis and T. swinhoei contained the cytotoxic and HIV-inhibitory depsipeptides papuamides A 372 and B 373 and papuamides C 374 and D 375, respectively.286 The cyclic heptapeptide phakellistatin 2 376, which is a cytotoxic constituent of Phakellia carteri from the Comoros Islands,287 has been synthesized in a stepwise manner but the synthetic product did not possess the biological activity of the natural product.288 Synthesis of the proposed structure of keramamide J 377, which was isolated from an Okinawan Theonella sp.,289 indicated that the structure of the natural product should be re-examined.290
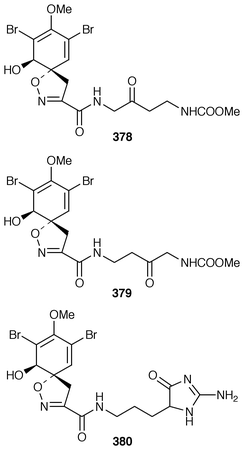
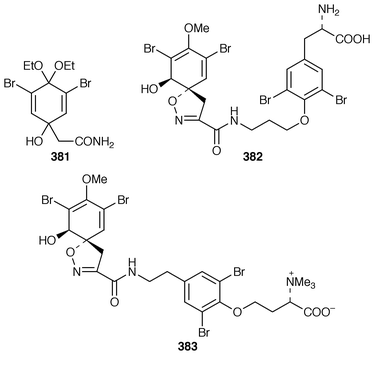
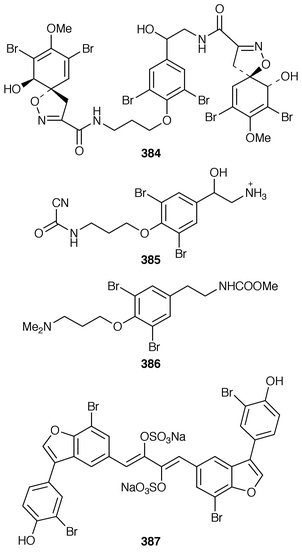
Two bromotyrosine derivatives 378 and 379 were described as constituents of Aplysina cauliformis from the Bahamas.291 14-Oxo-aerophobin-2 380 was among fourteen bromoisoxazole alkaloids isolated from A. insularis from the Bahamas.292 The diethyl ketal 381 was obtained from a Turkish specimen of Verongia aerophoba that had been extracted with ethanol.293 Two additional bromotyrosine-derived metabolites, araplysillin III 382 and hexadelin C 383, were isolated from Aiolochroia crassa (=![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) Ianthella ardis) from Belize.294 A specimen of Aplysina fistularis insularis from Venezuela contained the cytotoxic metabolite 11-deoxyfistularin-3 384.295 7-Hydroxyceratinamine 385 is an additional cyanoformamide from an Aplysinella sp. from Micronesia.296 The urethane 386 and the corresponding salt were isolated from Psammaplysilla purpurea from the Mandapam Coast of India.297 Iantheran A 387 is an unusual dimeric polybrominated benzofuran that was obtained as a Na,K-ATPase inhibitor from an Australian Ianthella species.298
Ianthella ardis) from Belize.294 A specimen of Aplysina fistularis insularis from Venezuela contained the cytotoxic metabolite 11-deoxyfistularin-3 384.295 7-Hydroxyceratinamine 385 is an additional cyanoformamide from an Aplysinella sp. from Micronesia.296 The urethane 386 and the corresponding salt were isolated from Psammaplysilla purpurea from the Mandapam Coast of India.297 Iantheran A 387 is an unusual dimeric polybrominated benzofuran that was obtained as a Na,K-ATPase inhibitor from an Australian Ianthella species.298
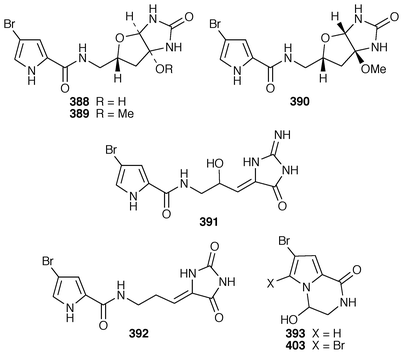
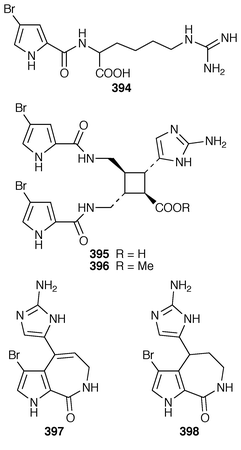
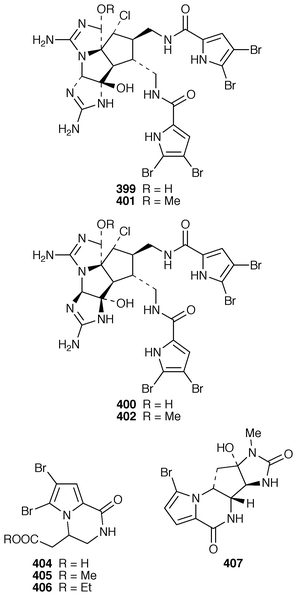
Six additional bromopyrrole alkaloids, slagenins A–C 388–390 and mukanadins A–C 391–393 were isolated from Agelas nakamurai from Okinawa: the planar structure of mukanadin C 393 is the same as that previously reported for dispacamide D.299,300A. wiedenmayeri from the Florida Keys contained an additional bromopyrrole alkaloid (4-bromopyrrole-2-carboxy)homoarginine 394.301 Two dimeric bromopyrrole alkaloids, nakamuric acid 395 and the corresponding methyl ester 396 were obtained from a methanolic extract of A. nakamurai from Indonesia.302Stylissa carteri from Indonesia contained debromostevensine 397 and debromohymenin 398 together with known congeners.303 Axinellamides A–D 399–402 were obtained as antibacterial agents from an Australian Axinella species.304 The racemic forms of the pyrrolodiketopiperazines, longamide 403, longamide B 404, longamide B methyl ester 405 and hanishin 406, which were isolated from Agelas longissima, A. dispar and Acanthella carteri,305–307 have all been synthesized in a concise manner.308 A second independent synthesis of (±)-longamide 403 was reported.309 Agelastatin A 407, a cytotoxic metabolite of Agelas dendromorpha from New Caledonia,310 has been synthesized as its racemate.311
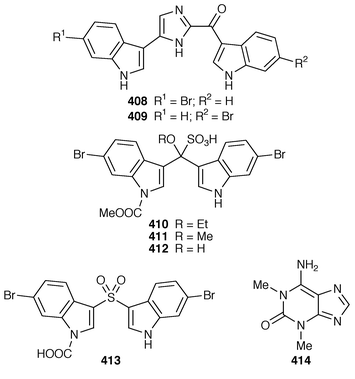
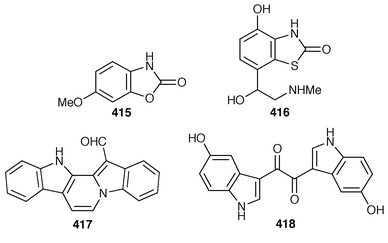
Two additional alkaloids, 408 and 409, of the topsentin class have been isolated as cytotoxic constituents of Spongosorites genitrix from Korea.312 A Southern Australian Echinodictyum sp. contained the antibacterial metabolites echinosulfonic acids A–C 410–412 and echinosulfone A 413.313 The X-ray structure of 1,3-dimethylisoguanine 414 from Amphimedon viridis,314 indicated that the compound crystallized as a trihydrate and in a different tautomeric form than that previously reported.315,316 Coixol (6-methoxy-2(3H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-benzoxazolinone 415) was isolated from an Indian Oceanapia sp. as a toxin to brine shrimp.317 A specimen of Dysidea sp. from Okinawa contained S1319 416, which was isolated as a β-adrenoceptor agonist.318 A synthesis of homofascaplysin C 417 from Fascaplysinopsis reticulatus
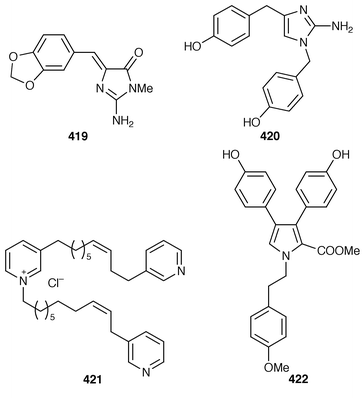
)-benzoxazolinone 415) was isolated from an Indian Oceanapia sp. as a toxin to brine shrimp.317 A specimen of Dysidea sp. from Okinawa contained S1319 416, which was isolated as a β-adrenoceptor agonist.318 A synthesis of homofascaplysin C 417 from Fascaplysinopsis reticulatus![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 319 involved a ditryptophan intermediate.320 The bis-indole alkaloid hyrtiosin B 418,321 from an Okinawan specimen of Hyrtios erecta, has been prepared in good yield.322 Two different routes were used to synthesize the alkaloid leucettamine B 419, which was obtained from Leucetta microraphis,323 in good yields.324 Isonaamine A 420, which was isolated from L. chagosensis,325 has been synthesized using a condensation reaction involving tosyl isocyanate to prepare the 2-aminoimidazole ring system.326 The structure of niphatoxin B 421 from a Red Sea Niphates sp.327 was confirmed by total synthesis.328 Lamellarin O 422, which is a metabolite of Dendrilla cactos,329 has been synthesized using a Diels–Alder strategy.330
319 involved a ditryptophan intermediate.320 The bis-indole alkaloid hyrtiosin B 418,321 from an Okinawan specimen of Hyrtios erecta, has been prepared in good yield.322 Two different routes were used to synthesize the alkaloid leucettamine B 419, which was obtained from Leucetta microraphis,323 in good yields.324 Isonaamine A 420, which was isolated from L. chagosensis,325 has been synthesized using a condensation reaction involving tosyl isocyanate to prepare the 2-aminoimidazole ring system.326 The structure of niphatoxin B 421 from a Red Sea Niphates sp.327 was confirmed by total synthesis.328 Lamellarin O 422, which is a metabolite of Dendrilla cactos,329 has been synthesized using a Diels–Alder strategy.330
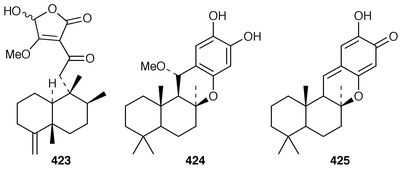
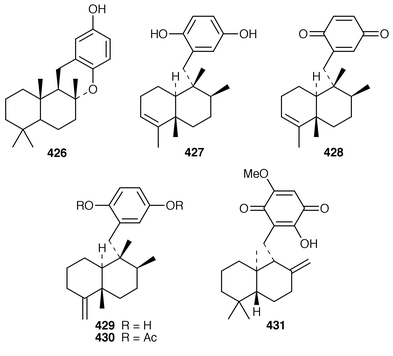
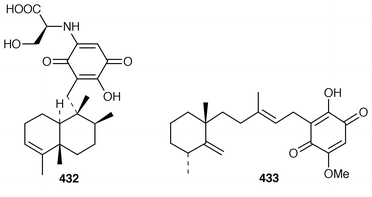
A Red Sea Smenospongia sp. contained smenotronic acid 423, which is an interesting tetronic acid thought to be derived from ilimaquinone.331 A single methanol-addition product, 15α-methoxypuupehenol 424, crystallized from methanol during the work-up of a New Caledonian Hyrtios sp. known to contain puupehenone 425.332 Puupehenone 425, first isolated from a Verongid sponge,333 was synthesized from (−)-sclareol in an enantiospecific manner.334 An efficient synthesis of ent-chromozonarol 426, which is a metabolite of Dysidea pallescens,335 also used (−)-sclareol as a starting material.336 Avarol 427 and the corresponding quinone, avarone 428, which are cytotoxic metabolites of Dysidea avara,337 have been synthesized in an enantioselective manner using a radical addition reaction to incorporate the aromatic ring.338 The structure of isoavarol 429, also known as neoavarol,339 which is a merosesquiterpene from a Pacific Dysidea sp.,340 has been confirmed by an X-ray crystallographic study of the corresponding diacetate 430.341 The absolute configuration of (+)-hyatellaquinone 431, which is a metabolite of Hyatella intestinalis,342 has been revised as the result of a synthesis of (−)-hyatellaquinone from (+)-sclareolide.343 Nakijiquinone C 432, which is a selective inhibitor of the Her-2/Neu protooncogene from an unidentified sponge of the family Spongiidae,344 has been synthesized by an enantioselective route.345 Both a stereoselective total synthesis and an enantioselective formal synthesis of metachromin A 433, which is a metabolite of Hippospongia metachromia,346 have been accomplished.347
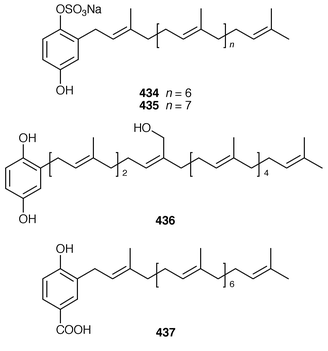
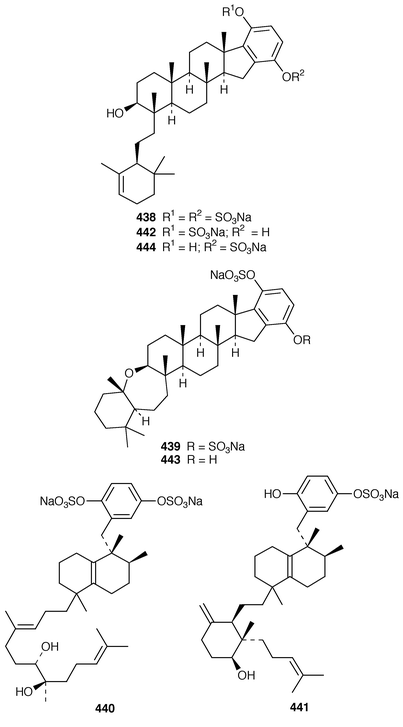
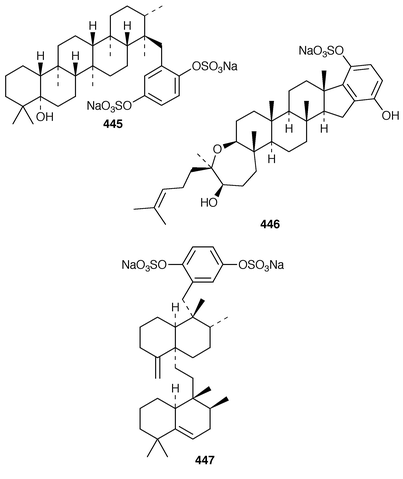
A number of meroterpenoid sulfates have recently been reported. An Australian Sarcotragus sp. contained octaprenylhydroquinone sulfate 434 and nonaprenylhydroquinone sulfate 435 as inhibitors of α1,3-fucosyltransferase VII.348 Two related but non-sulfated meroterpenoids, 1,4,44-trihydroxy-2-octaprenylbenzene 436 and 4-hydroxy-3-octaprenylbenzoic acid 437 were obtained from specimens of Spongia sp. and Ircinia sp., respectively, collected from the Aegean Sea.349 Adociasulfates 1–6 438–443 were obtained as inhibitors of kinesin motor proteins from a Palauan sponge of the genus Haliclona (aka Adocia).350 An Adocia sp. from the Great Barrier Reef contained adociasulfates 1 438, 7 444 and 8 445, which inhibit vacuolar H+-ATPase.351 Adociasulfates 5 442 and 9 446 were obtained from Adocia aculeata from the Great Barrier Reef.352 The structure of adociasulfate 1 438 has been confirmed by an enantioselective total synthesis.353 The relative stereochemistry of the ring junction in the upper decalin moiety of akaterpin 447, which is an inhibitor of phosphatidylinositol-specific phospholipase C from a Callyspongia sp.,354 was shown to be cis by synthesis of model compounds.355
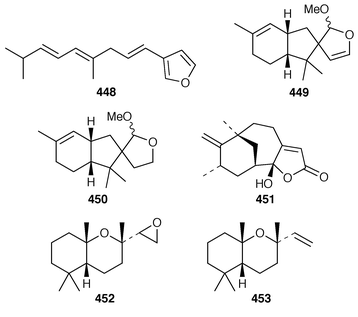
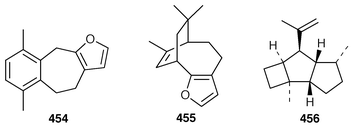
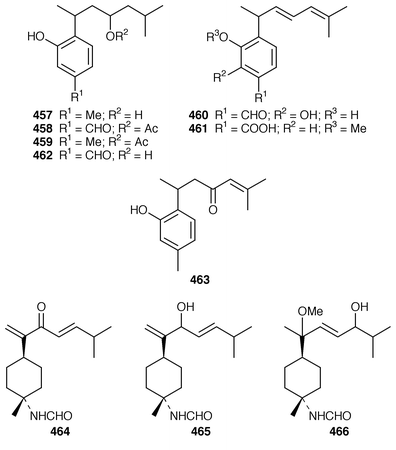
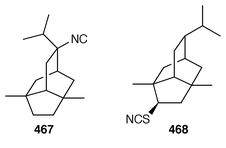
A new furanosesquiterpene, iso-dehydrodendrolasin 448, was obtained from Euryspongia deliculata from the Great Barrier Reef.356 The sequiterpenoids (−)-14-methoxy-14-deacetoxyspirodysin-1 449 and the corresponding 12,13-dihydro derivative 450 were isolated from an Indian specimen of Dysidea fragilis.357 The tricyclic sesquiterpene γ-hydroxybutenolide 451 was obtained as an antifouling agent from D. herbacea from Palau.358 Dysifragin 452 and caparrapi oxide 453 were isolated from a Taiwanese specimen of D. fragilis.359 Tavacpallescensin 454 from D. avara contaminated with Pleraplysilla spinifera![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 360 was synthesized as the racemate from a symmetrical precursor.361 Pallescensin B 455, which was isolated from Dysidea pallescens,362 has been synthesized as its racemate.363 1,5-Cyclooctadiene was employed as the starting material for a stereoselective total synthesis of kelsoene 456,364 which is a metabolite of Cymbastela hooperi.365 A Taiwanese sponge of the genus Parahigginsia contained parahigginols A–D 457–460, parahigginic acid 461, parahigginine 462 and parahigginone 463, of which 458–461 were moderately cytotoxic.365,367 Three 3-formamidobisabolene derivatives 464–466 were isolated from an Axinyssa sp. from Yap, Micronesia.368 The first total synthesis of 9-isocyanoneopupukeanane 467, a metabolite of Ciocalypta sp.,369 has been reported.370 (−)-4-Thiocyanatoneopupukaenane 468, which is a metabolite of an unidentified sponge from Pohnpei,371 has been synthesized in an enantiospecific manner.372 The guanidinoimidazole alkaloid stellettazole A 469 is an antibacterial and Ca
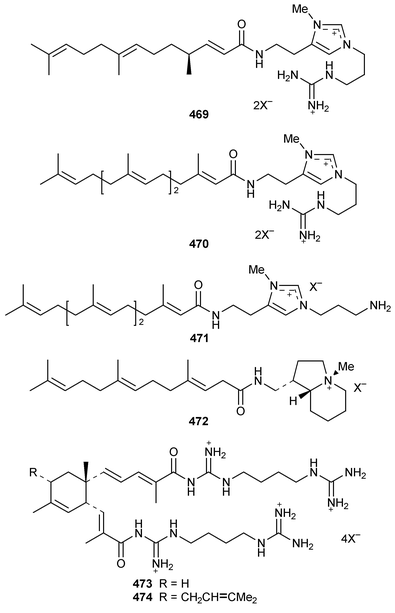
360 was synthesized as the racemate from a symmetrical precursor.361 Pallescensin B 455, which was isolated from Dysidea pallescens,362 has been synthesized as its racemate.363 1,5-Cyclooctadiene was employed as the starting material for a stereoselective total synthesis of kelsoene 456,364 which is a metabolite of Cymbastela hooperi.365 A Taiwanese sponge of the genus Parahigginsia contained parahigginols A–D 457–460, parahigginic acid 461, parahigginine 462 and parahigginone 463, of which 458–461 were moderately cytotoxic.365,367 Three 3-formamidobisabolene derivatives 464–466 were isolated from an Axinyssa sp. from Yap, Micronesia.368 The first total synthesis of 9-isocyanoneopupukeanane 467, a metabolite of Ciocalypta sp.,369 has been reported.370 (−)-4-Thiocyanatoneopupukaenane 468, which is a metabolite of an unidentified sponge from Pohnpei,371 has been synthesized in an enantiospecific manner.372 The guanidinoimidazole alkaloid stellettazole A 469 is an antibacterial and Ca![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2/calmodulin-dependent phosphodiesterase inhibitor from a Japanese species of Stelletta.373 The same Stelletta sp. also yielded stellettazoles B 470 and C 471, stellettamide C 472, and bistellettadines A 473 and B 474, which also inhibited Ca
2/calmodulin-dependent phosphodiesterase inhibitor from a Japanese species of Stelletta.373 The same Stelletta sp. also yielded stellettazoles B 470 and C 471, stellettamide C 472, and bistellettadines A 473 and B 474, which also inhibited Ca![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2/calmodulin-dependent phosphodiesterase.374,375
2/calmodulin-dependent phosphodiesterase.374,375
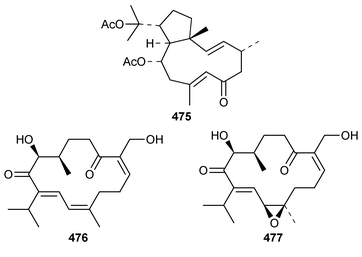
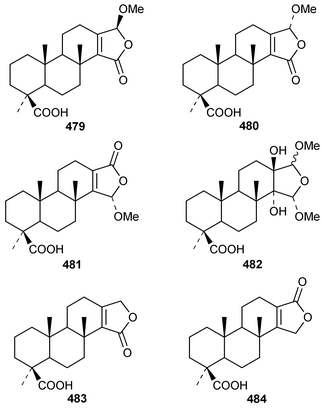
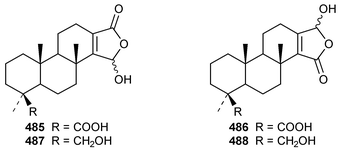
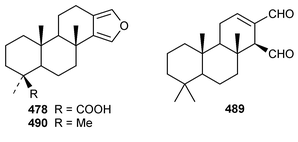
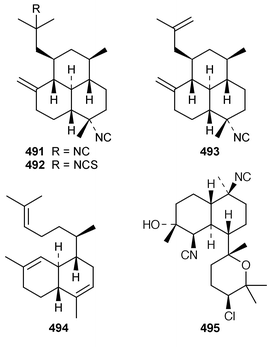
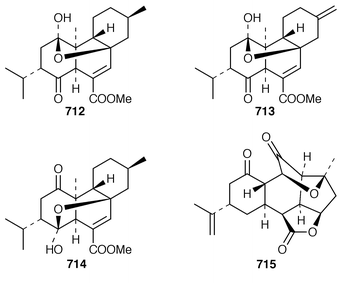
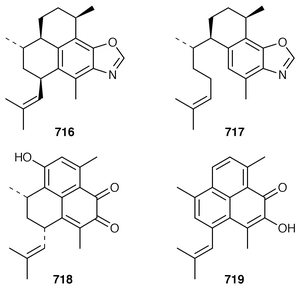
It is interesting that the cytotoxic diterpene 475, which is closely related to metabolites of brown algae, was obtained from Sigmosceptrella quadrilobata from the Comoros Islands.376 Two cytotoxic cembranoids, flabellatenes A 476 and B 477, which are compounds that are normally associated with soft corals, were isolated from the Antarctic sponge Lissodendoryx flabellata.377 Together with the known major metabolite, (+)-spongia-13(16),14-diene-19-oic acid 478, six additional spongian diterpenes, the epimeric γ-methoxybutenolides 479–481, the di(methoxy ketal) 482 and the epimeric butenolides 483 and 484 were isolated from Spongia matamata from Yap, Micronesia.378 A Spongia sp. from the Philippines contained spongiabutenolides A–D 485–488 in addition to the known major metabolite (+)-spongia-13(16),14-diene-19-oic acid 478: the structure of spongiabutenolide A 485 was confirmed by synthesis from the furan 478 using singlet oxygen oxidation.379 The X-ray crystallographic data for ent-isocopal-12-ene-15,16-dialdehyde 489, which is a metabolite of S. officinalis,380 has been presented.381 (−)-Spongia-13(16),14-diene 490, which is also a metabolite of S. officinalis,382 was synthesized in a diastereoselective manner.383 A trio of amphilectene isonitriles, 491–493, and the bicyclic diterpene hydrocarbon 494 were obtained from a Caribbean Cribochalina sp.384 The absolute configuration of kalihinol A 495, which is a metabolite of an Acanthella sp. from Guam,385 was determined by application of the CD exciton chirality method to a bis-p-bromobenzoate derivative.386
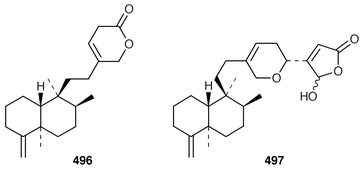
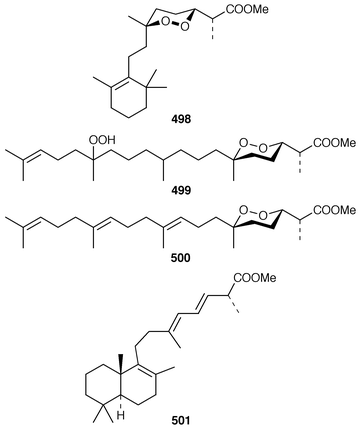
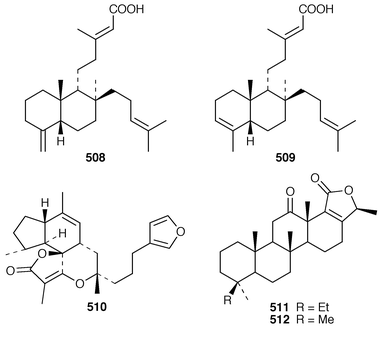
An additional C21 terpene lactone 496, that is possibly derived from cacospongionolide B 497, with which it was found in a Mediterranean specimen of Fasciospongia cavernosa, was identified by interpretation of spectral data.387 Methylation of the crude extract of a Sigmosceptrella sp. from Southern Australia with diazomethane produced a mixture of products from which nuapapuin methyl ester 498 and sigmosceptrellin D and E methyl esters 499 and 500 were isolated and identified.388 The methyl ester 501, which is a metabolite of Latrunculia brevis from Australia,389 has been synthesized in an enantiospecific manner.390
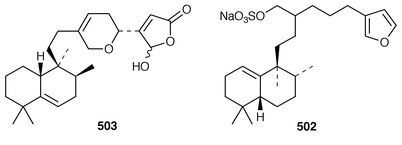
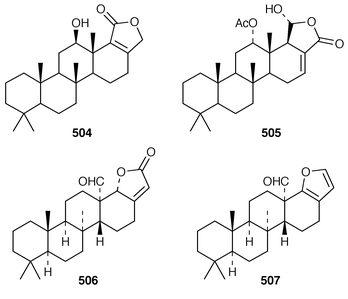
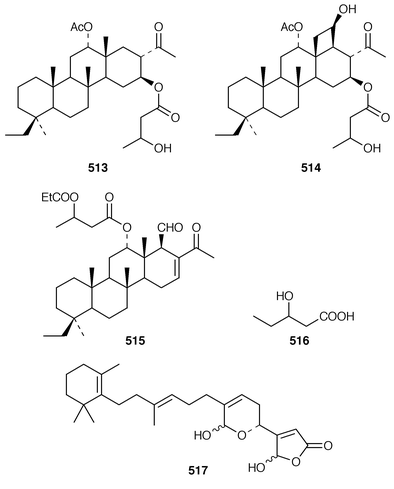
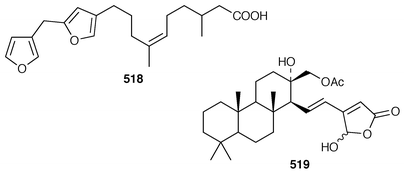
