A novel ferrocenyl diselenide for the catalytic asymmetric aryl transfer to aldehydes
Carsten
Bolm
*,
Martin
Kesselgruber
,
Achim
Grenz
,
Nina
Hermanns
and
Jens P.
Hildebrand
Institut für Organische Chemie der RWTH Aachen, Prof.-Pirlet-Str. 1, 52074, Aachen, Germany. E-mail: Carsten.Bolm@oc.rwth-aachen.de; Fax: + 49 241 888 8391
First published on 3rd October 2000
Abstract
An oxazolinyl ferrocenyl diselenide was synthesised by directed ortho-metalation and used as catalyst precursor in the asymmetric addition of diethyl- and diphenylzinc to various aldehydes, yielding synthetically useful secondary alcohols, of which some are difficult to access using other catalytic methodologies. Enantioselectivities of up to 44% for the former and up to 85% for the latter transformations were obtained.
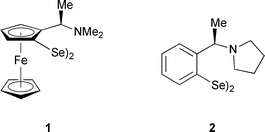
Organoselenium chemistry represents an established tool in organic synthesis.1 However, compared to sulfur, this area is much less investigated, a fact that the reduced stability towards oxidative conditions and light as well as the frequently encountered toxicity of selenium reagents may account for. Extensive use of selenium in organic synthesis was initiated with the discovery of olefin formation by the decomposition of selenoxides, a reaction that proceeds under very mild conditions.2 The chemistry of chiral selenium compounds is an even more undeveloped area.3 Early articles dealing with their application in asymmetric catalysis were published in 1996 by Uemura and coworkers.4 Ferrocene 1, derived from Ugi's amine,5 showed enantioselectivities of up to 88% in the rhodium(I)-catalysed hydrosilylation of ketones. This compound was also employed in the asymmetric transfer hydrogenation of ketones and in stoichiometric asymmetric syntheses like selenoxide eliminations,6 [2,3]sigmatropic rearrangements7 and intramolecular selenocyclisations.8 Wirth et al.9 demonstrated that 2 and related compounds are highly effective ligands in the asymmetric addition of diethylzinc to aromatic and aliphatic aldehydes.10 With only 1 mol% of catalyst enantioselectivities of up to 98% were achieved for a wide range of substrates.
Recently, we developed a new catalytic system for the asymmetric addition of dialkylzinc reagents to aldehydes11 based on 2-ferrocenyloxazoline, 3, which is capable of inducing enantioselectivities up to 97% ee.12 Even higher ee values were obtained in the analogous phenyl transfer reaction with a zinc reagent prepared in situ from diphenylzinc and di ethylzinc,12,13 leading to synthetically useful enantioenriched diarylmethanols with up to 98% ee.14–16
Encouraged by the fact that diselenide 2 displayed excellent selectivities in the alkylations, we assumed that compound 4 would also be useful for the asymmetric addition of diorganozinc reagents to aldehydes. Herein, we present the application of 4 as catalyst precursor in these reactions.
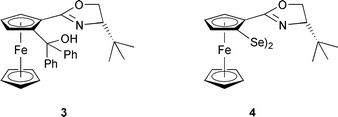
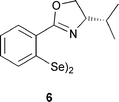
The synthesis of 4 was accomplished by directed ortho-lithiation17 of (S)-2-ferrocenyl-4-tert-butyloxazoline 5,18 followed by addition of selenium powder (Scheme 1). Oxidation by air for several minutes afforded the diselenide in good yield (69%).
