Hot off the press
Robert A.
Hill
and
Marie Claire
Parker
Department of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bobh@chem.gla.ac.uk; m.parker@chem.gla.ac.uk
First published on 9th January 2001
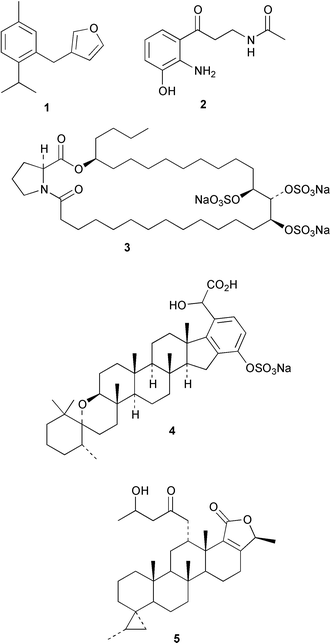
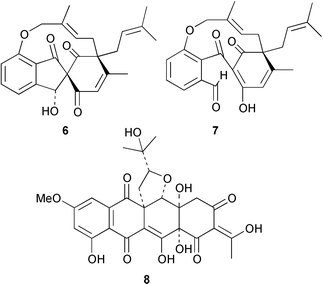
Marine sponges continue to be a source of intriguing natural products. A South African Dysidea sponge is the source of tsitsikammafuran 1, a member of a rare sesquiterpenoid class that includes penlanfuran (M. T. Davies-Coleman and co-workers, Tetrahedron, 2000, 56, 9391). A yellow pigment, erebusinone 2, has been isolated from the Antarctic sponge Isodictya erinacea (B. J. Baker and co-workers, Tetrahedron, 2000, 56, 9057). The Japanese Penares sponge contains penarolide sulfate A13, which inhibits α-glucosidase (N. Fusetani and co-workers, Tetrahedron, 2000, 56, 8977). The Palauan Haliclona (aka Adocia) sponge is a source of the meroterpenoid adociasulfate 10 4, an inhibitor of the kinesin family of microtubule motor proteins (C. L. Blackburn and D. J. Faulkner, Tetrahedron, 2000, 56, 8429). A group of cyclopropane bishomoscalarane sesterterpenoids, including honulactone A 5, has been isolated from the Indonesian sponge Strepsichordaia aliena (J. I. Jiménez et al., J. Org. Chem., 2000, 65, 6837).Coleophomones A 6 and B 7 are metabolites of a Coleophoma species that inhibit transglycolase, an enzyme involved in bacterial cell wall synthesis (K. E. Wilson et al., Tetrahedron Lett., 2000, 41, 8705). Coleophomones A 6 and B 7 can be interconverted by aldol/retroaldol reactions. The structure of coleophomone A 6 was confirmed by X-ray analysis. The relative stereochemistry of the fungal metabolite seragakinone A 8 has been revised on the basis of an X-ray analysis (J. Kobayashi and co-workers, Tetrahedron, 2000, 56, 8841). The biosynthesis of seragakinone A 8 was shown to involve prenylation of a decaketide.
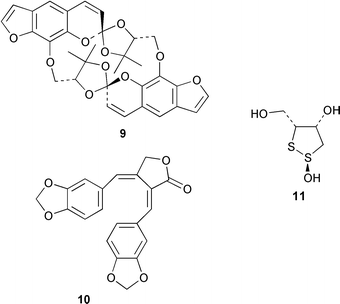
The first examples of cyclic spirobifuranocoumarins, such as cyclorivulobirin A 9, have been isolated from Pleurospermum rivulorum (K. Baba and co-workers, Chem. Pharm. Bull., 2000, 48, 1246). The highly cytotoxic taiwanin A 10 has been isolated from the heartwood of Taiwania cryptomeroides (S.-T. Chang et al., Phytochemistry, 2000, 55, 227). Zeylanoxide A 11 is a plant growth inhibitor isolated from the tropical weed Sphenocle zeylanica (N. Hirai et al., Phytochemistry, 2000, 55, 131).
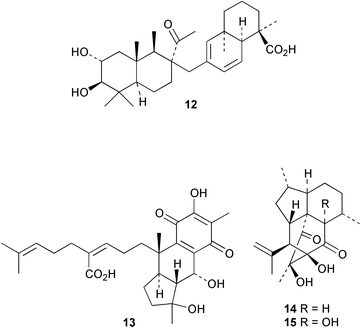
An anti-HIV triterpenoid, geumonoid 12, has been isolated from Geum japonicum (P. P.-H. But and co-workers, Chem. Pharm. Bull., 2000, 48, 1367). A new meroterpenoid skeleton is present in pycnanthuquinone A 13 from Pycnanthus angolensis (D. M. Fort et al., J. Org. Chem., 2000, 65, 6534). Cumbiasins A 14 and B 15, from Pseudopterogorgia elisabethae, have a novel tetracyclic diterpenoid skeleton named cumbiane (A. D. Rodríguez et al., J. Org. Chem., 2000, 65, 6682).