Halisulfate 7 502 is a sesterterpene sulfate from a species of Coscinoderma from Yap, Micronesia.391 An additional sesterterpene, cacospongionolide F 503, was isolated from Fasciospongia cavernosa from the Northern Adriatic Sea and its absolute stereochemistry was determined using the modified Mosher’s method.392 The X-ray crystallographic data for scalarolide 504 and scalarin 505, which are metabolites of sponges of the family Thorectidae,393,394 have been reported.395 A specimen of Coscinoderma mathewsi from Pohnpei contained two sesterterpenes 506 and 507 that have an unusual cis geometry about the B/C ring junction.396 Bilosespens A 508 and B 509, which were isolated as an inseparable mixture from an Eritrean sample of Dysidea cinerea, are cytotoxic sesterterpenes having an unprecedented carbon skeleton.397 (−)-Wistarin 510 from a Red Sea Ircinia sp. is the enantiomer of a metabolite previously isolated![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 398 from I. wistaria.399 Phyllofolactones C 511 and D 512 are minor homoscalarane sesterterpenes from a Chinese specimen of Phyllospongia foliascens.400 Three additional sesterterpenes 513–515 were isolated from Strepsichordaia lendenfeldi from the Great Barrier Reef.401 The polar material from a Great Barrier Reef Carteriospongia sp. contained (−)-3-hydroxypentanoic acid 516 which is sometimes found as an ester group on scalaranes.402 Manoalide 517, which is an antiinflammatory agent from Luffariella variabilis,403 has been synthesized in an enantioselective manner.404 A synthesis of ircinin-4 518, a metabolite of Ircinia oros,405 employed a palladium-catalyzed reaction as the key step.406 The absolute configuration of (−)-spongianolide A 519, which is a cytotoxic sesterterpene from a Spongia sp.,407 was determined by total synthesis.408
398 from I. wistaria.399 Phyllofolactones C 511 and D 512 are minor homoscalarane sesterterpenes from a Chinese specimen of Phyllospongia foliascens.400 Three additional sesterterpenes 513–515 were isolated from Strepsichordaia lendenfeldi from the Great Barrier Reef.401 The polar material from a Great Barrier Reef Carteriospongia sp. contained (−)-3-hydroxypentanoic acid 516 which is sometimes found as an ester group on scalaranes.402 Manoalide 517, which is an antiinflammatory agent from Luffariella variabilis,403 has been synthesized in an enantioselective manner.404 A synthesis of ircinin-4 518, a metabolite of Ircinia oros,405 employed a palladium-catalyzed reaction as the key step.406 The absolute configuration of (−)-spongianolide A 519, which is a cytotoxic sesterterpene from a Spongia sp.,407 was determined by total synthesis.408
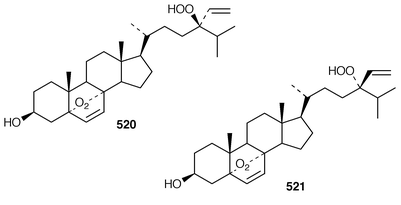
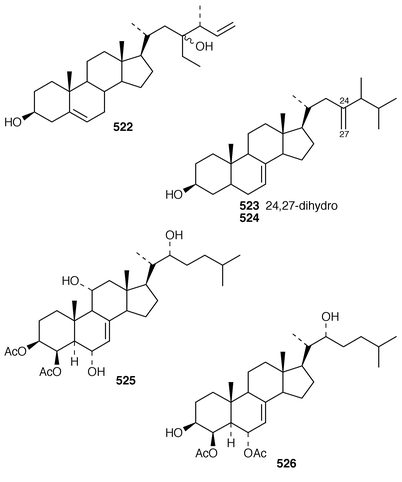
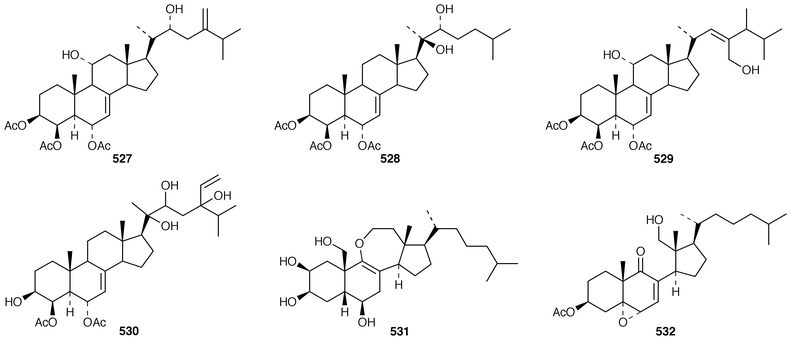
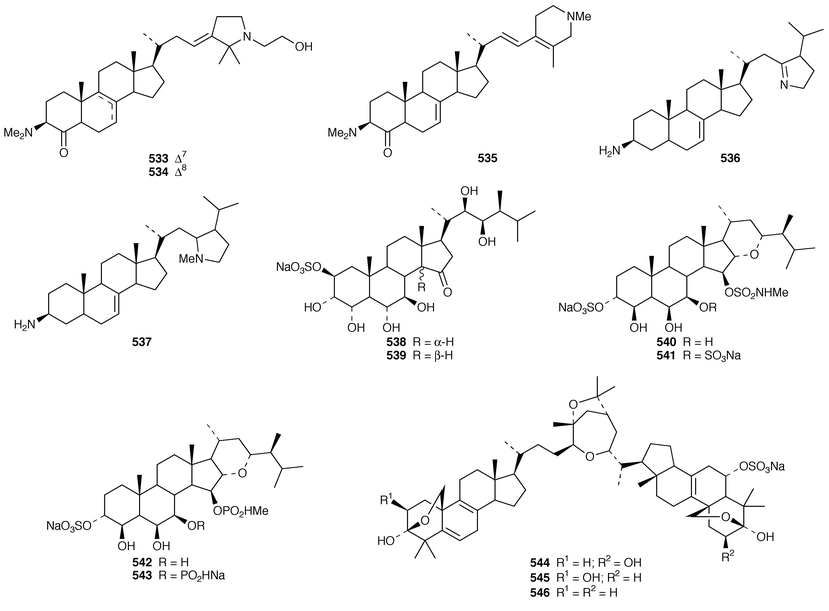
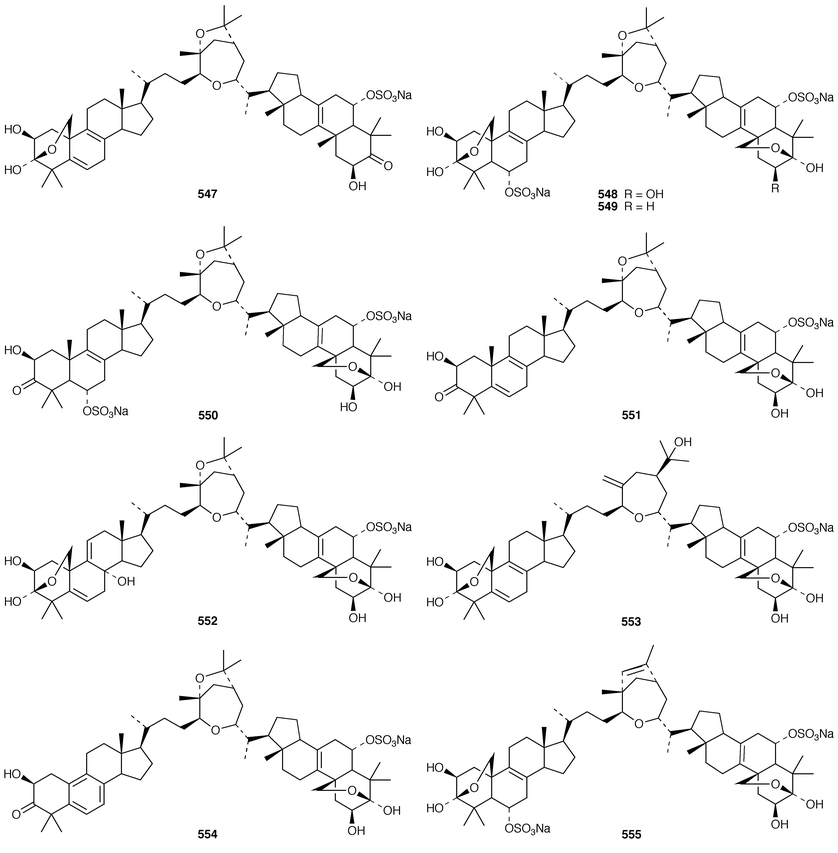
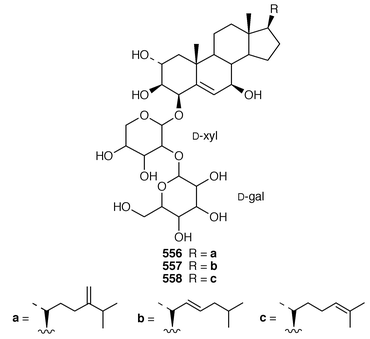
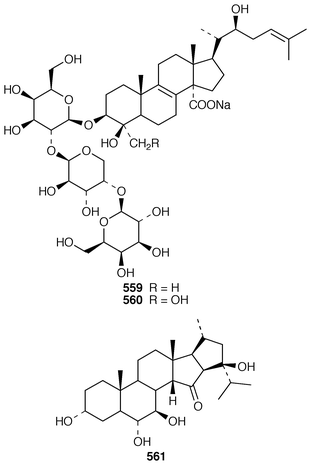
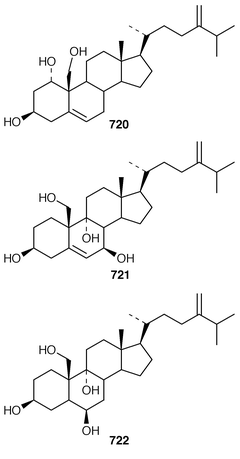
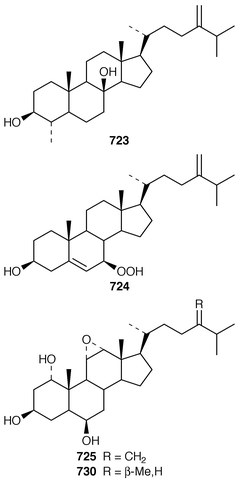
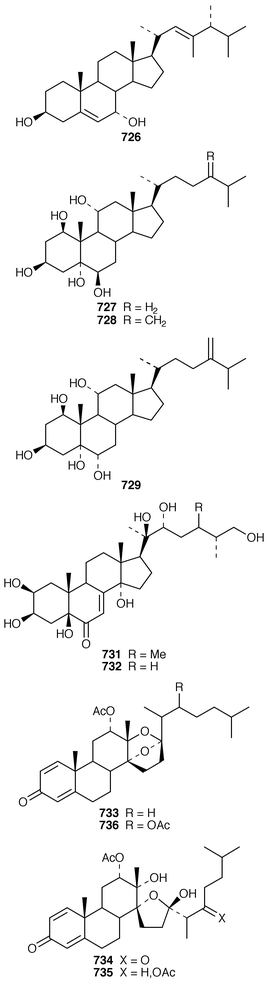
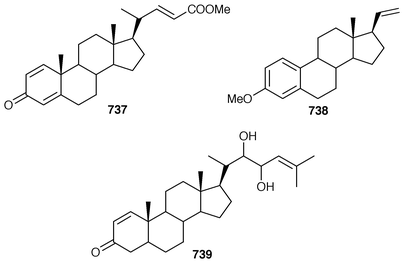
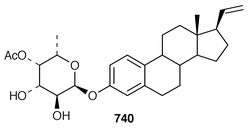
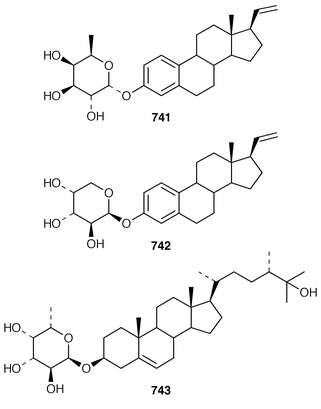
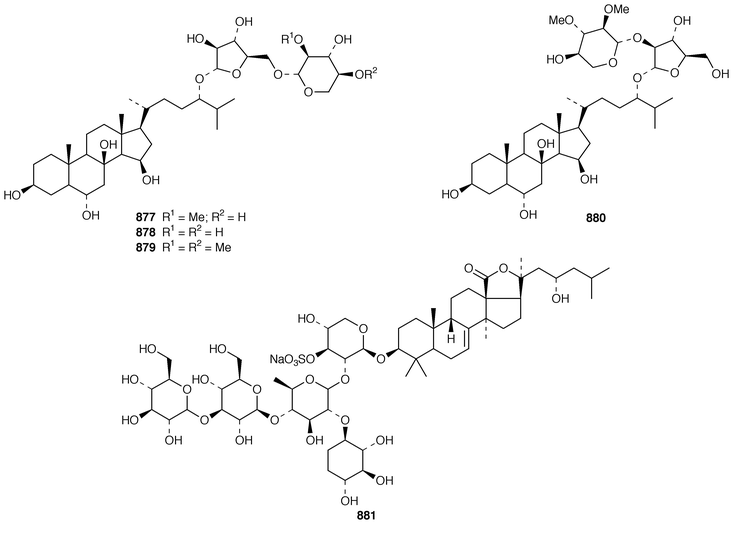
In addition to known sesterterpenes, an inseparable mixture of two epimeric epidioxy sterols 520 and 521 was obtained as an antifouling agent from Lendenfeldia chondrodes from Palau.409 An Indian specimen of Petrosia testudinaria yielded an unusual 27-nor sterol 522.410 Two C30 sterols, thymosiosterol 523 and 24,27-dehydrothymosiosterol 524 were isolated from an undescribed Thymosiopsis sp. from France.411 As part of a study of multidrug resistance reversal, six new agosterol derivatives, agosterols B 525, C 526, A4527, D2528, A5529 and C6530 were isolated from a Japanese species of Spongia.412 An unusual sterol enol ether 531, derived by cyclization of a 9,11-secosterol, was obtained from an Australian specimen of Euryspongia arenaria.413 Glaciasterol B 3-monoacetate 532 is a 9,11-secosterol from Fasciospongia cavernosa from the Mediterranean that was toxic to brine shrimp.414 A Corticium sp. from Vanuatu contained plakinamines C 533 and D 534 and three other steroidal alkaloids 535–537, all of which showed significant cytotoxicity.415 Tamasterone sulfates 538 and 539 are a C-14 epimeric pair of polyhydroxylated sterols from a new species of the genus Oceanapia.416 Although haplosamates A 540 and B 541, which are inhibitors of HIV-1 integrase from two Philippines Haplosclerid sponges, were reported to be the first naturally occurring sulfamates,417 re-examination of the spectral data has revealed that they are in fact phosphate esters and that the structures must be revised to 542 and 543, respectively.418 Crellastatins B–M 544–555 are twelve additional cytotoxic dimeric 4,4′-dimethylsterols from a Crella sp. from Vanuatu.419,420 A two sponge association of Poecillastra wondoensis and Jaspis wondoensis contained three cytotoxic and antimicrobial steroidal glycosides, wondosterols A–C 556–558.421 The Caribbean sponge Ectyoplasia ferox contained two triterpene oligoglycosides, ectyoplasides A 559 and B 560, that showed moderate cytotoxicity.422 Xestobergsterol A 561, which is a metabolite of Xestospongia bergquistii that inhibits the release of histamine from rat mast cells,423 has been synthesized in 24 steps from stigmasterol.424
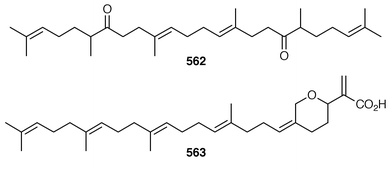
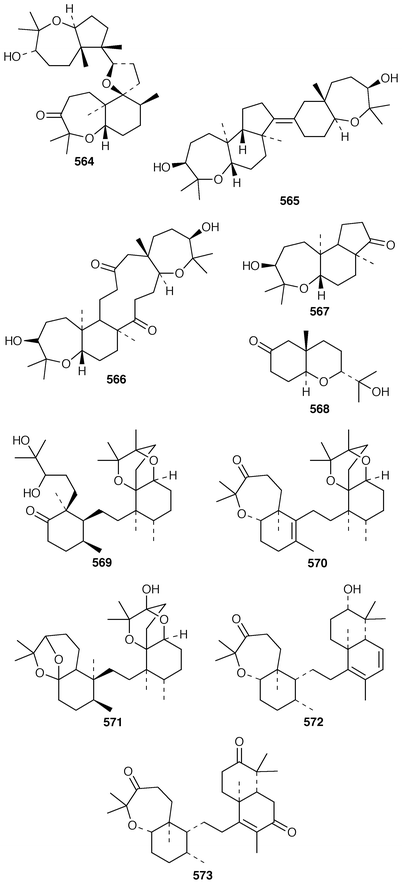
The symmetrical diketotriterpenoid 562 was obtained from an Indonesian specimen of Hyrtios erectus.425 The revised structure of hippospongic acid 563,426 which is a metabolite of a Japanese Hippospongia sp.,427 has been synthesized as the racemate.428,429 Three additional triterpenes, 22-dihydroyardenone 564, abudinol B 565 and muzitone 566, together with possible degradation products nakorone 567 and durgamone 568, were obtained from a Red Sea specimen of Ptilocaulis spiculifer and five new triterpenes, sodwanones N–R 569–573 were reported from Axinella weltneri from South Africa.430
7 Coelenterates
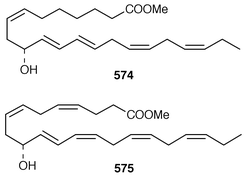
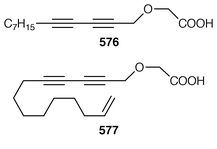
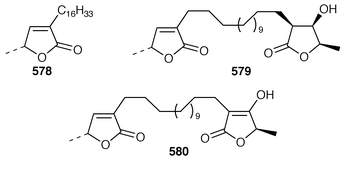
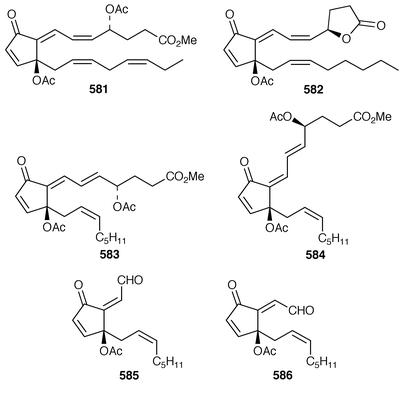
Although the chemistry of coelenterates continues to be dominated by terpenoids, there are a few interesting lipids and alkaloids to be reported. The scleractinian coral Madrepora oculata contained a mixture of 10-hydroxydocosapolyenoic acids that was methylated to obtain the methyl esters 574 and 575.431 Montiporic acids A 576 and B 577, which are cytotoxic polyacetylenes from the scleractinian coral Montipora digitata,432 have been synthesized in good yields.433 Three additional butenolides 578–580 related to ancepsenolide![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 434 were obtained from the Caribbean gorgonian Pterogorgia anceps.435 The Okinawan soft coral Clavularia viridis has yielded a number of new prostanoids: 17,18-dehydroclavulone I 581 and clavulactone I 582,436 4-epiclavulones II 583 and III 584,437 and the truncated derivatives clavirins I 585 and II 586.438 Batyl alcohol-3-O-α-L-fucoside 587 was obtained from a new species of Sinularia from Rangat Island in the Andaman group.439 An Indian specimen of Sinularia dissecta contained the known
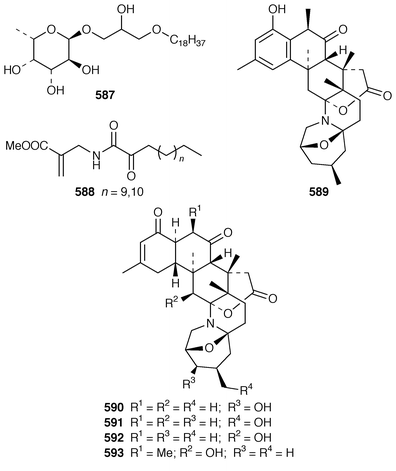
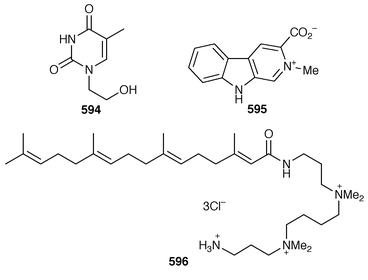
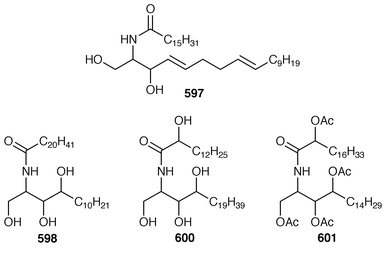
434 were obtained from the Caribbean gorgonian Pterogorgia anceps.435 The Okinawan soft coral Clavularia viridis has yielded a number of new prostanoids: 17,18-dehydroclavulone I 581 and clavulactone I 582,436 4-epiclavulones II 583 and III 584,437 and the truncated derivatives clavirins I 585 and II 586.438 Batyl alcohol-3-O-α-L-fucoside 587 was obtained from a new species of Sinularia from Rangat Island in the Andaman group.439 An Indian specimen of Sinularia dissecta contained the known![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 440 sponge metabolite 588.441 Five additional alkaloids, zoanthenol 589, 3-hydroxynorzoanthamine 590, 30-hydroxynorzoanthamine 591, 11-hydroxynorzoanthamine 592 and 11-hydroxyzoanthamine 593 have been isolated from a Zoanthus sp. from the Canary Islands.442 An Indian Zoanthus sp. contained known bases together with thyminol 594.126 The deep-water briareid soft coral Lignopsis spongiosum from South Georgia Island yielded the β-carboline alkaloid 595.443 The H,K-ATPase inhibitor sinulamide 596 is a tetraprenylated spermine derivative from a Japanese soft coral of the genus Sinularia.444 Two sphingosine derivatives, N-hexadecanoyl-1,3-dihydroxy-2-amino-4,8-octadecadiene 597 and N-heneicosanoyl-1,3,4-trihydroxy-2-aminotetradecane 598, were isolated from S. crassa from the Andaman and Nicobar Islands.445 As part of a pharmacological study that reported the cytotoxicity of africanene 599, it was shown that S. leptaclados from Southern India contained two new sphingolipids, 600 and 601.446
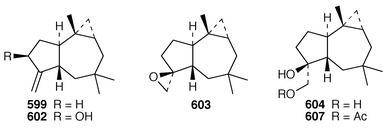
440 sponge metabolite 588.441 Five additional alkaloids, zoanthenol 589, 3-hydroxynorzoanthamine 590, 30-hydroxynorzoanthamine 591, 11-hydroxynorzoanthamine 592 and 11-hydroxyzoanthamine 593 have been isolated from a Zoanthus sp. from the Canary Islands.442 An Indian Zoanthus sp. contained known bases together with thyminol 594.126 The deep-water briareid soft coral Lignopsis spongiosum from South Georgia Island yielded the β-carboline alkaloid 595.443 The H,K-ATPase inhibitor sinulamide 596 is a tetraprenylated spermine derivative from a Japanese soft coral of the genus Sinularia.444 Two sphingosine derivatives, N-hexadecanoyl-1,3-dihydroxy-2-amino-4,8-octadecadiene 597 and N-heneicosanoyl-1,3,4-trihydroxy-2-aminotetradecane 598, were isolated from S. crassa from the Andaman and Nicobar Islands.445 As part of a pharmacological study that reported the cytotoxicity of africanene 599, it was shown that S. leptaclados from Southern India contained two new sphingolipids, 600 and 601.446
Three additional africanene derivatives, 10(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-hydroxy-9(15)-africanene 602, 9(S
)-hydroxy-9(15)-africanene 602, 9(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ),15-epoxyafricanane 603 and 9(S
),15-epoxyafricanane 603 and 9(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ),15-dihydroxyafricanane 604 were isolated as minor constituents of Sinularia dissecta from Southern India.447 10(S
),15-dihydroxyafricanane 604 were isolated as minor constituents of Sinularia dissecta from Southern India.447 10(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-Hydroxy-9(15)-africanene 602 and 9(S
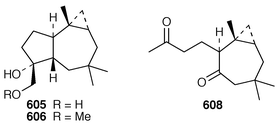
)-Hydroxy-9(15)-africanene 602 and 9(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ),15-epoxyafricanane 603 were also obtained by microbial oxidation of 9(15)-africanene 599 using Aspergillus niger and Rhizopusoryzae.448S. intacta from India contained four additional africanane derivatives, (9R)-9,15-dihydroxyafricanane 605, (9R)-9-methoxy-15-hydroxyafricanane 606, (9S
),15-epoxyafricanane 603 were also obtained by microbial oxidation of 9(15)-africanene 599 using Aspergillus niger and Rhizopusoryzae.448S. intacta from India contained four additional africanane derivatives, (9R)-9,15-dihydroxyafricanane 605, (9R)-9-methoxy-15-hydroxyafricanane 606, (9S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-9,15-dihydroxyafricanane 607 and the seco-diketone 608.449,450 A specimen of Gorgonia ventalina from the Bahamas contained 5,10-epoxymuurolane 609.451 During studies of fish feeding deterrents from the Brazilian gorgonian Heterogorgia uatumani, a new sesquiterpene, heterogorgiolide 610, was isolated as an active agent.452 Alertenone 611 is a dimer of the cytotoxin suberosenone 612, previously isolated from Subergorgia suberosa,453 that was obtained from an Alertigorgia sp. from Australia.454 Euplexides A–E 613–617 are cytotoxic farnesylhydroquinone glycosides from the Korean gorgonian Euplexaura anastomosans.455 Curcuhydroquinone 618, which is a metabolite of Pseudopterogorgia rigida,456 has been synthesized as its racemate.457 The absolute configuration of the secosesquiterpene (−)-anthoplalone 619, which is a cytotoxic constituent of the sea anemone Anthopleura pacifica,458 was determined by total synthesis.459 Clavularin B 620, which is a metabolite of the soft coral Clavularia koellikeri,460 has been synthesized by an enantio- and diastereo-controlled route.461
)-9,15-dihydroxyafricanane 607 and the seco-diketone 608.449,450 A specimen of Gorgonia ventalina from the Bahamas contained 5,10-epoxymuurolane 609.451 During studies of fish feeding deterrents from the Brazilian gorgonian Heterogorgia uatumani, a new sesquiterpene, heterogorgiolide 610, was isolated as an active agent.452 Alertenone 611 is a dimer of the cytotoxin suberosenone 612, previously isolated from Subergorgia suberosa,453 that was obtained from an Alertigorgia sp. from Australia.454 Euplexides A–E 613–617 are cytotoxic farnesylhydroquinone glycosides from the Korean gorgonian Euplexaura anastomosans.455 Curcuhydroquinone 618, which is a metabolite of Pseudopterogorgia rigida,456 has been synthesized as its racemate.457 The absolute configuration of the secosesquiterpene (−)-anthoplalone 619, which is a cytotoxic constituent of the sea anemone Anthopleura pacifica,458 was determined by total synthesis.459 Clavularin B 620, which is a metabolite of the soft coral Clavularia koellikeri,460 has been synthesized by an enantio- and diastereo-controlled route.461
In addition to several known compounds, a Taiwanese specimen of the soft coral Nephthea brassica contained both the sesquiterpene (−)-4α-O-acetylselin-11-ene 621 and the diterpenes brassicolide 622 and brassicolide acetate 623.462 Three additional cembranoids, sartol acetate B 624, epoxysartone B 625 and sartone E 626, were obtained from a Japanese Sarcophyton species.463 Sarcophytols A 627 and B 628, which are metabolites of the Okinawan soft coral S. glaucum,464 were synthesized in a concise and efficient manner.465 The synthesis of racemic sinulariol B 629, isolated from a Japanese specimen of Sinularia mayi,466 was accomplished in 10 steps and ca. 10% yield from geraniol.467 A subsequent paper recorded the synthesis of (−)-sinulariol B 629 from geraniol.468 Preverecynarmin 630, which was isolated from both Veretillum cynomorium and its specific predator, the opisthobranch mollusc Armina maculata,469 was synthesized from (E,E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-farnesol.470 Lobatrienolide 631 and lobatrientriol 632, which are metabolites of an Okinawan specimen of S. flexibilis,471 were prepared from (+)-nopinone.472
)-farnesol.470 Lobatrienolide 631 and lobatrientriol 632, which are metabolites of an Okinawan specimen of S. flexibilis,471 were prepared from (+)-nopinone.472
The Indonesian sea pen Veretillum malayense contained four diterpenes, malayenolides A–D 633–636, that were toxic to brine shrimp.473 Sandresolides A 637 and B 638 are nor-diterpenes that were isolated from the gorgonian Pseudopterogorgia elisabethae from Columbia.474 An Okinawan soft coral of the genus Xenia contained three additional xenicane diterpenes, xeniadiol 639, xeniaol 640 and xeniatriol 641.475 In addition to known metabolites, the Indian soft coral Sinularia maxima produced two furanocembranoids, sethukarailin 642 and sethukarailide 643.476 A specimen of the gorgonian Pseudopterogorgia bipinnata from Columbia contained bipinnapterolide A 644, bipinnatins G–I 645–647 and bipinnatolides F–J 648–652, among which the structures of bipinnapterolide A 644 and bipinnatolide F 648 were determined by X-ray crystallography.477 Bisgersolanolide 653 is a diterpenoid dimer that was also obtained from a specimen of P. bipinnata from the same location.478 A full account of the synthesis of eleutherobin 654, which is an anti-tumour agent from an Australian Eleutherobia sp.,479 has appeared.480
The Senegalese gorgonian Eunicella labiata contained two additional eunicellin-type diterpenes, labiatins D 655 and E 656.481 Obtained from E. cavolinii from the Mediterranean, massileunicellins A–C 657–659 are cytotoxic eunicellin-type diterpenes that contain a second ether bridge.482 The briarane class of diterpenoids have dominated reports of new metabolites from coelenterates. Five such compounds, (−)-4-deacetyljunceellolide D 660, (+)-11α,20α-epoxyjunceellolide D 661, (−)-11α,20α-epoxy-4-deacetyljunceellolide D 662, (−)-11α,20α-epoxy-4-deacetoxyjunceellolide D 663 and (+)-junceellolide A 664, an antipode of a known metabolite, were obtained from an Indonesian specimen of Junceella fragilis.483Erythropodium caribaeorum from Jamaica contained three additional briaranes, erythrolide E 3-acetate 665, erythrolide F 3-acetate 666 and erythrolide H 16-acetate 667.484 Violides C–I 668–674 are additional briarane diterpenes from a Japanese species of Briareum (aka Pachyclavularai violacea):![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 485 the authors propose that two previously reported
485 the authors propose that two previously reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 486 analogs be renamed violides A and B. A subsequent paper reported the isolation of violides J -M 675–678 from the same organism and the X-ray crystallographic structure determination of violide J 675.487 Excavatolides F–M 679–686 and U–Z 687–692, some of which were cytotoxic, were isolated from a Taiwanese specimen of Briareum excavatum
486 analogs be renamed violides A and B. A subsequent paper reported the isolation of violides J -M 675–678 from the same organism and the X-ray crystallographic structure determination of violide J 675.487 Excavatolides F–M 679–686 and U–Z 687–692, some of which were cytotoxic, were isolated from a Taiwanese specimen of Briareum excavatum![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 488,489 while excavatolides N–T 693–699 were obtained from a Western Australian specimen of B. excavatum.490 Having reached the end of the alphabet for the excavatolides, the next series of compounds from B. excavatum were named briaexcavatolides A–J 700–709 and the structure of briaexcavatolide B 701 was determined by X-ray crystallography.491
488,489 while excavatolides N–T 693–699 were obtained from a Western Australian specimen of B. excavatum.490 Having reached the end of the alphabet for the excavatolides, the next series of compounds from B. excavatum were named briaexcavatolides A–J 700–709 and the structure of briaexcavatolide B 701 was determined by X-ray crystallography.491
Bishomoisomandapamate 710, which was isolated from a new species of Sinularia from the Indian Ocean, is an analog of a known metabolite.492Sarcophyton elegans from the Indian Ocean has yielded an additional diterpene, 7-dehydrosarcophytin 711.493 A full paper has described the isolation of sarcophytin 712, 7-dehydrosarcophytin 711, 7(15)-dehydrosarcophytin 713 and isosarcophytin 714 from S. elegans.494 The structure of an unusual cytotoxic norditerpenoid, ineleganolide 715, which was obtained from Sinularia inelegans from Formosa, was determined by X-ray crystallography.495 Pseudopteroxazole 716, and to a lesser extent, secopseudopteroxazole 717 are inhibitors of Mycobacterium tuberculosis H37Rv that were isolated from Pseudopterogorgia elizabethae from Columbia.496P. elizabethae from the same location also contained elisabatins A 718 and B 719.497
Two 19-oxygenated polyhydroxy steroids, 24-methylenecholest-5-en-1α,3β,19-triol 720 and 24-methylenecholest-5-en-3β,7β,9α,19-tetraol 721, were isolated from an Indian Ocean specimen of Nephthea chabroli![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 498 while a Chinese specimen of N. brassica contained 24-methylenecholest-3β,6β,9α,19-tetraol 722.499 A specimen of N. brassica from the Indian Ocean contained 4α-methyl-24-methylenecholest-3β,8β-diol 723.500 A Formosan Sinularia sp. contained 7β-hydroperoxy-24-methylenecholesterol 724 together with a number of known compounds.501 A Sinularia sp. from Southern India yielded 11α,12α-epoxy-24-methylenecholest-1α,3β,6β-triol 725
498 while a Chinese specimen of N. brassica contained 24-methylenecholest-3β,6β,9α,19-tetraol 722.499 A specimen of N. brassica from the Indian Ocean contained 4α-methyl-24-methylenecholest-3β,8β-diol 723.500 A Formosan Sinularia sp. contained 7β-hydroperoxy-24-methylenecholesterol 724 together with a number of known compounds.501 A Sinularia sp. from Southern India yielded 11α,12α-epoxy-24-methylenecholest-1α,3β,6β-triol 725![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 502 while Lobophytum crassum from the same region contained 23,24(S
502 while Lobophytum crassum from the same region contained 23,24(S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-dimethylcholest-5,22-dien-3β,7α-diol 726, which was isolated as the corresponding diacetate.503 Four polyhydroxy steroids from an Indian specimen of S. dissecta, cholest-1β,3β,5α,6β,11α-pentaol 727, 24-methylenecholest-1β,3β,5α,6β,11α-pentaol 728, 24-methylenecholest-1β,3β,5α,6α,11α-pentaol 729 and 11α,12α-epoxy-24-β-methylcholest-1α,3β,6β-triol 730, were identified as their peracetate derivatives.504 Palythoalones A 731 and B 732 are ecdysteroids from an Okinawan specimen of the zoanthid Palythoa australiae.505 A Dendronephthya sp. from Japan contained four 13,17-secosteroids, isogosterones A–D 733–736, that inhibited settlement of the cyprid larvae of the barnacle Balanus amphitrite.506 Subsequently, methyl 3-oxochola-1,4,22-trien-24-oate 737 from the Dendronephthya sp. and 3-methoxy-19-norpregna-1,3,5(10),20-tetraene 738, 22,23-dihydroxycholesta-1,24-dien-3-one 739 and 3-(4-O-acetyl-6-deoxy-β-galactopyranosyloxy)-19-norpregna-1,3,5(10),20- tetraene 740 from a Japanese specimen of Alcyonium gracillimum were shown to be toxic to the cyprid larvae of B. amphitrite.507 Two norpregnane glycosides, 19-norpregna-1,3,5(10),20-tetraen-3-O-α-fucopyranoside 741 and 19-norpregna-1,3,5(10),20-tetraen-3-O-β-arabinopyranoside 742, were obtained from the soft coral Scleronephthya pallida from Thailand.508Sinularia gravis from Southern India contained (24S)-methylcholest-5-en-3β,25-diol-3-O-α-fucopyranoside 743.509
)-dimethylcholest-5,22-dien-3β,7α-diol 726, which was isolated as the corresponding diacetate.503 Four polyhydroxy steroids from an Indian specimen of S. dissecta, cholest-1β,3β,5α,6β,11α-pentaol 727, 24-methylenecholest-1β,3β,5α,6β,11α-pentaol 728, 24-methylenecholest-1β,3β,5α,6α,11α-pentaol 729 and 11α,12α-epoxy-24-β-methylcholest-1α,3β,6β-triol 730, were identified as their peracetate derivatives.504 Palythoalones A 731 and B 732 are ecdysteroids from an Okinawan specimen of the zoanthid Palythoa australiae.505 A Dendronephthya sp. from Japan contained four 13,17-secosteroids, isogosterones A–D 733–736, that inhibited settlement of the cyprid larvae of the barnacle Balanus amphitrite.506 Subsequently, methyl 3-oxochola-1,4,22-trien-24-oate 737 from the Dendronephthya sp. and 3-methoxy-19-norpregna-1,3,5(10),20-tetraene 738, 22,23-dihydroxycholesta-1,24-dien-3-one 739 and 3-(4-O-acetyl-6-deoxy-β-galactopyranosyloxy)-19-norpregna-1,3,5(10),20- tetraene 740 from a Japanese specimen of Alcyonium gracillimum were shown to be toxic to the cyprid larvae of B. amphitrite.507 Two norpregnane glycosides, 19-norpregna-1,3,5(10),20-tetraen-3-O-α-fucopyranoside 741 and 19-norpregna-1,3,5(10),20-tetraen-3-O-β-arabinopyranoside 742, were obtained from the soft coral Scleronephthya pallida from Thailand.508Sinularia gravis from Southern India contained (24S)-methylcholest-5-en-3β,25-diol-3-O-α-fucopyranoside 743.509
8 Bryozoans
Recent research on natural products from bryozoans has centered as much on synthesis as on structural elucidation. Six new brominated alkaloids, amathaspiramides A–F 744–749, were isolated from Amathia wilsoni from New Zealand.510 Convalutamines F 750, which is cytotoxic, and G 751 and convolutamydine E 752 are additional alkaloids from A. convoluta from Florida.511 Convalutamydine A 753, an alkaloid from A. convoluta,512 has been synthesized from isatin as the racemate.513,514 Debromoflustramines A 754 and B 755, the latter of which is a metabolite of Flustra foliacea,515 have been synthesized using a strategy that can be applied to other alkaloids in this group.516 A detailed account of the convergent synthesis of bryostatin 2 756, which is a cytotoxic macrolide from Bugula neritina,517 has been published.5189 Molluscs
There was only one new paper on the isolation of sea hare metabolites. In addition to known algal metabolites, four halogenated sesquiterpenes, algoane 757, the structure of which was established by X-ray crystallography, 1-deacetoxyalgoane 758, 1-deacetoxy-8-deoxyalgoane 759 and ibhayinol 760 were isolated from Aplysia dactylomela from the Eastern Cape of South Africa.519 Dolastatin 15 761, which is a cytotoxic depsipeptide from the Indian Ocean sea hare Dolabella auricularia,520 was prepared using a convergent synthesis.521 The stereostructure of dolastatin I 762, a cytotoxic metabolite of a Japanese specimen of D. auricularia,522 was confirmed by an enantioselective synthesis.523The absolute stereochemistry of kahalalide F 763, which is a bioactive metabolite of the Hawaiian sacoglossan Elysia rufescens and its food source Bryopsis sp.,524 has been determined by acid hydrolysis and hydrazinolysis, followed by chiral analysis of the fragments.525 Volvatellin 764 is an unstable sesquiterpene from the Indian sacoglossan Volvatella sp. that is formally related to the green algal metabolite caulerpenyne.526
In the process of defining the de novo biosynthesis of drimane terpenoids in dorid nudibranchs, an additional sesquiterpene, 7-deacetoxyolepupuane 765, was isolated from Dendrodoris arborescens.527 2-Isocyanoallopupukeanane 766, which is a metabolite of the nudibranch Phyllidia pustulosa from Japan,528 has been synthesized in good yield using an intramolecular Diels–Alder reaction to fuse the bridging cyclopentane ring to a bicyclo[3.2.1]octane unit.529 The Okinawan nudibranch Reticulidia fungia contained two additional members of a rare class of sponge metabolites, the carbonimidic dichlorides reticulidins A 767 and B 768.530 The absolute stereochemistry of the farnesic acid glyceride derivatives 769–771 from Archidoris odhneri![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 531 and the drimane derivatives 772 and 773 from A. montereyensis
531 and the drimane derivatives 772 and 773 from A. montereyensis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 532 were determined by total synthesis.533 Austrodorins A 774 and B 775 are tricyclic diterpenoid 2′-monoglyceryl esters from the skin of the Antarctic nudibranch Austrodoris kerguelenensis.534 Two additional glycerides 776 and 777 were isolated from A. kerguelenensis and the absolute configurations of 776 and the known
532 were determined by total synthesis.533 Austrodorins A 774 and B 775 are tricyclic diterpenoid 2′-monoglyceryl esters from the skin of the Antarctic nudibranch Austrodoris kerguelenensis.534 Two additional glycerides 776 and 777 were isolated from A. kerguelenensis and the absolute configurations of 776 and the known![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 535 glyceride 778 were established using the modified Mosher method.536 Similar compounds, anisidorins 1–5 779–783, were obtained from the Patagonian dorid Anisodoris fontaini.537 The absolute configuration of anisodorin 5 783 was established by synthesis of the enantiomer.538 The Japanese nudibranch Chromodoris inornata contained three cytotoxic sesterterpenes, inorolides A 784, B 785 and C 786, as well as five new scalaranes 787–791.539 Two additional scalaranes 792 and 793 were obtained from mantle dermal formation-like structures of the Indian nudibranch Glossodoris atromarginata and from the sponge, tentatively identified as a Spongia sp., on which it was grazing.540
535 glyceride 778 were established using the modified Mosher method.536 Similar compounds, anisidorins 1–5 779–783, were obtained from the Patagonian dorid Anisodoris fontaini.537 The absolute configuration of anisodorin 5 783 was established by synthesis of the enantiomer.538 The Japanese nudibranch Chromodoris inornata contained three cytotoxic sesterterpenes, inorolides A 784, B 785 and C 786, as well as five new scalaranes 787–791.539 Two additional scalaranes 792 and 793 were obtained from mantle dermal formation-like structures of the Indian nudibranch Glossodoris atromarginata and from the sponge, tentatively identified as a Spongia sp., on which it was grazing.540
Three new polypropionates, capensinone 794, capensifurannone 795 and (2E,4S,6S,8S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-2,4,6,8-tetramethyl-2-undecenoic acid 796, were obtained from the South African pulmonate mollusc Siphonaria capensis.541 The prosobranch mollusc Coriocella nigra contained an additional cytotoxic staurosporine analog, 4′-N-demethyl-11-hydroxystaurosporine 797.542 The synthesis of both dorimidazole A 798 and preclathridine A 799, which were isolated from the Indo-Pacific nudibranch Notodoris gardneri,543,544 have been reported.326
)-2,4,6,8-tetramethyl-2-undecenoic acid 796, were obtained from the South African pulmonate mollusc Siphonaria capensis.541 The prosobranch mollusc Coriocella nigra contained an additional cytotoxic staurosporine analog, 4′-N-demethyl-11-hydroxystaurosporine 797.542 The synthesis of both dorimidazole A 798 and preclathridine A 799, which were isolated from the Indo-Pacific nudibranch Notodoris gardneri,543,544 have been reported.326
The occurrence of human intoxication due to ingestion of shellfish is providing increasing opportunities for chemists to identify new toxins, which are often related to dinoflagellate metabolites. Turbotoxins A 800 and B 801 are toxic diiodotyramine derivatives from the (inedible) viscera of the Japanese gastropod Turbo marmorata.545 The Chinese bivalve Pinna attenuata contained isomeric bicyclic ketals, attenols A 802 and B 803, which exhibited moderate cytotoxicity.546 Azaspiracids-2 804 and -3 805 are additional toxins isolated from cultivated mussels (Mytilus edulis) that were the source of human intoxication in Ireland in 1995.547 An additional yessotoxin analog, 1-desulfoyessotoxin 806, was isolated from the digestive glands of Norwegian M. edulis.548 The major toxin involved in neurotoxic shellfish poisoning associated with the New Zealand greenshell mussel Perna canaliculus was identified as brevetoxin B4 807.549 Feeding experiments have shown that the scallop Patinopectin yessoensis converts dinophysistoxin-1 808 from the dinoflagellate Dinophysis fortii into dinophysistoxin-3 809.550
10 Tunicates (ascidians)
Although tunicates (ascidians) have yielded fewer new metabolites than usual during the last year, a greater variety of structural types has been described. For example, didemniserinols A–C 810–812 are an unprecedented group of serinolipids from an Indonesian species of Didemnum.551 Lissoclinolide 813, which is an antimicrobial metabolite from a Lissoclinum patella,552 has been synthesized in an efficient manner using organozirconium chemistry.553 A second stereoselective synthesis![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 554 of lissoclinolide 813 established that it was identical to ‘tetrenolin’, which had previously been isolated from the fungus Micropolyspora venezuelensis.555 An Okinawan species of Lissoclimum contained the cytotoxic macrolide haterumalide B 814 that inhibited cell division in fertilized sea urchin eggs.556 An additional monoterpene, hydroquinone 815, the geometrical isomer of a known metabolite, was isolated from Aplidium savignyi from the Comoro Islands.557 A specimen of A. longithorax from Palau contained the isomeric longithorols A 816 and B 817, which were isolated only as their pentaacetates,558 while longithorones J 818 and K 819
554 of lissoclinolide 813 established that it was identical to ‘tetrenolin’, which had previously been isolated from the fungus Micropolyspora venezuelensis.555 An Okinawan species of Lissoclimum contained the cytotoxic macrolide haterumalide B 814 that inhibited cell division in fertilized sea urchin eggs.556 An additional monoterpene, hydroquinone 815, the geometrical isomer of a known metabolite, was isolated from Aplidium savignyi from the Comoro Islands.557 A specimen of A. longithorax from Palau contained the isomeric longithorols A 816 and B 817, which were isolated only as their pentaacetates,558 while longithorones J 818 and K 819![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 559 and longithorols C–E 820–822
559 and longithorols C–E 820–822![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 560 were isolated from an Australian specimen of the same ascidian. Longithorone B 823 from A. logithorax
560 were isolated from an Australian specimen of the same ascidian. Longithorone B 823 from A. logithorax![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 561 has been synthesized as the racemate.562