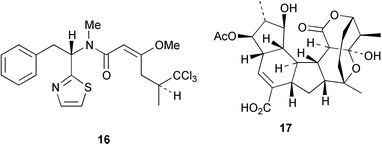
A triterpene synthase from Arabidopsis thaliana has been functionally expressed in yeast (Y. Ebizuka and co-workers, Tetrahedron Lett., 2000, 41, 7705). This synthase produces at least nine products such as the tetracyclic triterpenoids butyrospermol and tirucalla-7,21-dien-3β-ol and several pentacyclic triterpenoids such as lupeol, baureneol and β-amyrin. Detailed labelling studies have established that the biosynthetic pathway to barbamide 16 from the marine cyanobacterium Lyngbya majuscula involves 5,5,5-trichloroleucine, acetate, phenylalanine and cysteine (W. H. Gerwick and co-workers, Tetrahedron, 2000, 56, 9103).
A strain of Streptomyces cellulosae has been induced to produce different metabolites by altering the fermentation conditions (A. Zeeck and co-workers, Angew. Chem., Int. Ed., 2000, 39, 3258). When sodium bromide was added to the culture medium a new polyketide, hexacyclinic acid 17, was formed. The structure was confirmed by X-ray analysis.
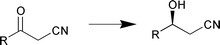
G. Cainelli et al. have studied the temperature and solvent effects on enzyme stereoselectivity (Chem. Commun., 2000, 2351). They observed an inversion temperature in kinetic resolution by lipases, demonstrating that re-organisation of different solute–solvent clusters depends on temperature. The data suggested that solvent molecules are still present in substrate–enzyme transition states. V. Gotor and J. R. Dehli have used methanol as co-solvent as a general methodology for the chemo- and enantio-selective bioreduction of β-keto nitriles using the fungus Curvularia lunata yielding the corresponding (S![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-β-hydroxynitriles (Scheme 1) with high enantioselectivity (Tetrahedron: Asymmetry, 2000, 11, 3693).
)-β-hydroxynitriles (Scheme 1) with high enantioselectivity (Tetrahedron: Asymmetry, 2000, 11, 3693).
 | ||
| Scheme 1 | ||
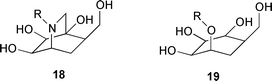
Polyhydroxylated isoquinuclidines mimicking the boat conformation of pyranosides have been found to be strong and selective inhibitors of a β-mannosidase (A. Vasella and co-workers, Chem. Commun., 2000, 1829). The cyclohexane ring of 18 mimics the tetrahydropyran ring of a β-mannoside 19 in a 1,4B conformation, in agreement with the postulate that a conformational change of the pyranose ring precedes or accompanies enzymatic cleavage of β-glycosides. The preparation of O-glycosides by enzymatic glycosylation in plasticised glass phases has been reported by I. Gill and R. Valivety (Angew. Chem., Int. Ed., 2000, 39, 3804). The plasticised glasses consisted of mixtures of monosaccharides, alkyl glycosides and various hydrophilic and hydrophobic compounds. Glycosidases were found to be active in the plasticised glasses, which can support a high concentration of both acceptor and sugar donor molecules, resulting in good yields.
Recent advances in protein engineering have been highlighted in Tetrahedron, 2000, 56, issue 48. The engineering of novel bioactive mini-proteins on natural scaffolds (C. Vita et al., Tetrahedron, 2000, 56, 9451) and the generation of active trypsin by chemical cleavage by T. Baird Jr. et al. (Tetrahedron, 2000, 56, 9477) have been detailed. The in vivo building and folding of protein shuttles for drug delivery and targeting of pharmacologically active amino acids (using non-canonical amino acids) has been presented (C. Minks et al., Tetrahedron, 2000, 56, 9431).
J. Zou and N. Sougimoto have investigated the role of the Trp–His interaction in the conformation of peptides in and the folding of globular proteins for a series of alanine-based peptides having a pair of Trp–His (J. Chem. Soc., Perkin Trans. 2, 2000, 2135). The Trp–His interaction has a different effect on the conformation of the peptide depending on its different geometrical spacing and positions in short alanine-based peptides.
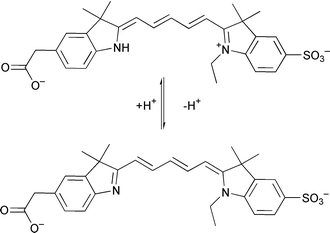
M. E. Cooper and co-workers have developed a pH sensitive fluorescent pentamethine cyanine dye (Scheme 2) that is fluorescent when protonated and non-fluorescent upon proton abstraction (Chem. Commun., 2000, 2323). The probe has a pKa of 7.5, and changes in the emission spectra can be monitored in the pH range 6.0–8.0.
 | ||
| Scheme 2 | ||
Single molecule spectroscopy of polymers and biomolecules probed by scanning probe microscopy (force spectroscopy) has been reviewed by H. Fuchs et al. (Angew. Chem., Int. Ed., 2000, 39, 3212). Experiments dealing with inter- and intramolecular forces that can provide the possibility of obtaining insights into the mechanical behaviour and the structural details of synthetic polymers and biomolecules on a molecular level are discussed. Such examples include simple electrostatic forces, ligand–receptor systems and the stretching of individual molecules.
The simple and efficient synthesis of supramolecular nanocircles consisting of streptavidin and DNA obtained by thermal denaturation and rapid cooling of the oligomeric DNA–streptavidin networks has been reported by C. M. Niemeyer et al. (Angew. Chem., Int. Ed., 2000, 39, 3056).
| This journal is © The Royal Society of Chemistry 2001 |