561 has been synthesized as the racemate.562
Aplidioxins A 824, the structure of which was determined by X-ray analysis, and B 825 were isolated from Aplidiopsis ocellata from the Philippines.563 The Australian ascidian Synoicum prunum contained three weakly cytotoxic bis-spiroketals, prunolides A–C 826–828, together with the known metabolite rubrolide A.564 Five additional lamellarins, the 20-sulfates of lamellarin B 829, C 830 and L 831, lamellarin G 8-sulfate 832 and lamellarin Z 833 were isolated from Didemnum chartaceum from the Great Barrier Reef: unusually long relaxation times were observed for certain signals in their ![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1H NMR spectra.565 Having exhausted the regular alphabet, an inhibitor of HIV-1 integrase from an unidentified ascidian from India was named lamellarin α 20-sulfate 834.566 Ningalin A 835, which is a metabolite of a Western Australian Didemnum sp.,567 and lukianol A 836 from an unidentified tunicate
1H NMR spectra.565 Having exhausted the regular alphabet, an inhibitor of HIV-1 integrase from an unidentified ascidian from India was named lamellarin α 20-sulfate 834.566 Ningalin A 835, which is a metabolite of a Western Australian Didemnum sp.,567 and lukianol A 836 from an unidentified tunicate![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 568 have been synthesized using a Diels–Alder strategy.330Distaplia regina from Palau contained the antibacterial agent 3,6-dibromoindole 837,569 a structure that had been erroneously proposed as a metabolite of acorn worms.570 The first marine ergoline alkaloid, pibocin 838, was isolated as a cytotoxic and antimicrobial agent from a Far-eastern Eudistoma species.571 The cyclized didemnimide alkaloid 839, earlier reported as isogranulatimide from Didemnum granulatum,572 was isolated in very low yield from D. conchyliatum from the Bahamas.573 Two additional staurosporine derivatives, 3′-demethoxy-3,3′-dihydroxystaurosporine 840 and 11-hydroxy-4′-N-demethylstaurosporine 841, were isolated from Eudistoma toealensis and a flatworm of the genus Pseudoceros that preys upon the ascidian.574 A Polycitorella sp. from Okinawa contained the cytotoxic alkaloids iheyamines A 842 and B 843, the latter being optically active and therefore unlikely to be an artifact of acetone extraction.575 Two pyrazine alkaloids, botryllazines A 844 and B 845, and 2-(p-hydroxybenzoyl)-4-(p-hydroxyphenyl)imidazole 846 were isolated from a Spanish specimen of Botryllus leachi.576 Trypargimine 847, 1-carboxytrypargine 848 and 3′,5′-dibromo-4′-methoxyphenylethylamine 849 were isolated from an undescribed Eudistoma sp. from Indonesia.577 Didemnolines A–D 850–853, which are β-carboline alkaloids from a Didemnum sp. from Rota, Northern Mariana Islands,578 have been synthesized in a straightforward manner.579
568 have been synthesized using a Diels–Alder strategy.330Distaplia regina from Palau contained the antibacterial agent 3,6-dibromoindole 837,569 a structure that had been erroneously proposed as a metabolite of acorn worms.570 The first marine ergoline alkaloid, pibocin 838, was isolated as a cytotoxic and antimicrobial agent from a Far-eastern Eudistoma species.571 The cyclized didemnimide alkaloid 839, earlier reported as isogranulatimide from Didemnum granulatum,572 was isolated in very low yield from D. conchyliatum from the Bahamas.573 Two additional staurosporine derivatives, 3′-demethoxy-3,3′-dihydroxystaurosporine 840 and 11-hydroxy-4′-N-demethylstaurosporine 841, were isolated from Eudistoma toealensis and a flatworm of the genus Pseudoceros that preys upon the ascidian.574 A Polycitorella sp. from Okinawa contained the cytotoxic alkaloids iheyamines A 842 and B 843, the latter being optically active and therefore unlikely to be an artifact of acetone extraction.575 Two pyrazine alkaloids, botryllazines A 844 and B 845, and 2-(p-hydroxybenzoyl)-4-(p-hydroxyphenyl)imidazole 846 were isolated from a Spanish specimen of Botryllus leachi.576 Trypargimine 847, 1-carboxytrypargine 848 and 3′,5′-dibromo-4′-methoxyphenylethylamine 849 were isolated from an undescribed Eudistoma sp. from Indonesia.577 Didemnolines A–D 850–853, which are β-carboline alkaloids from a Didemnum sp. from Rota, Northern Mariana Islands,578 have been synthesized in a straightforward manner.579
The New Zealand ascidian Botrylloides leachi contained a new purine base, 1,3-dimethylguanine 854.580 Another purine base, 6-methoxy-7-methyl-8-oxoguanine 855 was isolated from Symplegma rubra from the Southeastern Brazilian coast.581 A total synthesis of aplidiamine, which was isolated from an Aplidiopsis sp.,582 provided evidence that the structure should be revised from 856 to 857.583 Lumichrome 858 functions as a natural metamorphosis inducer in the larvae of Halocynthia roretzi.584
There have been a relatively large number of syntheses of ascidian alkaloids and peptides belonging to classes for which no new examples have appeared. The absolute configuration of lepadin B 859, which was obtained as a cytotoxic agent from Clavelina lepadiformis,585 was determined by total synthesis.586,587 The structures and absolute configurations of clavepictines A 860 and B 861 and pictamine 862 from C. picta![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 588,589 have been verified by total syntheses.590,591 The racemates of cylindricines A 863 and B 864, which are tricyclic alkaloids from an Australian specimen of C. cylindrica,592 have been synthesized in good yields.593 (−)-Cylindricine C 865 from C. cylindrica
588,589 have been verified by total syntheses.590,591 The racemates of cylindricines A 863 and B 864, which are tricyclic alkaloids from an Australian specimen of C. cylindrica,592 have been synthesized in good yields.593 (−)-Cylindricine C 865 from C. cylindrica![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 594 has been synthesized in 12% overall yield from (S
594 has been synthesized in 12% overall yield from (S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-butane-1,2,4-triol.595 A convergent stereoselective synthesis of the putative structure of lepadiformine 866, which was isolated as a cytotoxic agent from C. lepadiformis,596 indicated the need to re-examine the structure, stereochemistry and the claim of a zwitterionic form.597–599 In addition, syntheses of isomeric forms of lepidaformine indicate that it is not a stereoisomer at C-2 or C-13.598
)-butane-1,2,4-triol.595 A convergent stereoselective synthesis of the putative structure of lepadiformine 866, which was isolated as a cytotoxic agent from C. lepadiformis,596 indicated the need to re-examine the structure, stereochemistry and the claim of a zwitterionic form.597–599 In addition, syntheses of isomeric forms of lepidaformine indicate that it is not a stereoisomer at C-2 or C-13.598
Virenamide B 867, which is a cytotoxic thiazole-containing tripeptide from Diplosoma virens,600 has been synthesized and its optical rotation has been corrected.601 The macrocyclic hexapeptide bistratamide D 868 from Lissoclinum bistratum![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 602 has been synthesized using a convergent strategy using enantiomerically pure oxazole, thiazole and oxazoline segments.603 A total synthesis of the proposed structure of trunkamide A 869, which is a cyclic heptapeptide from a Lissoclinum sp.,604 revealed that the structure of the natural product should be reinvestigated.605 The total synthesis of mollamide 870, a cytotoxic cyclic peptide from Didemnum molle,606 has been reported.607 The synthesis of tamandarin A 871, which is a cyclic peptide related to the didemnins, was announced before the structural elucidation was published!
602 has been synthesized using a convergent strategy using enantiomerically pure oxazole, thiazole and oxazoline segments.603 A total synthesis of the proposed structure of trunkamide A 869, which is a cyclic heptapeptide from a Lissoclinum sp.,604 revealed that the structure of the natural product should be reinvestigated.605 The total synthesis of mollamide 870, a cytotoxic cyclic peptide from Didemnum molle,606 has been reported.607 The synthesis of tamandarin A 871, which is a cyclic peptide related to the didemnins, was announced before the structural elucidation was published!![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 608,609
608,609
11 Echinoderms
In comparison with recent years, very few new metabolites have been reported from echinoderms. Two major inositolphosphoceramides 872 and 873 were identified among the lipids of the crinoid Comanthus japonica.610 The neuritogenic ganglioside LMG-2, the major component of which is 874, was isolated from the Japanese seastar Luidia maculata as a mixture of closely related compounds having different alkyl chains.611 A similar situation applies to the ganglioside LLG-3 875, which is a metabolite of Linckia laevigata from Okinawa.612 The Japanese sea cucumber Stichopus japonicus contained SJG-1 876 as the major neuritogenic ganglioside.613 The lengths of the acyl chains in the ceramide moieties can be determined without chemical degradation by collision-induced dissociation mass spectrometry of the sodium ion complexes.614 Four additional steroidal glycosides, mediasterosides M1877, M2878, M3879 and M4880, were isolated from the deep-water seastar Mediaster murrayi as inhibitors of cell division in fertilized sea urchin eggs.615 Frondoside D 881 is an additional triterpenoid saponin from the holothurian Cucumaria frondosa.61612 Miscellaneous
Although there were no reports of novel compounds from marine worms, L-ovithiol A 882, which was isolated as the corresponding disulfide, was shown to be the egg release pheromone of the marine polychaete worm Platynereis dumerilii.617 Hallachrome 883, which is a pigment of the marine worms Halla parthenopeia![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 618 and Lumbriconereis impatiens,619 has been synthesized using a Diels–Alder strategy.620 One of the few reports of a new natural product from fishes is the isolation of α-tocomonoenol 884 from the eggs of the Pacific salmon Oncorhynchus keta.621 5-Deoxytetrodotoxin 885 was isolated as a minor metabolite from the ovaries of the puffer fish Fugu poecilonotus.622 Chinese studies of the puffer fish Fugu vermicularis described the isolation of tetrodoine 886 and a number of tetrodotoxin derivatives, which may or may not be artifacts.623,624
618 and Lumbriconereis impatiens,619 has been synthesized using a Diels–Alder strategy.620 One of the few reports of a new natural product from fishes is the isolation of α-tocomonoenol 884 from the eggs of the Pacific salmon Oncorhynchus keta.621 5-Deoxytetrodotoxin 885 was isolated as a minor metabolite from the ovaries of the puffer fish Fugu poecilonotus.622 Chinese studies of the puffer fish Fugu vermicularis described the isolation of tetrodoine 886 and a number of tetrodotoxin derivatives, which may or may not be artifacts.623,624
References
- D. J. Faulkner, Nat. Prod. Rep., 2000, 17, 7 RSC
.
- M. G. Haygood, E. W. Schmidt, S. K. Davidson and D. J. Faulkner, J. Mol. Microbiol. Biotechnol., 1999, 1, 33 Search PubMed
.
- B. S. Moore, Nat. Prod. Rep., 1999, 16, 653 RSC
.
- R. G. Kerr and S. S. Kerr, Exp. Opin. Ther. Patents, 1999, 9, 1207 Search PubMed
.
- G. Cimino, A. Fontana and M. Gavignin, Curr. Org. Chem., 1999, 3, 327 Search PubMed
.
- G. Cimino and M. T. Ghiselin, Chemoecology, 1999, 9, 187 CrossRef CAS
.
- Q. Ding, K. Chichak and J. W. Lown, Curr. Med. Chem., 1999, 6, 1 Search PubMed
.
- J. Poncet, Curr. Pharm. Design, 1999, 5, 139 Search PubMed
.
- W. H. Gerwick, M. A. Roberts, A. Vulpanovici and D. L. Ballantine, Adv. Exp. Mar. Biol., 1999, 447, 211 Search PubMed
.
- K. C. Nicolau, J. Pfefferkorn, J. Xu, N. Winssinger, T. Ohshima, S. Kim, S. Hosokawa, D. Vourloumis, F. van Delft and T. Li, Chem. Pharm. Bull., 1999, 47, 1199 Search PubMed
.
- V. A. Stonik, V. I. Kalanin and S. A. Avilov, J. Nat. Toxins, 1999, 8, 235 Search PubMed
.
- L. E. Fleming, J. Easom, D. Baden, A. Rowan and B. Levin, Toxicol. Pathol., 1999, 27, 573 CAS
.
- A. Aiello, E. Fattorusso and M. Menna, Steroids, 1999, 64, 687 CrossRef CAS
.
- G. M. Cragg and D. J. Newman, Cancer Invest., 1999, 17, 153 CAS
.
- G. W. Gribble, Chem. Soc. Rev., 1999, 28, 335 RSC
.
- D. A. Casteel, Nat. Prod. Rep., 1999, 16, 55 RSC
.
- M. Jaspars, Chem. Ind. (London), 1999, 51 Search PubMed
.
- P. J. Scheuer, J. Chem. Educ., 1999, 76, 1075 Search PubMed
.
- A. Kelecom, An. Acad. Bras. Cienc., 1999, 71, 249 Search PubMed
.
- Various authors
J. Biotechnol., 1999, 70, 1–415.
Search PubMed
.
- H. Yagi and A. Maruyama, J. Nat. Prod., 1999, 62, 631 CrossRef CAS
.
- J. Kobayashi, S. Mikami, H. Shigemori, T. Takao, Y. Shimonishi, S. Izuta and S. Yoshida, Tetrahedron, 1995, 51, 10487 CrossRef CAS
.
- H. Takikawa, D. Nozawa, A. Kayo, S. Muto and K. Mori, J. Chem. Soc., Perkin Trans. 1, 1999, 2467 RSC
.
- K. W. Cho, Y. Seo, T. Yoon and J. Shin, J. Microbiol. Biotechnol., 1999, 9, 709 Search PubMed
.
- V. Bultet-Poncé, J.-P. Berge, C. Debitus, J.-L. Nicolas and M. Guyot, Mar. Biotechnol., 1999, 1, 384 Search PubMed
.
- X. C. Cheng, P. R. Jensen and W. Fenical, J. Nat. Prod., 1999, 62, 605 CrossRef CAS
.
- Z.-D. Jiang, P. R. Jensen and W. Fenical, Bioorg. Med. Chem. Lett., 1999, 9, 2003 CrossRef CAS
.
- X. C. Cheng, P. R. Jensen and W. Fenical, J. Nat. Prod., 1999, 62, 608 CrossRef CAS
.
- L. M. Cañedo, J. A. de la Fuente, C. Gesto, M. J. Ferreiro, C. Jiménez and R. Riguera, Tetrahedron Lett., 1999, 40, 6841 CrossRef CAS
.
- C. Acebal, L. M. Cañedo, J. L. Fernández Puentes, J. P. Baz, F. Romero, F. de la Calle, M. D. García Grávalos and P. Rodriguez, J. Antibiot., 1999, 52, 983 Search PubMed
.
- J. M. Gerard, P. Haden, M. T. Kelly and R. J. Andersen, J. Nat. Prod., 1999, 62, 80 CrossRef CAS
.
- B. S. Moore, J. A. Trischman, D. Seng, D. Kho, P. R. Jensen and W. Fenical, J. Org. Chem., 1999, 64, 1145 CrossRef
.
- J. A. Trischman, D. M. Tapiolas, P. R. Jensen, W. Fenical, T. C. McKee, C. M. Ireland, T. J. Stout and J. Clardy, J. Am. Chem. Soc., 1994, 116, 757 CrossRef CAS
.
- M. K. Renner, Y.-C. Shen, X.-C. Cheng, P. R. Jensen, W. Frankmoelle, C. A. Kauffmann, W. Fenical, E. Lobkovsky and J. Clardy, J. Am. Chem. Soc., 1999, 121, 11273 CrossRef CAS
.
- D. E. Williams, V. S. Bernan, F. V. Ritacco, W. M. Maiese, M. Greenstein and R. J. Andersen, Terahedron Lett., 1999, 40, 7171 Search PubMed
.
- K. Yoshikawa, T. Takadera, K. Adachi, M. Nishijima and H. Sano, J. Antibiot., 1997, 50, 949 Search PubMed
.
- H. Uehara, T. Oishi, K. Yoshikawa, K. Mochida and M. Hirama, Tetrahedron Lett., 1999, 40, 8641 CrossRef CAS
.
- S. Ōmura, H. Tomoda, N. Tabata, Y. Ohyama, T. Abe and M. Namikoshi, J. Antibiot., 1999, 52, 586 Search PubMed
.
- H. Tomoda, Y. Ohyama, T. Abe, N. Tabata, M. Namikoshi, Y. Yamaguchi, R. Masuma and S. Ōmura, J. Antibiot., 1999, 52, 689 Search PubMed
.
- N. Tabata, Y. Ohyama, H. Tomoda, T. Abe, M. Namikoshi and S. Ōmura, J. Antibiot., 1999, 52, 815 Search PubMed
.
- C. Takahashi, A. Numata, T. Yamada, K. Minoura, S. Enomoto, K. Konishi, M. Nakai, C. Matsuda and K. Nomoto, Tetrahedron Lett., 1996, 37, 655 CrossRef CAS
.
- C. Iwamoto, K. Minoura, T. Oka, T. Ohta, S. Hagashita and A. Numata, Tetrahedron, 1999, 55, 14353 CrossRef CAS
.
- Z. Shao, Y. Lin, G. Jiang, S. Zhou, L. L. P. Vrijmoed and E. B. G. Jones, Zhongsan Daxue Xuebao Ziran Kexueban, 1999, 38, 131 Search PubMed
.
- U. Höller, G. M. König and A. D. Wright, J. Nat. Prod., 1999, 62, 114 CrossRef
.
- T. Amagata, Y. Usami, K. Minoura, T. Ito and A. Numata, J. Antibiot., 1998, 51, 33 Search PubMed
.
- Y. Usami and A. Numata, Synlett, 1999, 723 CAS
.
- J. Nielsen, P. H. Nielsen and J. C. Frisvad, Phytochemistry, 1999, 50, 263 CrossRef CAS
.
- L. A. McDonald, D. R. Abbanat, L. R. Barbieri, V. S. Bernan, C. M. Discafani, M. Greenstein, K. Janota, J. D. Korshalla, P. Lassota, M. Tischler and G. T. Carter, Tetrahedron Lett., 1999, 40, 2489 CrossRef CAS
.
- H. Shigemori, K. Komatsu, Y. Mikami and J. Kobayashi, Tetrahedron, 1999, 55, 14925 CrossRef CAS
.
- U. Höller, G. M. König and A. D. Wright, Eur. J. Org. Chem., 1999, 2949 CrossRef CAS
.
- U. Höller, A. D. Wright, G. M. König and P. G. Jones, Acta Crystallogr., Sect. C, 1999, 55, 1310 CrossRef
.
- B. W. Son, P. R. Jensen, C. A. Kauffman and W. Fenical, Nat. Prod. Lett., 1999, 13, 213 Search PubMed
.
- C.-B. Cui, H. Kakeya, G. Okuda, R. Onose, M. Ubukata, I. Takahashi, K. Inoso and H. Osada, J. Antibiot., 1995, 48, 1382 Search PubMed
.
- J. M. Schkeryantz, J. C. G. Woo, P. Siliphaivanh, K. M. Depew and S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 11964 CrossRef CAS
.
- G. N. Belofsky, P. R. Jensen and W. Fenical, Tetrahedron Lett., 1999, 40, 2913 CrossRef CAS
.
- Y. Hwang, D. Rowley, D. Rhodes, J. Gertsch, W. Fenical and F. Bushman, Mol. Pharmacol., 1999, 55, 1049 Search PubMed
.
- J. Malmstrøm, J. Nat. Prod., 1999, 62, 787 CrossRef CAS
.
- M. Daferner, S. Mensch, T. Anke and O. Sterner, Z. Naturforsch., Teil C, 1999, 54, 474 Search PubMed
.
- C. Le, Y. Lin, G. Jiang, S. Zhou, L. Guan and W. Luo, Chin. J. Mar. Drugs, 1999, 18(2), 12 Search PubMed
.
- T. Amagata, M. Doi, M. Tohgo, K. Minoura and A. Numata, Chem. Commun., 1999, 1321 RSC
.
- D. I. Lee, J. S. Choi, M.-R. Yang, W. K. Lee, D.-S. Kim, H. D. Choi and B. W. Son, Nat. Prod. Sci., 1999, 5, 93 Search PubMed
.
- A. Guerriero, M. D’Ambrosio, V. Cuomo, F. Vanzanella and F. Pietra, Helv. Chim. Acta, 1989, 72, 438 CrossRef CAS
.
- H. Akao, H. Kiyota, T. Nakajima and T. Kitahara, Tetrahedron, 1999, 55, 7757 CrossRef CAS
.
- K. M. Jenkins, P. R. Jensen and W. Fenical, Tetrahedron Lett., 1999, 40, 7637 CrossRef CAS
.
- V. Mesguiche, R. Valls, L. Piovetti and G. Peiffer, Tetrahedron Lett., 1999, 40, 7473 CrossRef CAS
.
- F. Wan and K. L. Erickson, J. Nat. Prod., 1999, 62, 1696 CrossRef CAS
.
- I. P. Singh, K. E. Milligan and W. H. Gerwick, J. Nat. Prod., 1999, 62, 1333 CrossRef CAS
.
- D. Klein, J. C. Braekman, D. Daloze, L. Hoffmann and V. Demoulin, Tetrahedron Lett., 1996, 37, 7519 CrossRef CAS
.
- D. Klein, J. C. Braekman, D. Daloze, L. Hoffmann, G. Castillo and V. Demoulin, J. Nat. Prod., 1999, 62, 934 CrossRef CAS
.
- D. Klein, J. C. Braekman, D. Daloze, L. Hoffmann, G. Castillo and V. Demoulin, Tetrahedron Lett., 1999, 40, 695 CrossRef CAS
.
- G. G. Harrigan, H. Luesch, W. Y. Yoshida, R. E. Moore, D. G. Nagle, J. Biggs, P. U. Park and V. J. Paul, J. Nat. Prod., 1999, 62, 464 CrossRef CAS
.
- T. Mutou, T. Kondo, M. Ojika and K. Yamada, J. Org. Chem., 1996, 61, 6340 CrossRef CAS
.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 1999, 62, 1702 CrossRef CAS
.
- G. R. Pettit, Y. Kamano, C. L. Herald, C. Dufresne, R. L. Cerny, D. L. Herald, J. M. Schmidt and H. Kizu, J. Am. Chem. Soc., 1989, 111, 5015 CrossRef CAS
.
- G. G. Harrigan, H. Luesch, W. Y. Yoshida, R. E. Moore, D. G. Nagle and V. J. Paul, J. Nat. Prod., 1999, 62, 655 CrossRef CAS
.
- J. Orjala, D. G. Nagle, V. L. Hsu and W. H. Gerwick, J. Am. Chem. Soc., 1995, 117, 8281 CrossRef CAS
.
- J. D. White, R. Hanselmann and D. J. Wardrop, J. Am. Chem. Soc., 1999, 121, 1106 CrossRef CAS
.
- F. Yokokawa, H. Fujiwara and T. Shioiri, Tetrahedron Lett., 1999, 40, 1915 CrossRef CAS
.
- S.-C. Lee, G. A. Williams and G. D. Brown, Phytochemistry, 1999, 52, 537 CrossRef CAS
.
- J. H. Cardellina II, M. P. Kirkup, R. E. Moore, J. S. Mynderse, K. Seff and C. J. Simmons, Tetrahedron Lett., 1979, 4915 CrossRef
.
- H.-J. Knölker, E. Baum and T. Hopfmann, Tetrahedron, 1999, 55, 10391 CrossRef CAS
.
- A. H. Daranas, J. J. Fernández and M. Norte, Nat. Prod. Lett., 1999, 14, 107 Search PubMed
.
- M. P. Mansour, J. K. Volkman, D. G. Holdsworth, A. E. Jackson and S. I. Blackburn, Phytochemistry, 1999, 50, 541 CrossRef CAS
.
- M. Tsuda, T. Endo and J. Kobayashi, Tetrahedron, 1999, 55, 14565 CrossRef CAS
.
- J. Kobayashi, T. Kubota, M. Takahashi, M. Ishibashi, M. Tsuda and H. Naoki, J. Org. Chem., 1999, 64, 1478 CrossRef CAS
.
- T. Kubota, M. Tsuda, M. Takahashi, M. Ishibashi, H. Naoki and J. Kobayashi, J. Chem. Soc., Perkin Trans. 1, 1999, 3483 RSC
.
-
G. K. Paul
, N. Matsumori
, K. Konoki
, M. Sakai
, M. Murata
and K. Tachibana
, in Harmful and Toxic Algal Blooms, eds. T. Yasumoto, Y. Oshima and Y. Fukuyo, UNESCO, Paris, 1996, pp. 503–506.
Search PubMed
.
- M. Murata, S. Matsuoka, N. Matsumori, G. K. Paul and K. Tachibana, J. Am. Chem. Soc., 1999, 121, 870 CrossRef CAS
.
- T. Hu, J. M. Curtis, J. A. Walter and J. L. C. Wright, Tetrahedron Lett., 1999, 40, 3977 CrossRef CAS
.
- M. Satake, M. Murata and T. Yasumoto, J. Am. Chem. Soc., 1993, 115, 361 CrossRef CAS
.
- A. Morohashi, M. Satake and T. Yasumoto, Tetrahedron Lett., 1999, 40, 97 CrossRef CAS
.
- T. Igarashi, M. Satake and T. Yasumoto, J. Am. Chem. Soc., 1996, 118, 479 CrossRef CAS
.
- T. Igarashi, M. Satake and T. Yasumoto, J. Am. Chem. Soc., 1999, 121, 8499 CrossRef CAS
.
- Y. Shimizu, H.-N. Chou, H. Bando, G, van Duyne and J. Clardy, J. Am. Chem. Soc., 1986, 108, 514 CrossRef CAS
.
- K. C. Nicolaou, J. L. Gunzner, G. Shi, K. A. Agrios, P. Gärtner and Z. Yang, Chem. Eur. J., 1999, 5, 646 CrossRef CAS
.
- A. B. Dounay, R. A. Urbanek, S. F. Sabes and C. J. Forsyth, Angew. Chem., Int. Ed., 1999, 38, 2258 CrossRef CAS
.
- G. Guella, F. Dini and F. Pietra, Angew. Chem., Int. Ed., 1999, 38, 1134 CrossRef
.
- T. Ohshiro, S. Nakano, Y. Takahashi, M. Suzuki and Y. Izumi, Phytochemistry, 1999, 52, 1211 CrossRef CAS
.
- C. Flodin and F. B. Whitfield, Phytochemistry, 1999, 51, 249 CrossRef CAS
.
- Y. Kan, T. Fujita, B. Sakamoto, Y. Hokama and H. Nagai, J. Nat. Prod., 1999, 62, 1169 CrossRef CAS
.
- J.-Y. Su, Y. Zhu, L.-M. Zeng and X.-H. Xu, J. Nat. Prod., 1997, 60, 1043 CrossRef CAS
.
- P. M. Fresneda, P. Molina and M. A. Saez, Synlett, 1999, 1651 CAS
.
- D. J. Bourne, S. E. Pilchowski and P. T. Murphy, Aust. J. Chem., 1999, 52, 69 CAS
.
- M. S. Ali, V. U. Ahmad, F. Mazhar, I. Azhar and K. Usmanghani, Turk. J. Chem., 1999, 23, 181 Search PubMed
.
- L. M. Murray, R. A. Barrow and R. J. Capon, Aust. J. Chem., 1991, 44, 843 CAS
.
- Y. Mori, T. Sawada and H. Furukawa, Tetrahedron Lett., 1999, 40, 731 CrossRef CAS
.
- K.-W. Glombitza and A. Schmidt, J. Nat. Prod., 1999, 62, 1238 CrossRef CAS
.
- K.-W. Glombitza and A. Schmidt, Phytochemistry, 1999, 51, 1095 CrossRef CAS
.
- B. Sailler and K.-W. Glombitza, Phytochemistry, 1999, 50, 869 CrossRef CAS
.
- B. Sailler and K.-W. Glombitza, Nat. Toxins, 1999, 7, 57 CrossRef CAS
.
- In reference 112, the alga is called S. vachell but in Chem. Abstr. ( 1999, 131, 283698) the species name is given as S. vachellianum. Search PubMed.
- S. H. Xu, Y. Z. Cen, Y. L. Li and S. Y. Xu, Chin. Chem. Lett., 1999, 10, 401 Search PubMed
.
- Atta-ur-Rahman, M. I. Choudhary, S. Hayat, A. M. Khan, A. Ahmad and S. Malik, Phytochemistry, 1999, 52, 495 CrossRef
.
- M. Wessels, G. M. König and A. D. Wright, J. Nat. Prod., 1999, 62, 927 CrossRef CAS
.
- A. Bennamara, A. Abourriche, M. Berrada, M. Charrouf, N. Chaib, M. Boudouma and F. X. Garneau, Phytochemistry, 1999, 52, 37 CrossRef CAS
.
- Y.-C. Shen, P. I. Tsai, W. Fenical and M. E. Hay, Phytochemistry, 1993, 32, 71 CrossRef
.
- A. Fadel and L. Vandromme, Tetrahedron: Asymmetry, 1999, 10, 1153 CrossRef CAS
.
- G. Culioli, M. Daoudi, V. Mesguiche, R. Valls and L. Piovetti, Phytochemistry, 1999, 52, 1447 CrossRef CAS
.
- R. Valls, B. Banaigs, C. Francisco, L. Codomier and A. Cave, Phytochemistry, 1986, 25, 751 CrossRef CAS
.
- S. Di Guardia, R. Valls, V. Mesguiche, J.-M. Brunel and G. Culioli, Tetrahedron Lett., 1999, 40, 8359 CrossRef CAS
.
- S. De Rosa, C. Iodice, M. Khalaghdoust, S. Oryan and A. Rustaiyan, Phytochemistry, 1999, 51, 1009 CrossRef CAS
.
- B. N. Ravi and R. J. Wells, Aust. J. Chem., 1982, 35, 129 CAS
.
- H. Yamase, K. Umemoto, T. Ooi and T. Kusumi, Chem. Pharm. Bull., 1999, 47, 813 Search PubMed
.
- The species name is spelt okamurae, rather than okamurai in the paper..
- M. Ninomiya, H. Hirohara, J. Onishi and T. Kusumi, J. Org. Chem., 1999, 64, 5436 CrossRef CAS
.
- Atta-ur-Rahman, M. Shabbir, A. M. Khan, S. Hayat, A. Nasreen, S. Malik and M. I. Choudhary, Nat. Prod. Lett., 1999, 13, 255 Search PubMed
.
- J.-H. Sheu, G.-H. Wang, P.-J. Sung and C.-Y. Duh, J. Nat. Prod., 1999, 62, 224 CrossRef CAS
.
- Y. Takahashi, M. Suzuki, T. Abe and M. Masuda, Phytochemistry, 1999, 50, 799 CrossRef CAS
.
- M. Suzuki, S. Nakano, Y. Takahashi, T. Abe and M. Masuda, Phytochemistry, 1999, 51, 657 CrossRef CAS
.
- N. Mihopoulos, C. Vagias, M. Scoullos and V. Roussis, Nat. Prod. Lett., 1999, 13, 151 Search PubMed
.
- Z. Aydoǧmuş and S. Imre, Acta Pharm. Turc., 1999, 41, 93 Search PubMed
.
- B. M. Howard, G. R. Schulte, W. Fenical, B. A. Solheim and J. Clardy, Tetrahedron, 1980, 36, 1747 CrossRef CAS
.
- T. J. King, S. Imre, A. Öztunc and R. H. Thompson, Tetrahedron Lett., 1979, 17, 1453 CrossRef
.
- K. Fujiwara, D. Awakura, M. Tsunashima, A. Nakamura, T. Honma and A. Murai, J. Org. Chem., 1999, 64, 2616 CrossRef CAS
.
- M. Norte, A. G. González, F. Cataldo, M. L. Rodríguez and I. Brito, Tetrahedron, 1991, 47, 9411 CrossRef CAS
.
- D. Awakura, K. Fujiwara and A. Murai, Chem. Lett., 1999, 461 CrossRef CAS
.
- T. Suzuki, K. Koizumi, M. Suzuki and E. Kurosawa, Chem. Lett., 1983, 1639 CAS
.
- P. A. Evans, V. S. Murthy, J. D. Roseman and A. L. Rheingold, Angew. Chem., Int. Ed., 1999, 38, 3175 CrossRef CAS
.
- T. Irie, M. Suzuki and T. Masamune, Tetrahedron, 1968, 24, 4193 CrossRef CAS
.
- M. T. Crimmins and K. A. Emmitte, Org. Lett., 1999, 1, 2029 CrossRef CAS
.
- H. Kurihara, T. Mitani, J. Kawabata and K. Takahashi, J. Nat. Prod., 1999, 62, 882 CrossRef CAS
.
- D. J. Faulkner, M. O. Stallard, J. Fayos and J. Clardy, J. Am. Chem. Soc., 1973, 95, 3413 CrossRef CAS
.
- G. M. König, A. D. Wright and R. de Nys, J. Nat. Prod., 1999, 62, 383 CrossRef
.
- G. M. König, A. D. Wright and A. Linden, Phytochemistry, 1999, 52, 1047 CrossRef CAS
.
- A. A. L. Gunatilaka, V. J. Paul, P. U. Park, M. P. Puglisi, A. D. Gitler, D. S. Eggleston, R. C. Haltiwanger and D. G. I. Kingston, J. Nat. Prod., 1999, 62, 1376 CrossRef CAS
.
- J. Rovirosa, H. Soto, M. Cueto, J. Dárias, J. Herrera and A. San-Martín, Phytochemistry, 1999, 50, 745 CrossRef CAS
.
- J. Kimura, N. Kamada and Y. Tsujimoto, Bull. Chem. Soc. Jpn., 1999, 72, 289 CrossRef CAS
.
- K. Agatsuma, J. Sugiyama, S. Nishinaka and J. Kimura, Fish. Sci., 1999, 65, 494 Search PubMed
.
- T. Irie, M. Suzuki and Y. Hayakawa, Bull. Chem. Soc. Jpn., 1969, 42, 843 CAS
.
- T. Irie, M. Suzuki, E. Kurosawa and T. Masamune, Tetrahedron, 1970, 26, 3271 CrossRef CAS
.
- J. A. McMillan and I. C. Paul, Tetrahedron Lett., 1976, 17, 4219 CrossRef
.
- D. C. Harrowven, M. C. Lucas and P. D. Howes, Tetrahedron Lett., 1999, 40, 8271 CrossRef CAS
.
- D. C. Harrowven, M. C. Lucas and P. D. Howes, Tetrahedron Lett., 1999, 40, 4443 CrossRef CAS
.
- T. Suzuki, M. Suzuki, A. Furusaki, T. Matsumoto, A. Kato, Y. Imanaka and E. Kurosawa, Tetrahedron Lett., 1985, 26, 1329 CrossRef CAS
.
- Y. Morimoto, T. Iwai and T. Kinoshita, J. Am. Chem. Soc., 1999, 121, 6792 CrossRef CAS
.
- M. Yotsu-Yamashita, R. L. Haddock and T. Yasumoto, J. Am. Chem. Soc., 1993, 115, 1147 CrossRef CAS
.
- L. A. Paquette, L. Barriault and D. Pissarnitski, J. Am. Chem. Soc., 1999, 121, 4542 CrossRef CAS
.
- K. Fujiwara and A. Murai, J. Synth. Org. Chem., Jpn., 1999, 57, 993 Search PubMed
.
- S. Murakami, T. Takemoto and Z. Shimizu, J. Pharm. Soc. Jpn., 1953, 73, 1026 Search PubMed
.
- M. V. Chevliakov and J. Montgomery, J. Am. Chem. Soc., 1999, 121, 11139 CrossRef CAS
.
- B. A. Shin, Y. R. Kim, I.-S. Lee, C. K. Sung, J. Hong, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 1999, 62, 1554 CrossRef CAS
.
- D.-K. Kim, Y. J. Lim, J. S. Kim, J. H. Park, N. D. Kim, K. S. Im, J. Hong and J. H. Jung, J. Nat. Prod., 1999, 62, 773 CrossRef CAS
.
- G. M. Nicholas, T. W. Hong, T. F. Molinski, M. L. Lerch, M. T. Cancilla and C. B. Lebrilla, J. Nat. Prod., 1999, 62, 1678 CrossRef CAS
.
- V. Costantino, E. Fattorusso, A. Mangoni, M. Di Rosa and A. Ianaro, Bioorg. Med. Chem. Lett., 1999, 9, 271 CrossRef CAS
.
- G. R. Pettit, J. Xu, D. E. Gingrich, M. D. Williams, D. L. Doubek, J.-C. Chapuis and J. M. Schmidt, Chem. Commun., 1999, 915 RSC
.
- S. Aoki, K. Higuchi, A. Kato, N. Murakami and M. Kobayashi, Tetrahedron, 1999, 55, 14865 CrossRef CAS
.
- J. Kobayashi, J.-F. Cheng, M. Ishibashi, M. R. Walchli, S. Yamamura and Y. Ohizumi, J. Chem. Soc., Perkin Trans. 1, 1991, 1135 RSC
.
- J. Kobayashi, M. Tsuda, J. Cheng, M. Ishibashi, H. Takikawa and K. Mori, Tetrahedron Lett., 1996, 37, 6775 CrossRef CAS
.
- D.-G. Liu and G.-Q. Lin, Tetrahedron Lett., 1999, 40, 337 CrossRef CAS
.
- K. Honma, M. Tsuda, Y. Mikami and J. Kobayashi, Tetrahedron, 1995, 51, 3745 CrossRef CAS
.
- S. G. Davies, H. J. Sanganee and P. Szolcsanyi, Tetrahedron, 1999, 55, 3337 CrossRef CAS
.
- K. L. Erickson, J. A. Beutler, J. H. Cardellina II and M. R. Boyd, Tetrahedron, 1995, 51, 11953 CrossRef CAS
.
- I. R. Czuba and M. A. Rizzacasa, Chem. Commun., 1999, 1419 RSC
.
- Y. Seo, K. W. Cho, H.-S. Lee, J.-R. Rho and J. Shin, J. Nat. Prod., 1999, 62, 122 CrossRef CAS
.
- J. S. Kim, Y. J. Lim, K. S. Im, J. H. Jung, C. J. Shim, C. O. Lee, J. Hong and H. Lee, J. Nat. Prod., 1999, 62, 554 CrossRef CAS
.
- Y. J. Lim, J. S. Kim, K. S. Im, J. H. Jung, C.-O. Lee, J. Hong and D. Kim, J. Nat. Prod., 1999, 62, 1215 CrossRef CAS
.
- X. Fu, F. J. Schmitz and M. Kelly, J. Nat. Prod., 1999, 62, 1336 CrossRef CAS
.
- N. B. Pham, M. S. Butler, J. N. A. Hooper, R. W. Moni and R. J. Quinn, J. Nat. Prod., 1999, 62, 1439 CrossRef CAS
.
- K. Morishita, M. Kamezawa, T. Ohtani, H. Tachibana, M. Kawase, M. Kishimoto and Y. Naoshima, J. Chem. Soc., Perkin Trans. 1, 1999, 513 RSC
.
- Y. F. Hallock, J. H. Cardellina II, M. S. Balaschak, M. R. Alexander, T. R. Prather, R. H. Shoemaker and M. R. Boyd, J. Nat. Prod., 1995, 58, 1801 CrossRef CAS
.
- S. P. Gunasekera and G. T. Faircloth, J. Org. Chem., 1990, 55, 6223 CrossRef CAS
.
- A. Aiello, E. Fattorusso, M. Menna and M. Pansini, J. Nat. Prod., 1992, 55, 1275 CrossRef CAS
.
- W. Lu, G. Zheng and J. Cai, Tetrahedron, 1999, 55, 4649 CrossRef CAS
.
- J. Garcia, M. López and J. Romeu, Tetrahedron: Asymmetry, 1999, 10, 2617 CrossRef CAS
.
- T. Higa, J. Tanaka, A. Kitamura, T. Koyama, M. Takahashi and T. Uchida, Pure Appl. Chem., 1994, 66, 2227 CrossRef CAS
.
- M. Kobayashi, T. Mahmud, H. Tajima, W. Wang, S. Aoki, S. Nakagawa, T. Mayumi and I. Kitagawa, Chem. Pharm. Bull., 1996, 44, 720 Search PubMed
.
- J. Garcia, M. López and J. Romeu, Synlett, 1999, 429 CAS
.
- F. Cafieri, E. Fattorusso, O. Taglialatela-Scafati and A. Ianaro, Tetrahedron, 1999, 55, 7045 CrossRef CAS
.
- F. Cafieri, E. Fattorusso, O. Taglialatela-Scafati, M. Di Rosa and A. Ianaro, Tetrahedron, 1999, 55, 13831 CrossRef CAS
.
- R. J. Wells, Tetrahedron Lett., 1976, 17, 2637 CrossRef
.
- S. Sakemi, T. Higa, U. Anthoni and C. Christophersen, Tetrahedron, 1987, 43, 263 CrossRef CAS
.
- T. Murayama, Y. Ohizumi, H. Nakamura, T. Sasaki and J. Kobayashi, Experientia, 1989, 45, 898 Search PubMed
.
- P. H. Dussault, C. T. Eary and K. R. Woller, J. Org. Chem., 1999, 64, 1789 CrossRef CAS
.
- E. G. Juagdan, R. S. Kalindindi, P. J. Scheuer and M. Kelly-Borges, Tetrahedron Lett., 1995, 36, 2905 CrossRef CAS
.
- R. C. Hoye, A. S. Baigorria, M. E. Danielson, A. A. Pragman and H. A. Rajapakse, J. Org. Chem., 1999, 64, 2450 CrossRef CAS
.
- A. Qureshi, C. S. Stevenson, C. L. Albert, R. S. Jacobs and D. J. Faulkner, J. Nat. Prod., 1999, 62, 1205 CrossRef CAS
.
- H. W. Lam, P. A. Cooke, G. Pattenden, W. M. Bandaranayake and W. A. Wickramasinghe, J. Chem. Soc., Perkin Trans. 1, 1999, 847 RSC
.
- S. Tsukamoto, S. Matsunaga, N. Fusetani and A. Toh-e, Tetrahedron, 1999, 55, 13697 CrossRef CAS
.
-
S. P. Gunasekera,
M. Gunasekera,
R. E. Longley
and G. K. Schulte,
J. Org. Chem.,
1990, 55, 4912 [erratum,
1991, 56, 1346].
Search PubMed
.
- A. B. Smith III, M. D. Kaufman, T. J. Beauchamp, M. J. LaMarche and H. Arimoto, Org. Lett., 1999, 1, 1823 CrossRef
.
- S. P. B. Ovenden, R. J. Capon, E. Lacey, J. H. Gill, T. Friedel and D. Wadsworth, J. Org. Chem., 1999, 64, 1140 CrossRef CAS
.
- R. J. Capon, C. Skene, E. Lacey, J. H. Gill, D. Wadsworth and T. Friedel, J. Nat. Prod., 1999, 62, 1256 CrossRef CAS
.
- N. Takada, H. Sato, K. Suenaga, H. Arimoto, K. Yamada, K. Ueda and D. Uemura, Tetrahedron Lett., 1999, 40, 6309 CrossRef CAS
.
- M. D’Ambrosio, M. Tat, G. Pocsfalvi, C. Debitus and F. Pietra, Helv. Chim. Acta, 1999, 82, 347 CrossRef CAS
.
- S. Carbonelli, A. Zampella, A. Randazzo, C. Debitus and L. Gomez-Paloma, Tetrahedron, 1999, 55, 14665 CrossRef CAS
.
- N. Fusetani, K. Yasumuro, S. Matsunaga and K. Hashimoto, Tetrahedron Lett., 1989, 30, 2809 CrossRef CAS
.
-
S. Matsunaga
, P. Liu
, C. A. Celatka
, J. S. Panek
and N. Fusetani
, J. Am. Chem. Soc., 1999, 121, 5605 [erratum, 1999, 121, 8969].
Search PubMed
.
- D. Wolf, F. J. Schmitz, F. Qui and M. Kelly-Borge, J. Nat. Prod., 1999, 62, 170 CrossRef CAS
.
- N. U. Sata, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 1999, 62, 969 CrossRef CAS
.
- N. U. Sata, S. Wada, S. Matsunaga, S. Watabe, R. W. M. van Soest and N. Fusetani, J. Org. Chem., 1999, 64, 2331 CrossRef CAS
.
- T. Higa, J. Tanaka, M. Komesu, D. G. Gravalos, J. L. F. Puentes, G. Bernardinelli and C. W. Jefford, J. Am. Chem. Soc., 1992, 114, 7587 CrossRef CAS
.
- D. A. Evans, D. H. B. Ripin, D. P. Halstead and K. R. Campos, J. Am. Chem. Soc., 1999, 121, 6816 CrossRef CAS
.
- M. Kobayashi, S. Aoki, H. Sakai, N. Kihara, T. Sasaki and I. Kitagawa, Chem. Pharm. Bull., 1993, 41, 989 Search PubMed
.
- G. R. Pettit, Z. A. Cichacz, F. Gao, J. M. Schmidt and M. R. Boyd, J. Chem. Soc., Chem. Commun., 1993, 1166 RSC
.
- D. A. Evans, B. W. Trotter, P. J. Coleman, B. Côté, L. C. Dias, H. A. Rajapakse and A. N. Tyler, Tetrahedron, 1999, 55, 8671 CrossRef CAS
.
- H. Arimoto, I. Hayakawa, M. Kuramoto and D. Uemura, Tetrahedron Lett., 1998, 39, 861 CrossRef CAS
.
- D. Trauner, J. B. Schwarz and S. J. Danishefsky, Angew. Chem., Int. Ed., 1999, 38, 3542 CrossRef CAS
.
- T. Ichiba, W. Y. Yoshida, P. J. Scheuer, T. Higa and D. G. Gravalos, J. Am. Chem. Soc., 1991, 113, 3173 CrossRef CAS
.
- D. R. Williams, D. A. Brooks and M. A. Berliner, J. Am. Chem. Soc., 1999, 121, 4924 CrossRef CAS
.
- A. B. Smith III, G. K. Freistad, J. Barbosa, E. Bertounesque, J. J.-W. Duan, K. G. Hull, M. Iwashima, Y. Qui, P. G. Spoors and B. A. Salvatore, J. Am. Chem. Soc., 1999, 121, 10478 CrossRef
.
- Y. Kato, N. Fusetani, S. Matsunaga, K. Hashimoto, S. Fujita and T. Furuya, J. Am. Chem. Soc., 1986, 108, 2780 CrossRef CAS
.
- Y. Kato, N. Fusetani, S. Matsunaga, K. Hashimoto and K. Koseki, J. Org. Chem., 1988, 53, 3930 CrossRef CAS
.
- K. E. Volter, G. K. Pierens, R. J. Quinn, T. Wakimoto, S. Matsunaga and N. Fusetani, Bioorg. Med. Chem. Lett., 1999, 9, 717 CrossRef CAS
.
- R. S. Compagnone, M. C. Oliveri, I. C. Piña, S. Marques, H. R. Rangel, F. Dagger, A. I. Suárez and M. Gómez, Nat. Prod. Lett., 1999, 13, 203 Search PubMed
.
- M. J. Ortega, E. Zubía, J. L. Carballo and J. Salvá, Tetrahedron, 1997, 53, 331 CrossRef CAS
.
- B. K. Nabbs and A. D. Abell, Bioorg. Med. Chem. Lett., 1999, 9, 505 CrossRef CAS
.
- M. Tsuda, K. Hirano, T. Kubota and J. Kobayashi, Tetrahedron Lett., 1999, 40, 4819 CrossRef CAS
.
- A. Kitamura, J. Tanaka, I. I. Ohtani and T. Higa, Tetrahedron, 1999, 55, 2487 CrossRef CAS
.
- A. Groweiss, J. J. Newcomer, B. R. O′Keefe, A. Blackman and M. R. Boyd, J. Nat. Prod., 1999, 62, 1691 CrossRef CAS
.
- R. Fernández, M. Dherbomez, Y. Letourneux, M. Nabil, J. F. Verbist and J. F. Biard, J. Nat. Prod., 1999, 62, 678 CrossRef CAS
.
- M. Adamczeski, E. Quiñoà and P. Crews, J. Am. Chem. Soc., 1988, 110, 1598 CrossRef CAS
.
- R. J. Mulder, C. M. Shafer and T. F. Molinski, J. Org. Chem., 1999, 64, 4995 CrossRef CAS
.
- Y. Venkateswarlu, M. V. R. Reddy, P. Ramesh and J. V. Rao, Indian J. Chem., Sect. B, 1999, 38, 254 Search PubMed
.
- R. G. S. Berlinck, J. C. Braekman, D. Daloze, I. Bruno, R. Riccio, S. Ferri, S. Scampinato and E. Speroni, J. Nat. Prod., 1993, 56, 1007 CrossRef CAS
.
- D. S. Coffey, A. I. McDonald, L. E. Overman and F. Stappenbeck, J. Am. Chem. Soc., 1999, 121, 6944 CrossRef CAS
.
- A. D. Patil, A. J. Freyer, P. B. Taylor, B. Carte, G. Zuber, R. K. Johnson and D. J. Faulkner, J. Org. Chem., 1997, 62, 1814 CrossRef CAS
.
- G. P. Black, P. J. Murphy, A. J. Thornhill, N. D. A. Walshe and C. Zanetti, Tetrahedron, 1999, 55, 6547 CrossRef CAS
.
- B. B. Snider and M. V. Busuyek, J. Nat. Prod., 1999, 62, 1707 CrossRef CAS
.
- A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh, D. J. Faulkner, B. Carte, A. L. Breen, R. P. Herzberg, R. K. Johnson, J. W. Westley and B. C. M. Potts, J. Org. Chem., 1995, 60, 1182 CrossRef CAS
.
- F. Cohen, L. E. Overman and S. K. Ly Sakata, Org. Lett., 1999, 1, 2169 CrossRef CAS
.
- M. Tsuda, D. Watanabe and J. Kobayashi, Heterocycles, 1999, 50, 485 Search PubMed
.
- K. Kondo, H. Shigemori, Y. Kikuchi, M. Ishibashi, T. Sasaki and J. Kobayashi, J. Org. Chem., 1992, 57, 2480 CrossRef CAS
.
- S. F. Martin, J. M. Humphrey, A. Ali and M. C. Hillier, J. Am. Chem. Soc., 1999, 121, 866 CrossRef CAS
.
- J. E. Baldwin, T. D. W. Claridge, A. J. Culshaw, F. A. Heupel, V. Lee, D. R. Spring and R. C. Whitehead, Chem. Eur. J., 1999, 5, 3154 CrossRef
.
- M. Tsuda, K. Inaba, N. Kawasaki, K. Honma and J. Kobayashi, Tetrahedron, 1996, 52, 2319 CrossRef CAS
.
- Y. Kashman, G. Koren-Goldshlager, M. D. G. Gravalos and M. Schleyer, Tetrahedron Lett., 1999, 40, 997 CrossRef CAS
.
- J. E. Baldwin, H. R. Vollmer and V. Lee, Tetrahedron Lett., 1999, 40, 5401 CrossRef CAS
.
- W. P. D. Goldring and L. Weiler, Org. Lett., 1999, 1, 1471 CrossRef CAS
.
- D. E. Williams, P. Lassota and R. J. Andersen, J. Org. Chem., 1998, 63, 4838 CrossRef CAS
.
- P. Ramesh, N. S. Reddy and Y. Venkateswarlu, J. Nat. Prod., 1999, 62, 780 CrossRef CAS
.
- T. McKee and C. M. Ireland, J. Nat. Prod., 1987, 50, 754 CrossRef CAS
.
- N. Kuwabara, H. Hayashi, N. Hiramatsu, T. Choshi, E. Sugino and S. Hibino, Chem. Pharm. Bull., 1999, 47, 1805 Search PubMed
.
- S. P. Gunasekera, P. J. McCarthy, R. E. Longley, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 1999, 62, 1208 CrossRef CAS
.
- S. P. Gunasekera, P. J. McCarthy, R. E. Longley, S. A. Pomponi, A. E. Wright, E. Lobkovsky and J. Clardy, J. Nat. Prod., 1999, 62, 173 CrossRef CAS
.
- M.-G. Dijoux, W. R. Gamble, Y. F. Hallock, J. H. Cardellina II, R. van Soest and M. R. Boyd, J. Nat. Prod., 1999, 62, 636 CrossRef
.
- N. B. Perry, J. W. Blunt and M. H. G. Munro, Tetrahedron, 1988, 44, 1727 CrossRef CAS
.
- B. R. Copp, K. F. Fulton, N. B. Perry, J. W. Blunt and M. H. G. Munro, J. Org. Chem., 1994, 59, 8233 CrossRef CAS
.
- K. M. Aubart and C. H. Heathcock, J. Org. Chem., 1999, 64, 16 CrossRef CAS
.
- S. Sakemi, H. H. Sun, C. W. Jefford and G. Bernardinelli, Tetrahedron Lett., 1989, 30, 2517 CrossRef CAS
.
- H. H. Sun, S. Sakemi, N. Burres and P. McCarthy, J. Org. Chem., 1990, 55, 4964 CrossRef CAS
.
- M. Alvarez, M. A. Bros, G. Gras, W. Ajana and J. A. Joule, Eur. J. Org. Chem., 1999, 1173 CrossRef CAS
.
- D. C. Radisky, E. S. Radisky, L. R. Barrows, B. R. Copp, R. A. Kramer and C. M. Ireland, J. Am. Chem. Soc., 1993, 115, 1632 CrossRef CAS
.
- Y. Kita, M. Egi and H. Tohma, Chem. Commun., 1999, 143 RSC
.
- Y. Kita, M. Egi, T. Takada and H. Tohma, Synthesis, 1999, 885 CrossRef CAS
.
- D. A. Venables, L. R. Barrows, P. Lassota and C. M. Ireland, Tetrahedron Lett., 1997, 38, 721 CrossRef
.
- Y. Moro-oka, T. Fukuda and M. Iwao, Tetrahedron Lett., 1999, 40, 1713 CrossRef CAS
.
- F. S. de Guzman, B. Carte, N. Troupe, D. J. Faulkner, M. K. Harper, G. P. Concepcion, G. C. Mangalindan, S. S. Matsumoto, L. R. Barrows and C. M. Ireland, J. Org. Chem., 1999, 64, 1400 CrossRef CAS
.
- R. Sakai, C. Oiwa, K. Takaishi, H. Kamiya and M. Tagawa, Tetrahedron Lett., 1999, 40, 6941 CrossRef CAS
.
- A. Zampella, C. Giannini, C. Debitus, C. Roussakis and M. V. D’Auria, J. Nat. Prod., 1999, 62, 332 CrossRef CAS
.
- J. E. Coleman, R. van Soest and R. J. Andersen, J. Nat. Prod., 1999, 62, 1137 CrossRef CAS
.
- W. R. Gamble, N. A. Durso, R. W. Fuller, C. K. Westergaard, T. R. Johnson, D. L. Sackett, E. Hamel, J. H. Cardellina II and M. R. Boyd, Bioorg. Med. Chem., 1999, 7, 1611 CrossRef CAS
.
- N. Fusetani, M. Fujita, Y. Nakao and S. Matsunaga and R W. M. van Soest, Bioorg. Med. Chem. Lett., 1999, 9, 3397 CrossRef CAS
.
- N. Fusetani, K. Warabi, Y. Nogata, Y. Nakao, S. Matsunaga and R. W. M. van Soest, Tetrahedron Lett., 1999, 40, 4687 CrossRef CAS
.
- Y. Nakao, A. Masuda, S. Matsunaga and N. Fusetani, J. Am. Chem. Soc., 1999, 121, 2425 CrossRef CAS
.
- E. D. de Silva, D. E. Williams, R. J. Andersen, H. Klix, C. F. B. Holmes and T. M. Allen, Tetrahedron Lett., 1992, 33, 1561 CrossRef
.
- R. Samy, H. Y. Kim, M. Brady and P. L. Toogood, J. Org. Chem., 1999, 64, 2711 CrossRef CAS
.
- T. Hu and J. S. Panek, J. Org. Chem., 1999, 64, 3000 CrossRef CAS
.
- S. M. Bauer and R. W. Armstrong, J. Am. Chem. Soc., 1999, 121, 6355 CrossRef CAS
.
- M. Kobayashi, M. Kurosu, N. Ohyabu, W. Wang, S. Fujii and I. Kitagawa, Chem. Pharm. Bull., 1994, 42, 2196 Search PubMed
.
- J. D. White, J. Hong and L. A. Robarge, J. Org. Chem., 1999, 64, 6206 CrossRef CAS
.
- M. Tsuda, H. Ishiyama, K. Masuko, T. Takao, Y. Shimonishi and J. Kobayashi, Tetrahedron, 1999, 55, 12543 CrossRef CAS
.
- M. Tsuda, K. Shimbo, T. Kubota, Y. Mikami and J. Kobayashi, Tetrahedron, 1999, 55, 10305 CrossRef CAS
.
- M. Kobayashi, N. K. Lee, H. Shibuya, T. Momose and I. Kitagawa, Chem. Pharm. Bull., 1991, 39, 1177 Search PubMed
.
- M. Doi, T. Ishida, M. Kobayashi, J. R. Deschamps and J. L. Flippen-Anderson, Acta Crystallogr., Sect. C, 1999, 55, 796 CrossRef
.
- M. C. Roy, I. I. Ohtani, J. Tanaka, T. Higa and R. Satari, Tetrahedron Lett., 1999, 40, 5373 CrossRef CAS
.
- P. W. Ford, K. R. Gustafson, T. C. McKee, N. Shigematsu, L. K. Maurizi, L. K. Pannell, D. E. Williams, E. D. de Silva, P. Lassota, T. M. Allen, R. van Soest, R. J. Andersen and M. R. Boyd, J. Am. Chem. Soc., 1999, 121, 5899 CrossRef CAS
.
- G. R. Pettit, R. Tan, M. D. Williams, L. Tackett, J. M. Schmidt, R. L. Cerny and J. N. A. Hooper, Bioorg. Med. Chem. Lett., 1993, 3, 2869 CrossRef CAS
.
- G. R. Pettit, M. R. Rhodes and R. Tan, J. Nat. Prod., 1999, 62, 409 CrossRef CAS
.
- J. Kobayashi, F. Itagaki, H. Shigemori, T. Takao and Y. Shimonishi, Tetrahedron, 1995, 51, 2525 CrossRef CAS
.
- J. A. Sowinski and P. L. Toogood, Chem. Commun., 1999, 981 RSC
.
- P. Ciminiello, C. Dell′Aversano, E. Fattorusso, S. Magno and M. Pansini, J. Nat. Prod., 1999, 62, 590 CrossRef CAS
.
- T. Fendert, V. Wray, R. W. M. van Soest and P. Proksch, Z. Naturforsch., Sect. C, 1999, 54, 246 Search PubMed
.
- Z. Aydoǧmuş, N. Ersoy and S. Imre, Turk. J. Chem., 1999, 23, 339 Search PubMed
.
- H. Gao, M. Kelly and M. T. Hamann, Tetrahedron Lett., 1999, 55, 9717 CrossRef CAS
.
- R. S. Compagnone, R. Avila, A. I. Suárez, O. V. Abrams, H. R. Rangel, F. Arvelo, I. C. Piña and E. Merentes, J. Nat. Prod., 1999, 62, 1443 CrossRef CAS
.
- X. Fu and F. J. Schmitz, J. Nat. Prod., 1999, 62, 1072 CrossRef CAS
.
- Y. Venkateswarlu, U. Venkatesham and M. R. Rao, J. Nat. Prod., 1999, 62, 893 CrossRef CAS
.
- Y. Okamoto, M. Ojika and Y. Sakagami, Tetrahedron Lett., 1999, 40, 507 CrossRef CAS
.
- M. Tsuda, H. Uemoto and J. Kobayashi, Tetrahedron Lett., 1999, 40, 5709 CrossRef CAS
.
-
H. Uemoto
, M. Tsuda
and J. Kobayashi
, J. Nat. Prod., 1999, 62, 1581 [erratum: 2000, 63, 1050].
Search PubMed
.
- M. Assmann, E. Lichte, R. W. M. van Soest and M. Köck, Org. Lett., 1999, 1, 455 CrossRef CAS
.
- C. Eder, P. Proksch, V. Wray, R. W. M. van Soest, E. Ferdinandus and L. A. Pattisina and Sudarsono, J. Nat. Prod., 1999, 62, 1295 CrossRef CAS
.
- C. Eder, P. Proksch, V. Wray, K. Steube, G. Bringman, R. W. M. van Soest, Sudarsano, E. Ferdinandus, L. A. Pattisina, S. Wiryowidagdo and W. Moka, J. Nat. Prod., 1999, 62, 184 CrossRef CAS
.
- S. Urban, P. de A. Leone, A. R. Carroll, G. A. Fechner, J. Smith, J. N. A. Hooper and R. J. Quinn, J. Org. Chem., 1999, 64, 731 CrossRef CAS
.
- F. Cafieri, E. Fattorusso, A. Mangoni and O. Taglialatela-Scafati, Tetrahedron Lett., 1995, 36, 7893 CrossRef CAS
.
- F. Cafieri, E. Fattorusso and O. Taglialatela-Scafati, J. Nat. Prod., 1998, 61, 122 CrossRef CAS
.
- I. Mancini, G. Guella, P. Amade, C. Roussakis and F. Pietra, Tetrahedron Lett., 1997, 38, 6271 CrossRef CAS
.
- M. G. Banwell, A. M. Bray, A. C. Willis and D. J. Wong, New J. Chem., 1999, 23, 687 RSC
.
- S. Marchais, A. Al Mourabit, A. Ahond, C. Poupat and P. Potier, Tetrahedron Lett., 1999, 40, 5519 CrossRef CAS
.
- M. D’Ambrosio, A. Guerriero, G. Chiasera and F. Pietra, Helv. Chim. Acta, 1994, 77, 1895 CrossRef CAS
.
- D. Stein, G. T. Anderson, C. E. Chase, Y. Koh and S. M. Weinrab, J. Am. Chem. Soc., 1999, 121, 9574 CrossRef
.
- J. Shin, Y. Seo, K. W. Cho, J.-R. Rho and C. J. Sim, J. Nat. Prod., 1999, 62, 647 CrossRef CAS
.
- S. P. B. Ovenden and R. J. Capon, J. Nat. Prod., 1999, 62, 1246 CrossRef CAS
.
- M. T. P. Gambardella, R. L. A. Dias, C. C. Chehade and R. G. S. Berlink, Acta Crystallogr., Sect. C, 1999, 55, 1585 CrossRef
.
- S. S. Mitchell, A. B. Whitehill, H. G. Trapido-Rosenthal and C. M. Ireland, J. Nat. Prod., 1997, 60, 727 CrossRef CAS
.
- C. C. Chehade, R. L. A. Dias, R. G. S. Berlinck, A. G. Ferreira, L. V. Costa, M. Rangel, E. L. A. Malpezzi, J. C. de Freitas and E. Hajdu, J. Nat. Prod., 1997, 60, 729 CrossRef CAS
.
- Y. Venkateswarlu, N. S. Reddy, P. Ramesh and J. V. Rao, Biochem. Syst. Ecol., 1999, 27, 519 CrossRef CAS
.
- H. Suzuki, K. Shindo, A. Ueno, T. Miura, M. Takei, M. Sakakibara, H. Fukamachi, J. Tanaka and T. Higa, Bioorg. Med. Chem. Lett., 1999, 9, 1361 CrossRef CAS
.
- C. Jiménez, E. Quiñoa, M. Adamczeski, L. M. Hunter and P. Crews, J. Org. Chem., 1991, 56, 3403 CrossRef CAS
.
- D. S. Carter and D. L. Van Vranken, J. Org. Chem., 1999, 64, 8537 CrossRef CAS
.
- J. Kobayashi, T. Murayama, M. Ishibashi, S. Kosuge, M. Takamatsu, Y. Ohizumi, H. Kobayashi, T. Ohta and S. Nozoe, Tetrahedron, 1990, 46, 7699 CrossRef CAS
.
- J. Bergman, T. Janosik and A.-L. Johnsson, Synthesis, 1999, 580 CrossRef CAS
.
- G. W. Chan, S. Mong, M. E. Hemling, A. J. Freyer, P. H. Offen, C. W. DeBrosse, H. M. Sarau and J. W. Westley, J. Nat. Prod., 1993, 56, 116 CAS
.
- N. Roué and J. Bergman, Tetrahedron, 1999, 55, 14729 CrossRef CAS
.
- S. Carmely, M. Ilan and Y. Kashman, Tetrahedron, 1989, 45, 2193 CrossRef CAS
.
- P. Molina, P. M. Fresneda and M. A. Sanz, J. Org. Chem., 1999, 64, 2540 CrossRef CAS
.
- R. Talpir, A. Rudi, M. Ilan and Y. Kashman, Tetrahedron Lett., 1992, 33, 3033 CrossRef CAS
.
- A. Kaiser, C. Marazano and M. Maier, J. Org. Chem., 1999, 64, 3778 CrossRef CAS
.
- S. Urban, M. S. Butler and R. J. Capon, Aust. J. Chem., 1994, 47, 1919 CAS
.
- D. L. Boger, C. W. Boyce, M. A. Labroli, C. A. Sehon and Q. Jin, J. Am. Chem. Soc., 1999, 121, 54 CrossRef CAS
.
- M.-L. Bourguet-Kondracki and M. Guyot, Tetrahedron Lett., 1999, 40, 3149 CrossRef CAS
.
- M.-L. Bourguet-Kondracki, F. Lacombe and M. Guyot, J. Nat. Prod., 1999, 62, 1304 CrossRef CAS
.
- M. T. Hamann, P. J. Scheuer and M. Kelly-Borges, J. Org. Chem., 1993, 58, 6565 CrossRef CAS
.
- A. F. Barrero, E. J. Alvarez-Manzaneda, R. Chahboun, M. Cortés and V. Armstrong, Tetrahedron, 1999, 55, 15181 CrossRef CAS
.
- G. Cimino, S. De Stefano and L. Minale, Experientia, 1975, 31, 1117 Search PubMed
.
- A. F. Barrero, E. J. Alvarez-Manzaneda, M. M. Herrador, R. Chahboun and P. Galera, Bioorg. Med. Chem. Lett., 1999, 9, 2325 CrossRef CAS
.
- L. Minale, R. Riccio and G. Sodano, Tetrahedron Lett., 1974, 15, 3401 CrossRef
.
- T. Ling, A. X. Xiang and E. A. Theodorakis, Angew. Chem., Int. Ed., 1999, 38, 3089 CrossRef CAS
.
- K. Iguchi, A. Sahashi, J. Kohno and Y. Yamada, Chem. Pharm. Bull., 1990, 38, 1121 Search PubMed
.
- L. K. Shubina, S. N. Federov, V. A. Stonik, A. S. Dmitrenok and V. V. Isakov, Khim. Prir. Soedin., 1990, 358 Search PubMed
.
- S. G. Ilyin, L. K. Shubina, V. A. Stonik and M. Y. Antipin, Acta Crystallogr., Sect. C, 1999, 55, 266 CrossRef
.
- R. Talpir, A. Rudi, Y. Kashman, Y. Loya and A. Hizi, Tetrahedron, 1994, 50, 4179 CrossRef CAS
.
- S. Poigny, T. Huor, M. Guyot and M. Samadi, J. Org. Chem., 1999, 64, 9318 CrossRef CAS
.
- J. Kobayasi, T. Madono and H. Shigumori, Tetrahedron, 1995, 51, 10867 CrossRef
.
- P. Stahl and H. Waldmann, Angew. Chem., Int. Ed., 1999, 38, 3710 CrossRef CAS
.
- M. Ishibashi, Y. Ohizumi, J. Cheng, H. Nakamura, Y. Hirata, T. Sasaki and J. Kobayashi, J. Org. Chem., 1988, 53, 2855 CrossRef CAS
.
- W. P. Almeida and C. R. D. Correia, J. Braz. Chem. Soc., 1999, 10, 401 Search PubMed
.
- T. Wakimoto, A. Maruyama, S. Matsunaga, N. Fusetani, K. Shinoda and P. T. Murphy, Bioorg. Med. Chem. Lett., 1999, 9, 727 CrossRef CAS
.
- I. Erdogan, J. Tanaka, T. Higa and B. Sener, Nat. Prod. Sci., 1999, 5, 177 Search PubMed
.
- C. L. Blackburn, C. Hopmann, R. Sakowicz, M. S. Berdelis, L. S. B. Goldstein and D. J. Faulkner, J. Org. Chem., 1999, 64, 5565 CrossRef CAS
.
- J. A. Kalaitzis, P. de A. Leone, L. Harris, M. S. Butler, A. Ngo, J. N. A. Hooper and R. J. Quinn, J. Org. Chem., 1999, 64, 5571 CrossRef CAS
.
- J. A. Kalaitzis and R. J. Quinn, J. Nat. Prod., 1999, 62, 1682 CrossRef CAS
.
- M. Bogenstätter, A. Limberg, L. E. Overman and A. L. Tomasi, J. Am. Chem. Soc., 1999, 121, 12206 CrossRef
.
- A. Fukami, Y. Ikeda, S. Kondo, H. Naganawa, T. Takeuchi, S. Furuya, K. Hirabayashi, K. Shimoike, S. Hosaka, Y. Watanabe and K. Umezawa, Tetrahedron Lett., 1997, 38, 1201 CrossRef CAS
.
- N. Kawai, K. Takao and S. Kobayashi, Tetrahedron Lett., 1999, 40, 4193 CrossRef CAS
.
- R. J. Clark, M. J. Garson, I. M. Brereton and J. A. Kennedy, J. Nat. Prod., 1999, 62, 915 CrossRef CAS
.
- N. S. Reddy and Y. Venkateswarlu, Indian J. Chem., Sect. B, 1999, 38, 1002 Search PubMed
.
- Y. Sera, K. Adachi, F. Nishida and Y. Shizuri, J. Nat. Prod., 1999, 62, 395 CrossRef CAS
.
- Y.-C. Shen and P.-W. Hsieh, Chin. Pharm. J., 1999, 51, 213 Search PubMed
.
- G. Guella, I. Mancini, A. Guerriero and F. Pietra, Helv. Chim. Acta, 1985, 68, 1276 CrossRef CAS
.
- T.-L. Ho and Y.-J. Lin, J. Chem. Soc., Perkin Trans. 1, 1999, 1207 RSC
.
- G. Cimino, S. De Stefano, A. Guerriero and L. Minale, Tetrahedron Lett., 1975, 16, 1425 CrossRef
.
- W.-C. Liu and C.-C. Liao, Chem. Commun., 1999, 117 RSC
.
- G. Mehta and K. Srinivas, Tetrahedron Lett., 1999, 40, 4877 CrossRef CAS
.
- G. M. König and A. D. Wright, J. Org. Chem., 1997, 62, 3837 CrossRef
.
- C.-Y. Chen, Y.-C. Shen, Y.-J. Chen, J.-H. Sheu and C.-Y. Duh, J. Nat. Prod., 1999, 62, 573 CrossRef CAS
.
- Y.-C. Shen, C.-Y. Chen and C.-Y. Duh, J. Chin. Chem. Soc., 1999, 46, 201 Search PubMed
.
- C.-J. Li, F. J. Schmitz and M. Kelly, J. Nat. Prod., 1999, 62, 1330 CrossRef CAS
.
- P. Karuso, A. Poiner and P. J. Scheuer, J. Org. Chem., 1989, 54, 2095 CrossRef CAS
.
- T.-L. Ho and G. H. Jana, J. Org. Chem., 1999, 64, 8965 CrossRef CAS
.
- A. T. Pham, T. Ichiba, W. Y. Yoshida, P. J. Scheuer, T. Uchida, J. Tanaka and T. Higa, Tetrahedron Lett., 1991, 32, 4843 CrossRef CAS
.
- A. Srikrishna and S. J. Gharpure, Tetrahedron Lett., 1999, 40, 1035 CrossRef CAS
.
- S. Tsukamoto, T. Yamashita, S. Matsunaga and N. Fusetani, Tetrahedron Lett., 1999, 40, 737 CrossRef CAS
.
- S. Matsunaga, T. Yamashita, S. Tsukamoto and N. Fusetani, J. Nat. Prod., 1999, 62, 1202 CrossRef CAS
.
- S. Tsukamoto, T. Yamashita, S. Matsunaga and N. Fusetani, J. Org. Chem., 1999, 64, 3794 CrossRef CAS
.
- V. Costantino, E. Fattorusso, A. Mangoni, M. Di Rosa, A. Ianaro, M. Aknin and E. M. Gaydou, Eur. J. Org. Chem., 1999, 227 CrossRef CAS
.
- A. Fontana, M. L. Ciavatta, P. Amodeo and G. Cimino, Tetrahedron, 1999, 55, 1143 CrossRef CAS
.
- C.-J. Li, F. J. Schmitz and M. Kelly-Borges, J. Nat. Prod., 1999, 62, 287 CrossRef CAS
.
- S. S. Mitchell, M. K. Harper and D. J. Faulkner, Tetrahedron, 1999, 55, 10887 CrossRef CAS
.
- G. Cimino, R. Morrone and G. Sodano, Tetrahedron Lett., 1982, 23, 4139 CrossRef CAS
.
- R. Puliti and C. A. Mattia, Acta Crystallogr., Sect. C, 1999, 55, 2160 CrossRef
.
- N. Capelle, J. C. Braekman, D. Daloze and B. Tursch, Bull. Soc. Chim. Belg., 1980, 89, 399 Search PubMed
.
- M. Arnó, M. A. González and R. J. Zaragozá, Tetrahedron, 1999, 55, 12419 CrossRef CAS
.
- M. L. Ciavatta, A. Fontana, R. Puliti, G. Scognamiglio and G. Cimino, Tetrahedron, 1999, 55, 12629 CrossRef CAS
.
- C. W. J. Chang, A. Patra, D. M. Roll, P. J. Scheuer, G. K. Matsumoto and J. Clardy, J. Am. Chem. Soc., 1984, 106, 4644 CrossRef CAS
.
- M. Shimomura, H. Miyaoka and Y. Yamada, Tetrahedron Lett., 1999, 40, 8015 CrossRef CAS
.
- S. De Rosa, A. Crispino, A. De Giulio, C. Iodice, G. Tommonaro, R. Pronzato and M. Sidri, Tetrahedron, 1999, 55, 13805 CrossRef CAS
.
- S. P. B. Ovenden and R. J. Capon, J. Nat. Prod., 1999, 62, 214 CrossRef CAS
.
- M. S. Butler and R. J. Capon, Aust. J. Chem., 1991, 44, 77 CAS
.
- M. G. B. Drew, L. M. Harwood, A. Jahans, J. Robertson and S. Swallow, Synlett, 1999, 185 CAS
.
- X. Fu, M. L. G. Ferreira, F. J. Schmitz and M. Kelly, J. Nat. Prod., 1999, 62, 1190 CrossRef CAS
.
- S. De Rosa, A. Crispino, A. De Giulio, C. Iodice, P. Amodeo and T. Tancredi, J. Nat. Prod., 1999, 62, 1316 CrossRef
.
- R. P. Walker, J. E. Thompson and D. J. Faulkner, J. Org. Chem., 1980, 45, 4976 CrossRef CAS
.
- E. Fattorusso, S. Magno, C. Santacroce and D. Sica, Tetrahedron, 1972, 28, 5993 CrossRef CAS
.
- R. C. Cambie, C. E. F. Rickard, P. S. Rutledge and X.-S. Yang, Acta Crystallogr., Sect. C, 1999, 55, 112 CrossRef
.
- J. Kimura and M. Hyosu, Chem. Lett., 1999, 61 CrossRef CAS
.
- A. Rudi, T. Yosief, M. Schleyer and Y. Kashman, Org. Lett., 1999, 1, 471 CrossRef CAS
.
- R. P. Gregson and D. J. Ouvrier, J. Nat. Prod., 1982, 45, 412 CrossRef CAS
.
- A. Fontana, I. Fakhr, E. Mollo and G. Cimino, Tetrahedron: Asymmetry, 1999, 10, 3869 CrossRef CAS
.
- X. Fu, L. Zeng, J. Su and F. J. Schmitz, J. Nat. Prod., 1999, 62, 644 CrossRef CAS
.
- T. Jahn, G. M. König and A. D. Wright, J. Nat. Prod., 1999, 62, 375 CrossRef CAS
.
- G. M. König and A. D. Wright, Planta Med., 1999, 65, 679 CAS
.
- E. D. de Silva and P. J. Scheuer, Tetrahedron Lett., 1980, 21, 1611 CrossRef CAS
.
- A. Soriente, M. De Rosa, A. Apicella, A. Scettri and G. Sodano, Tetrahedron: Asymmetry, 1999, 10, 4481 CrossRef CAS
.
- G. Cimino, S. De Stefano and L. Minale, Tetrahedron, 1972, 28, 5983 CrossRef CAS
.
- A. Fürstner, T. Gastner and J. Rust, Synlett, 1999, 29 CrossRef
.
- H. He, P. Kulanthaivel and B. J. Baker, Tetrahedron Lett., 1994, 35, 7189 CrossRef CAS
.
- T. Hata, K. Tanaka and S. Katsumura, Tetrahedron Lett., 1999, 40, 1731 CrossRef CAS
.
- Y. Sera, K. Adachi and Y. Shizuri, J. Nat. Prod., 1999, 62, 152 CrossRef CAS
.
- N. S. Reddy, P. Ramesh and Y. Venkateswarlu, Nat. Prod. Lett., 1999, 14, 131 Search PubMed
.
- V. Bultel-Poncé, J.-P. Brouard, J. Vacelet and M. Guyot, Tetrahedron Lett., 1999, 40, 2955 CrossRef CAS
.
- S. Aoki, A. Setiawan, Y. Yoshioka, K. Higuchi, R. Fudetani, Z.-S. Chen, T. Sumizawa, S. Akiyama and M. Kobayashi, Tetrahedron, 1999, 55, 13965 CrossRef CAS
.
- I. A. van Altena, A. J. Butler and S. J. Dunne, J. Nat. Prod., 1999, 62, 1154 CrossRef CAS
.
- S. De Rosa, A. De Giulio, A. Crispino, C. Iodice and G. Tommonaro, Nat. Prod. Lett., 1999, 13, 15 Search PubMed
.
- S. De Marino, M. Iorizzi, F. Zollo, C. Roussakis and C. Debitus, Eur. J. Org. Chem., 1999, 697 CrossRef CAS
.
- X. Fu, M. L. G. Ferreira, F. J. Schmitz and M. Kelly, J. Org. Chem., 1999, 64, 6706 CrossRef CAS
.
- A. Qureshi and D. J. Faulkner, Tetrahedron, 1999, 55, 8323 CrossRef CAS
.
- The structure of haplosamate A was corrected by Prof. Nobuhiro Fusetani. The revised structures will be published in the near future..
- A. Zampella, C. Giannini, C. Debitus, C. Roussakis and M. V. D’Auria, Eur. J. Org. Chem., 1999, 949 CrossRef CAS
.
- C. Giannini, A. Zampella, C. Debitus, J.-L. Menou, C. Roussakis and M. V. D’Auria, Tetrahedron, 1999, 55, 13749 CrossRef CAS
.
- G. Ryu, B. W. Choi, B. H. Lee, K.-H. Hwang, U. C. Lee, D. S. Jeong and N. H. Lee, Tetrahedron, 1999, 55, 13171 CrossRef CAS
.
- F. Cafieri, E. Fattorusso and O. Taglialatela-Scafati, Eur. J. Org. Chem., 1999, 231 CrossRef CAS
.
- N. Shoji, A. Umeyama, K. Shin, K. Takeda, S. Arihara, J. Kobayashi and M. Takei, J. Org. Chem., 1992, 57, 2996 CrossRef CAS
.
- M. E. Jung and T. W. Johnson, Org. Lett., 1999, 1, 1671 CrossRef CAS
.
- D. E. Williams, A. Tahir and R. J. Andersen, J. Nat. Prod., 1999, 62, 653 CrossRef CAS
.
- H. Hioki, M. Hamano, Y. Mimura, M. Kodama, S. Ohta, M. Yanai and S. Ikegami, Tetrahedron Lett., 1998, 39, 7745 CrossRef CAS
.
- S. Ohta, M. Uno, M. Tokumasu, Y. Hiraga and S. Ikegami, Tetrahedron Lett., 1996, 37, 7765 CrossRef CAS
.
- M. Tokumasu, H. Ando, Y. Hiraga, S. Kojima and K. Ohkata, J. Chem. Soc., Perkin Trans. 1, 1999, 489 RSC
.
- H. Takikawa, J. Koizumi, Y. Kato and K. Mori, J. Chem. Soc., Perkin Trans. 1, 1999, 2271 RSC
.
- A. Rudi, T. Yosief, M. Schleyer and Y. Kashman, Tetrahedron, 1999, 55, 5555 CrossRef CAS
.
- I. Mancini, A. Guerriero, G. Guella, T. Bakken, H. Zibrowius and F. Pietra, Helv. Chim. Acta, 1999, 82, 677 CrossRef CAS
.
- N. Fusetani, T. Toyoda, N. Asai, S. Matsunaga and T. Maruyama, J. Nat. Prod., 1996, 59, 796 CrossRef CAS
.
- H. A. Stefani, I. M. Costa and G. Zeni, Tetrahedron Lett., 1999, 40, 9215 CrossRef CAS
.
- F. J. Schmitz, E. D. Lorance and L. S. Ciereszko, J. Org. Chem., 1969, 34, 1989 CrossRef CAS
.
- Y.-W. Guo, M. Gavagnin, E. Mollo, E. Trivellone and G. Cimino, J. Nat. Prod., 1999, 62, 1194 CrossRef CAS
.
- M. Iwashima, K. Okamoto, F. Konno and K. Iguchi, J. Nat. Prod., 1999, 62, 352 CrossRef CAS
.
- M. Iwashima, K. Okamoto, Y. Miyai and K. Iguchi, Chem. Pharm. Bull., 1999, 47, 884 Search PubMed
.
- M. Iwashima, K. Okamoto and K. Iguchi, Tetrahedron Lett., 1999, 40, 6455 CrossRef CAS
.
- C. Subrahmanyam and R. Kulatheeswaran, Indian J. Chem., Sect. B, 1999, 38, 1388 Search PubMed
.
- M. B. Yunker and P. J. Scheuer, Tetrahedron Lett., 1978, 19, 4651 CrossRef
.
- P. Ramesh, N. S. Reddy, T. P. Rao, J. V. Rao and Y. Venkateswarlu, Biochem. Syst. Ecol., 1999, 27, 661 CrossRef CAS
.
- A. H. Daranas, J. J. Fernández, J. A. Gavín and M. Norte, Tetrahedron, 1999, 55, 5539 CrossRef CAS
.
- G. M. Cabrera and A. M. Seldes, J. Nat. Prod., 1999, 62, 759 CrossRef CAS
.
- N. U. Sata, M. Sugano, S. Matsunaga and N. Fusetani, Tetrahedron Lett., 1999, 40, 719 CrossRef CAS
.
- V. Anjaneyulu and P. Radhika, Indian J. Chem., Sect. B, 1999, 38, 457 Search PubMed
.
- G. B. S. Reddy, D. V. Rao, C. B. Rao, N. Dhananjaya, R. Kuttan and T. D. Babu, Chem. Pharm. Bull., 1999, 47, 1214 Search PubMed
.
- P. Ramesh, N. S. Reddy, T. P. Rao and Y. Venkareswarlu, J. Nat. Prod., 1999, 62, 1019 CrossRef CAS
.
- Y. Venkateswarlu, P. Ramesh, P. S. Reddy and K. Jamil, Phytochemistry, 1999, 52, 1275 CrossRef CAS
.
- A. S. R. Anjaneyulu and P. M. Gowri, Indian J. Chem., Sect. B, 1999, 38, 4 Search PubMed
.
- A. S. R. Anjaneyulu, P. M. Gowri and M. V. R. Krishna Murthy, J. Nat. Prod., 1999, 62, 1600 CrossRef CAS
.
- C. Tsitsimpikou, C. Vagias and V. Roussis, Nat. Prod. Lett., 1999, 14, 17 Search PubMed
.
- L. F. Maia, R. de A. Epifanio, T. Eve and W. Fenical, J. Nat. Prod., 1999, 62, 1322 CrossRef CAS
.
- H. R. Bokesch, T. C. McKee, J. H. Cardellina II and M. R. Boyd, Tetrahedron Lett., 1996, 37, 3259 CrossRef CAS
.
- H. R. Bokesch, J. W. Blunt, C. K. Westergaard, J. H. Cardellina II, T. R. Johnson, J. A. Michael, T. C. McKee, M. G. Hollingshead and M. R. Boyd, J. Nat. Prod., 1999, 62, 633 CrossRef CAS
.
- J. Shin, Y. Seo, K. W. Cho, S.-S. Moon and Y. J. Cho, J. Org. Chem., 1999, 64, 1853 CrossRef CAS
.
- F. J. McEnroe and W. Fenical, Tetrahedron, 1978, 34, 1661 CrossRef CAS
.
- G. L. Kad, A. Kurana, V. Singh and J. Singh, J. Chem. Res. (S), 1999, 164 RSC
.
- G.-C. Zheng, M. Hatano, M. O. Ishitsuka, T. Kusumi and H. Kakisawa, Tetrahedron Lett., 1990, 31, 2617 CrossRef CAS
.
- S. Hanessian, L.-D. Cantin and D. Andreotti, J. Org. Chem., 1999, 64, 4893 CrossRef CAS
.
- M. Endo, M. Nakagawa, Y. Hamamoto and T. Nakanishi, Chem. Commun., 1983, 980 RSC
.
- K. Hiroya, H. Zhang and K. Ogasawara, Synlett, 1999, 529 CAS
.
- C.-Y. Duh, S.-K. Wang, Y.-L. Weng, M. Y. Chiang and C.-F. Dai, J. Nat. Prod., 1999, 62, 1518 CrossRef CAS
.
- T. Iwagawa, R. Nakashima, K. Takayama, H. Okamura, M. Nakatani, M. Doe and K. Shibata, J. Nat. Prod., 1999, 62, 1046 CrossRef CAS
.
- M. Kobayashi, T. Nakagawa and H. Mitsuhashi, Chem. Pharm. Bull., 1979, 27, 2382 Search PubMed
.
- W.-D. Z. Li, Y. Li and Y. Li, Tetrahedron Lett., 1999, 40, 965 CrossRef CAS
.
- M. Kobayashi, T. Ishizaka, N. Miura and H. Mitsuhashi, Chem. Pharm. Bull., 1987, 35, 2314 Search PubMed
.
- X. Yue, J. Lan, J. Li, Z. Liu and Y. Lin, Tetrahedron, 1999, 55, 133 CrossRef CAS
.
- J. Lan, J. Li, Z. Liu, Y. Li and A. S. C. Chan, Tetrahedron: Asymmetry, 1999, 10, 1877 CrossRef CAS
.
- A. Guerriero, M. D’Ambrosio and F. Pietra, Helv. Chim. Acta, 1990, 73, 277 CrossRef CAS
.
- J. Lan, Z. Lui, W. Cen, Y. Xing and Y. Li, Tetrahedron Lett., 1999, 40, 1963 CrossRef CAS
.
- T. Hamada, T. Kusumi, M. O. Ishitsuka and H. Kakisawa, Chem. Lett., 1992, 33 CAS
.
- M. Kato, H. Kosugi, T. Ichiyanagi and O. Yamabe, J. Chem. Soc., Perkin Trans. 1, 1999, 783 RSC
.
- X. Fu, F. J. Schmitz and G. C. Williams, J. Nat. Prod., 1999, 62, 584 CrossRef CAS
.
- A. D. Rodríguez, C. Ramírez and I. I. Rodríguez, Tetrahedron Lett., 1999, 40, 7627 CrossRef CAS
.
- H. Miyaoka, M. Nakano, K. Iguchi and Y. Yamada, Tetrahedron, 1999, 55, 12977 CrossRef CAS
.
- Y. Venkareswarlu, K. V. Sridevi and M. R. Rao, J. Nat. Prod., 1999, 62, 756 CrossRef
.
- A. D. Rodríguez, J.-G. Shi and S. D. Huang, J. Nat. Prod., 1999, 62, 1228 CrossRef CAS
.
- A. D. Rodríguez and J.-G. Shi, Org. Lett., 1999, 1, 337 CrossRef CAS
.
- T. Lindel, P. R. Jensen, W. Fenical, B. H. Long, A. M. Casazza, J. Carboni and C. R. Fairchild, J. Am. Chem. Soc., 1997, 119, 8744 CrossRef CAS
.
- X.-T. Chen, S. K. Bhattacharya, B. Zhou, C. E. Gutteridge, T. R. R. Pettus and S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 6563 CrossRef CAS
.
- C. Kakonikos, C. Vagias, V. Roussis, C. Roussakis and J.-M. Kornprobst, Nat. Prod. Lett., 1999, 13, 89 Search PubMed
.
- I. Mancini, G. Guella, H. Zibrowius, D. Laurent and F. Pietra, Helv. Chim. Acta, 1999, 82, 1681 CrossRef CAS
.
- M. García, J. Rodríguez and C. Jiménez, J. Nat. Prod., 1999, 62, 257 CrossRef CAS
.
- D. Maharaj, K. O. Pascoe and W. F. Tinto, J. Nat. Prod., 1999, 62, 313 CrossRef CAS
.
-
T. Iwagawa
, K. Takayama
, H. Okamura
, M. Nakatani
and M. Doe
, Heterocycles, 1999, 51, 1653 [erratum: 2000, 53, 1237].
Search PubMed
.
-
T. Iwagawa
, K. Takenoshita
, H. Okamura
, M. Nakatani
, M. Doe
, K. Shibata
and M. Shiro
, Heterocycles, 1999, 48, 123 [erratum: 2000, 53, 1237].
Search PubMed
.
-
T. Iwagawa
, K. Takayama
, H. Okamura
, M. Nakatani
, M. Doe
, K. Takemura
and M. Shiro
, Heterocycles, 1999, 51, 2619 [erratum: 2000, 53, 1237].
Search PubMed
.
- P.-J. Sung, J.-H. Su, G.-H. Wang, S. F. Lin, C.-Y Duh and J.-H. Sheu, J. Nat. Prod., 1999, 62, 457 CrossRef CAS
.
- J.-H. Sheu, P.-J. Sung, J.-H. Su, G.-H. Wang, C.-Y. Duh, Y.-C. Shen, M. Y. Chiang and I.-T. Chen, J. Nat. Prod., 1999, 62, 1415 CrossRef CAS
.
- J. E. Neve, B. J. McCool and B. F. Bowden, Aust. J. Chem., 1999, 52, 359 CAS
.
- J.-H. Sheu, P.-J. Sung, J.-H. Su, H.-Y. Liu, C.-Y. Duh and M. Y. Chiang, Tetrahedron, 1999, 55, 14555 CrossRef CAS
.
- A. S. R. Anjaneyulu and P. Sarada, J. Chem. Res. (S), 1999, 600 RSC
.
- A. S. R. Anjaneyulu, P. M. Gowri and M. V. R. Krishna Murthy, J. Chem. Res. (S), 1999, 140 RSC
.
- A. S. R. Anjaneyulu, P. M. Gowri, M. J. R. V. Venugopal, P. Sarada, M. V. R. Krishna Murthy, G. V. Rao, P. S. N. Murthy, C. V. Rao and G. Kumar, J. Indian Chem. Soc., 1999, 76, 651 Search PubMed
.
- C.-Y. Duh, S.-K. Wang, M.-C. Chia and M. Y. Chiang, Tetrahedron Lett., 1999, 40, 6033 CrossRef CAS
.
- A. D. Rodríguez, C. Ramírez, I. I. Rodríguez and E. González, Org. Lett., 1999, 1, 527 CrossRef CAS
.
- A. D. Rodríguez, C. Ramírez and I. I. Rodríguez, J. Nat. Prod., 1999, 62, 997 CrossRef CAS
.
- M. R. Rao, U. Venkatesham and Y. Venkateswarlu, J. Nat. Prod., 1999, 62, 1584 CrossRef CAS
.
- Q. Liu and R. Li, Chin. J. Mar. Drugs, 1999, 18(1), 13 Search PubMed
.
- G. Mehta, Y. Venkateswarlu, M. R. Rao and R. Uma, J. Chem. Res. (S), 1999, 628 RSC
.
- J.-H. Sheu, K.-C. Chang, P.-J. Sung, C.-Y. Duh and Y.-C. Shen, J. Chin. Chem. Soc., 1999, 46, 253 Search PubMed
.
-
Y. Venkateswarlu
P. Ramesh
N. S. Reddy
T. P. Rao
Nat. Prod. Lett.,
1999, 13, 11 and 229 Search PubMed
(this paper was published twice in the same journal)..
- M. R. Rao, U. Venkatesham, M. V. R. Reddy and Y. Venkateswarlu, J. Nat. Prod., 1999, 62, 785 CrossRef CAS
.
- P. Ramesh and V. Venkateswarlu, Steroids, 1999, 64, 785 CrossRef CAS
.
- H. Shigemori, Y. Sato, T. Kagata and J. Kobayashi, J. Nat. Prod., 1999, 62, 372 CrossRef CAS
.
- Y. Tomono, H. Hirota and N. Fusetani, J. Org. Chem., 1999, 64, 2272 CrossRef CAS
.
- Y. Tomono, H. Hirota, Y. Imahara and N. Fusetani, J. Nat. Prod., 1999, 62, 1538 CrossRef CAS
.
- P. Kittakoop, R. Suttisri, C. Chaichantipyuth, S. Vethchagarun and K. Suwanborirux, J. Nat. Prod., 1999, 62, 318 CrossRef CAS
.
- V. Anjaneyulu, P. V. S. Rao and P. Radhika, Indian J. Chem., Sect. B, 1999, 38, 357 Search PubMed
.
- B. D. Morris and M. R. Prinsep, J. Nat. Prod., 1999, 62, 688 CrossRef CAS
.
- Y. Kamano, A. Kotake, H. Hashima, I. Hayakawa, H. Hiraide, H. Zhang, H. Kizu, K. Komiyama, M. Hayashi and G. R. Pettit, Collect. Czech. Chem. Commun., 1999, 64, 1147 CrossRef CAS
.
- Y. Kamano, H.-P. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Tetrahedron Lett., 1995, 36, 2783 CrossRef CAS
.
- G. K. Jnaneshwara and V. H. Deshpande, J. Chem. Res. (S), 1999, 632 RSC
.
- G. K. Jnaneshwara, A. V. Bedekar and V. H. Deshpande, Synth. Comm., 1999, 29, 3627
.
- P. B. Holst, U. Anthoni, C. Christophersen and P. H. Nielsen, J. Nat. Prod., 1994, 57, 997 CrossRef CAS
.
- M. S. Morales-Ríos, O. R. Suárez-Castillo and P. Joseph-Nathan, J. Org. Chem., 1999, 64, 1086 CrossRef CAS
.
- G. R. Pettit, C. L. Herald, Y. Kamano, D. Gust and R. Aoyagi, J. Nat. Prod., 1983, 46, 528 CrossRef CAS
.
- D. A. Evans, P. H. Carter, E. M. Carreira, A. B. Charette, J. A. Prunet and M. Lautens, J. Am. Chem. Soc., 1999, 121, 7540 CrossRef CAS
.
- K. L. McPhail, M. T. Davies-Coleman, R. C. B. Copley and D. S. Eggleston, J. Nat. Prod., 1999, 62, 1618 CrossRef CAS
.
- G. R. Pettit, Y. Kamano, C. Dufresne, R. L. Cerny, C. L. Herald and J. M. Schmidt, J. Org. Chem., 1989, 54, 6005 CrossRef CAS
.
- K. Akaji, Y. Hayashi, Y. Kiso and N. Kuriyama, J. Org. Chem., 1999, 64, 405 CrossRef CAS
.
- H. Sone, H. Kigoshi and K. Yamada, Tetrahedron, 1997, 53, 8149 CrossRef CAS
.
- H. Kigoshi and S. Yamada, Tetrahedron, 1999, 55, 12301 CrossRef CAS
.
- M. T. Hamann and P. J. Scheuer, J. Am. Chem. Soc., 1993, 115, 5825 CrossRef CAS
.
- G. Goetz, W. Y. Yoshida and P. J. Scheuer, Tetrahedron, 1999, 55, 7739 CrossRef CAS
.
- A. Fontana, M. L. Ciavatta, E. Mollo, C. G. Naik, S. Wahidulla, L. D’Souza and G. Cimino, J. Nat. Prod., 1999, 62, 931 CrossRef CAS
.
- A. Fontana, M. L. Ciavatta, T. Miyamoto, A. Spinella and G. Cimino, Tetrahedron, 1999, 55, 5937 CrossRef CAS
.
- N. Fusetani, H. J. Wolstenholme and S. Matsunaga, Tetrahedron Lett., 1991, 32, 7291 CrossRef CAS
.
- T.-L. Ho and L.-R. Kung, Org. Lett., 1999, 1, 1051 CrossRef CAS
.
- J. Tanaka and T. Higa, J. Nat. Prod., 1999, 62, 1339 CrossRef CAS
.
- R. J. Andersen and F. W. Sum, Tetrahedron Lett., 1980, 21, 797 CrossRef CAS
.
- K. Gustafson and R. J. Andersen, Tetrahedron, 1985, 41, 1101 CrossRef CAS
.
- N. Ungar, M. Gavagnin, A. Fontano and G. Cimino, Tetrahedron: Asymmetry, 1999, 10, 1263 CrossRef CAS
.
- M. Gavagnin, A. De Napoli, F. Castelluccio and G. Cimino, Tetrahedron Lett., 1999, 40, 8471 CrossRef CAS
.
- M. T. Davies-Coleman and D. J. Faulkner, Tetrahedron, 1991, 47, 9743 CrossRef CAS
.
- M. Gavagnin, A. De Napoli, G. Cimino, K. Iken, C. Avila and F. J. Garcia, Tetrahedron: Asymmetry, 1999, 10, 2647 CrossRef CAS
.
- M. Gavagnin, N. Ungur, F. Castelluccio, C. Muniain and G. Cimino, J. Nat. Prod., 1999, 62, 269 CrossRef CAS
.
- N. Ungar, M. Gavagnin, E. Mollo and G. Cimino, Tetrahedron: Asymmetry, 1999, 10, 1635 CrossRef
.
- T. Miyamoto, K. Sakamoto, H. Amano, Y. Arakawa, Y. Nagarekawa, T. Komori, R. Higuchi and T. Sasaki, Tetrahedron, 1999, 55, 9133 CrossRef CAS
.
- A. Fontana, P. Cavaliere, N. Ugur, L. D’Souza, P. S. Paramaswaram and G. Cimino, J. Nat. Prod., 1999, 62, 1367 CrossRef CAS
.
- D. R. Beukes and M. T. Davies-Coleman, Tetrahedron, 1999, 55, 4051 CrossRef CAS
.
- C. L. Cantrell, A. Groweiss, K. R. Gustafson and M. R. Boyd, Nat. Prod. Lett., 1999, 14, 39 Search PubMed
.
- K. A. Alvi, P. Crews and D. G. Loughhead, J. Nat. Prod., 1991, 54, 1509 CrossRef CAS
.
- K. A. Alvi, B. M. Peters, L. M. Hunter and P. Crews, Tetrahedron, 1993, 49, 329 CrossRef
.
- H. Kigoshi, K. Kanematsu and D. Uemura, Tetrahedron Lett., 1999, 40, 5745 CrossRef CAS
.
- N. Takada, K. Suenaga, S. Zheng, H. Chen and D. Uemura, Chem. Lett., 1999, 1025 CAS
.
- K. Ofuji, M. Satake, T. McMahon, J. Silke, K. J. James, H. Naoki, Y. Oshima and T. Yasumoto, Nat. Toxins, 1999, 7, 99 CrossRef CAS
.
- M. Daiguji, M. Satake, H. Ramestad, T. Aune, H. Naoki and T. Yasumoto, Nat. Toxins, 1998, 6, 235 CrossRef CAS
.
- A. Morohashi, M. Satake, H. Naoki, H. F. Kaspar, Y. Oshima and T. Yasumoto, Nat. Toxins, 1999, 7, 45 CrossRef CAS
.
- T. Suzuki, H. Ota and M. Yamasaki, Toxicon, 1999, 37, 187 CrossRef CAS
.
- N. González, J. Rodríguez and C. Jiménez, J. Org. Chem., 1999, 64, 5705 CrossRef CAS
.
- B. S. Davidson and C. M. Ireland, J. Nat. Prod., 1990, 53, 1036 CAS
.
- C. Xu and E. Negishi, Tetrahedron Lett., 1999, 40, 431 CrossRef CAS
.
- F. C. Görth and R. Brückner, Synthesis, 1999, 1520 CrossRef CAS
.
- G. G. Gallo, C. Coronelli, A. Vigevani and G. C. Lancini, Tetrahedron, 1969, 25, 5677 CrossRef CAS
.
- K. Ueda and Y. Hu, Tetrahedron Lett., 1999, 40, 6305 CrossRef CAS
.
- M. Aknin, T. L.-A. Dayan, A. Rudi, Y. Kashman and E. M. Gaydou, J. Agric. Food Chem., 1999, 47, 4175 CrossRef CAS
.
- X. Fu, M. L. G. Ferreira and F. J. Schmitz, J. Nat. Prod., 1999, 62, 1306 CrossRef CAS
.
- R. A. Davis, A. R. Carroll and R. J. Quinn, J. Nat. Prod., 1999, 62, 158 CrossRef CAS
.
- R. A. Davis, A. R. Caroll and R. J. Quinn, J. Nat. Prod., 1999, 62, 1405 CrossRef CAS
.
- X. Fu, M. B. Hossain, F. J. Schmitz and D. van der Helm, J. Org. Chem., 1997, 62, 3810 CrossRef CAS
.
- T. Kato, K. Nagae and M. Hoshikawa, Tetrahedron Lett., 1999, 40, 1941 CrossRef CAS
.
- D. Wolf, F. J. Schmitz, M. B. Hossain and D. van der Helm, J. Nat. Prod., 1999, 62, 167 CrossRef CAS
.
- A. R. Carroll, P. C. Healy, R. J. Quinn and C. J. Tranter, J. Org. Chem., 1999, 64, 2680 CrossRef CAS
.
- R. A. Davis, A. R. Carroll, G. K. Pierens and R. J. Quinn, J. Nat. Prod., 1999, 62, 419 CrossRef CAS
.
- M. V. R. Reddy, M. R. Rao, D. Rhodes, M. S. T. Hansen, K. Rubins, F. D. Bushman, Y. Venkateswarlu and D. J. Faulkner, J. Med. Chem., 1999, 42, 1901 CrossRef CAS
.
- H. Kang and W. Fenical, J. Org. Chem., 1997, 62, 3254 CrossRef CAS
.
- W. Y. Yoshida, K. K. Lee, A. R. Carroll and P. J. Scheuer, Helv. Chim. Acta, 1992, 75, 1721 CrossRef CAS
.
- A. Qureshi and D. J. Faulkner, Nat. Prod. Lett., 1999, 13, 59 Search PubMed
.
- T. Higa, T. Fujiyama and P. J. Scheuer, Comp. Biochem. Physiol., Sect. B, 1980, 65, 525 Search PubMed
.
- T. N. Makarieva, S. G. Ilyin, V. A. Stonik, K. A. Lyssenko and V. A. Denisenko, Tetrahedron Lett., 1999, 40, 1591 CrossRef CAS
.
- R. G. S. Berlinck, R. Britton, E. Piers, L. Lim, M. Roberge, R. M. da Rocha and R. J. Andersen, J. Org. Chem., 1998, 63, 9850 CrossRef CAS
.
- H. C. Vervoort, W. Fenical and P. A. Keifer, J. Nat. Prod., 1999, 62, 389 CrossRef CAS
.
- P. Schupp, C. Eder, P. Proksch, V. Wray, B. Schneider, M. Herderich and V. Paul, J. Nat. Prod., 1999, 62, 959 CrossRef CAS
.
- T. Sasaki, I. I. Ohtani, J. Tanaka and T. Higa, Tetrahedron Lett., 1999, 40, 303 CrossRef CAS
.
- R. Durán, E. Zubía, M. J. Ortega, S. Naranjo and J. Salvá, Tetrahedron, 1999, 55, 13225 CrossRef CAS
.
- R. M. Van Wagoner, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 1999, 62, 794 CrossRef CAS
.
- R. W. Schumacher and B. S. Davidson, Tetrahedron, 1995, 51, 10125 CrossRef CAS
.
- R. W. Schumacher and B. S. Davidson, Tetrahedron, 1999, 55, 935 CrossRef CAS
.
- B. S. Lindsay, C. N. Battershill and B. R. Copp, J. Nat. Prod., 1999, 62, 638 CrossRef CAS
.
- B. S. Lindsay, A. M. P. Almeida, C. J. Smith, R. G. S. Berlinck, R. M. da Rocha and C. M. Ireland, J. Nat. Prod., 1999, 62, 1573 CrossRef CAS
.
- H. Kang and W. Fenical, Tetrahedron Lett., 1997, 38, 941 CrossRef CAS
.
- T. Itaya, Y. Hozumi, T. Kanai and T. Ohta, Chem. Pharm. Bull., 1999, 47, 1297 Search PubMed
.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Eur. J. Biochem., 1999, 264, 785 CrossRef CAS
.
- J. Kubanek, D. E. Williams, E. D. de Silva, T. Allen and R. J. Andersen, Tetrahedron Lett., 1995, 36, 6189 CrossRef CAS
.
- N. Toyooka, M. Okumura and H. Takahata, J. Org. Chem., 1999, 64, 2182 CrossRef CAS
.
- N. Toyooka, M. Okumura, H. Takahata and H. Nemoto, Tetrahedron, 1999, 55, 10673 CrossRef CAS
.
- M. F. Raub, J. H. Cardellina II, M. I. Choudhary, C.-Z. Ni, J. Clardy and M. C. Alley, J. Am. Chem. Soc., 1991, 113, 3178 CrossRef CAS
.
- F. Kong and D. J. Faulkner, Tetrahedron Lett., 1991, 32, 3667 CrossRef CAS
.
- J. D. Ha and J. K. Cha, J. Am. Chem. Soc., 1999, 121, 10012 CrossRef CAS
.
- N. Toyooka, Y. Yotsui, Y. Yoshida, T. Momose and H. Nemoto, Tetrahedron, 1999, 55, 15209 CrossRef CAS
.
- A. J. Blackman, C. Li, D. C. R. Hockless, B. W. Skelton and A. H. White, Tetrahedron, 1993, 49, 8645 CrossRef CAS
.
- J. F. Liu and C. H. Heathcock, J. Org. Chem., 1999, 64, 8263 CrossRef CAS
.
- C. Li and A. J. Blackman, Aust. J. Chem., 1994, 47, 1355 CAS
.
- G. A. Molander and M. Rönn, J. Org. Chem., 1999, 64, 5183 CrossRef CAS
.
- J. F. Biard, S. Guyot, C. Roussakis, J. F. Verbist, J. Vercauteren, J. F. Weber and K. Boukef, Tetrahedron Lett., 1994, 35, 2691 CrossRef CAS
.
- K. M. Werner, J. M. de los Santos, S. M. Weinreb and M. Shang, J. Org. Chem., 1999, 64, 686 CrossRef CAS
.
- W. H. Pearson and Y. Ren, J. Org. Chem., 1999, 64, 688 CrossRef CAS
.
- K. M. Werner, J. M. de los Santos, S. M. Weinreb and M. Shang, J. Org. Chem., 1999, 64, 4865 CrossRef
.
- A. R. Carroll, Y. J. Feng, B. F. Bowden and J. C. Coll, J. Org. Chem., 1996, 61, 4059 CrossRef CAS
.
- C. J. Moody and J. C. A. Hunt, J. Org. Chem., 1999, 64, 8715 CrossRef CAS
.
- M. P. Foster, G. P. Concepcíon, G. B. Caraan and C. M. Ireland, J. Org. Chem., 1992, 57, 6671 CrossRef CAS
.
- S. V. Downing, E. Aguilar and A. I. Meyers, J. Org. Chem., 1999, 64, 826 CrossRef CAS
.
- A. R. Carroll, J. C. Coll, D. J. Bourne, J. K. MacLeod, T. M. Zabriskie, C. M. Ireland and B. F. Bowden, Aust. J. Chem., 1996, 49, 659
.
- P. Wipf and Y. Uto, Tetrahedron Lett., 1999, 40, 5165 CrossRef CAS
.
- A. R. Carroll, B. F. Bowden, J. C. Coll, D. C. R. Hockless, B. W. Skelton and A. H. White, Aust. J. Chem., 1994, 47, 61 CAS
.
- B. McKeever and G. Pattenden, Tetrahedron Lett., 1999, 40, 9317 CrossRef CAS
.
- B. Liang, P. Portonovo, M. D. Vera, D. Xiao and M. M. Joullié, Org. Lett., 1999, 1, 1319 CrossRef CAS
.
- The structural elucidation was recently reported: H. Vervoort, W. Fenical and R. de A. Epifanio, J. Org. Chem., 2000, 65, 782 CrossRef CAS
.
- K. Arao, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 1999, 47, 687 Search PubMed
.
- S. Kawatake, M. Inagaki, T. Miyamoto, R. Isobe and R. Higuchi, Eur. J. Org. Chem., 1999, 765 CrossRef CAS
.
- M. Inagaki, R. Isobe and R. Higuchi, Eur. J. Org. Chem., 1999, 771 CrossRef CAS
.
- M. Kaneko, F. Kisa, K. Yamada, T. Miyamoto and R. Higuchi, Eur. J. Org. Chem., 1999, 3171 CrossRef CAS
.
- M. Inagaki, R. Isobe, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 1999, 47, 1184 Search PubMed
.
- A. A. Kicha, A. I. Kalinovsky, N. V. Ivanchina and V. A. Stonik, J. Nat. Prod., 1999, 62, 279 CrossRef CAS
.
- N. Yayli and J. A. Findlay, Phytochemistry, 1999, 50, 135 CrossRef CAS
.
- I. Röhl, B. Schneider, B. Schmidt and E. Zeeck, Z. Naturforsch., Sect. C, 1999, 54, 1145 Search PubMed
.
- G. Prota, M. D’Agostino and G. Misuraca, J. Chem. Soc., Perkin Trans. 1, 1972, 1614 RSC
.
- G. Cimino, S. De Rosa, S. De Stefano and G. Sodano, J. Nat. Prod., 1985, 48, 828 CrossRef CAS
.
- D. W. Cameron, D. R. Coller and C. A. McDonald, Aust. J. Chem., 1999, 52, 833 CAS
.
- Y. Yamamoto, N. Maita, A. Fujisawa, J. Takashima, Y. Ishii and W. C. Dunlap, J. Nat. Prod., 1999, 62, 1685 CrossRef CAS
.
- M. Yotsu-Yamashita, B. Schimmele and T. Yasumoto, Biosci. Biotechnol. Biochem., 1999, 63, 961 Search PubMed
.
- X. Wang, H. Fu and W. Lin, Chin. J. Mar. Drugs, 1999, 18(1), 7 Search PubMed
.
- X. Wang, H. Fu and W. Lin, Chin. J. Mar. Drugs, 1999, 18(2), 4 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2001 |