 | ||
| Scheme 1 Synthesis of ligand 4 | ||
Initially, 4 was used in the addition of diethylzinc to aromatic and aliphatic aldehydes. The most significant results are summarised in Table 1. Although the results in the diethylzinc addition to aldehydes are highly unsatisfying (eemax = 44% in the reaction with benzaldehyde), it is interesting to note that a structurally very similar compound, phenyloxazolinyl diselenide 6,9 devoid of the metal fragment, gives 1-phenylpropanol with only 8% ee in 1% yield.19 The element of planar chirality present in 4 seems to have a decisive influence on both the enantioselectivity and yield of the reaction.20
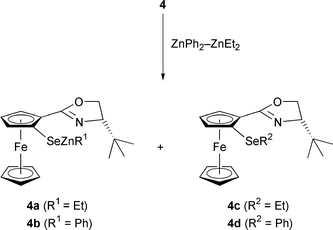
Superior results were obtained in the asymmetric aryl transfer to aldehydes using a zinc reagent prepared by mixing diphenylzinc and diethylzinc in a 1:2 ratio. Compared to the addition of dialkylzincs, this reaction gave ee's of up to 85% and uniformly high yields between 65 and 96% (Table 2). Aliphatic aldehydes were less suitable substrates, resulting in an ena ntiomeric excess of only 65% for 2,2-dimethyl-1-phenylpropanol. The catalytically active species is believed to be formed by heterolytic cleavage of the Se–Se bond of diselenide 4 (Scheme 2).9
|
|
||||
|---|---|---|---|---|
| Entry | R | Yield/% | ee/% | Abs. config. |
| a Tentatively assigned by assumption of an identical reaction pathway. | ||||
| 1 | 4-Chlorophenyl | 85 | 84 | R |
| 2 | 2-Naphthyl | 96 | 76 | R |
| 3 | 4-Biphenyl | 86 | 85 | R a |
| 4 | 2-Bromophenyl | 65 | 77 | R |
| 5 | 4-Tolyl | 80 | 76 | R a |
| 6 | tert-Butyl | 85 | 65 | S |
 | ||
| Scheme 2 Cleavage of the Se–Se bond in 4 | ||
Most likely, zinc selenides such as 4a or 4b serve as catalysts. Based on the results by Wirth et al.9 who demonstrated the inferiority of selenoethers in diethylzinc additions as concerns the rate and enantioselectivity compared to selenols, we believe that 4c and 4d play only a minor role in the stereochemical outcome of the catalysis.
In summary we have introduced a new catalyst for asymmetric addition reactions, capable of inducing moderate to high ee's with a zinc reagent prepared by mixing diethyl- and diphenylzinc. Further studies concerning the influence of the chalcogenide atom and the mechanistic details of the reaction are currently in progress.
Experimental
All manipulations except workup and purification were conducted under an inert atmosphere of Ar using standard Schlenk techniques. s-BuLi was purchased from Fluka as a 1.3 N solution in hexane. Diphenylzinc was obtained from Strem and handled in a glovebox under Ar. Tetrahydrofuran and toluene were distilled from sodium/benzophenone ketyl radical prior to use. 1H- and 13C-NMR spectra were recorded on a Varian Gemini 300 spectrometer at 300 and 75 MHz, respectively, in CDCl3, chemical shifts are given in ppm. All expe riments were conducted at least twice to ensure reproducibility.Preparation of compound 4
A solution of 5 (700 mg, 2.25 mmol) in 15 ml of THF is cooled to − 78°C and treated with s-BuLi (1.9 ml, 2.48 mmol). The resulting dark red solution is stirred for 30 min at this temperature, and then selenium powder (216 mg, 2.70 mmol) is added in one portion. The mixture is stirred for a further 10 min at − 78°C and subsequently warmed to room temperature within 15 min. After quenching with 30 ml of deionised water and extraction with dichloromethane (3 × 20 ml), air is bubbled through the combined organic layers for 5 min. The solution is dried over MgSO4 and filtered. The solvent is evaporated and the product purified by column chromatography (pentane–diethyl ether, 2:1) to afford 600 mg of 4 as an orange solid (69% yield). Mp: 192°C (dec.). [α]D = − 1270 [c = 1.0, CHCl3]. 1H NMR: δ 1.05 (s, 18H), 3.99 (dd, J = 9.9 Hz, 7.4 Hz, 2H), 4.16 (s, 10H), 4.22–4.29 (m, 6H), 4.65–4.68 (m, 4H). 13C NMR: δ = 25.9, 33.6, 69.2, 69.4, 70.2, 71.6, 72.0, 73.7, 76.7, 79.7, 165.2. MS (EI, 70 eV): m/z (%) 780.3 (7, M+), 713.2 (6), 511.2 (16), 390.2 (100), 309.3 (35), 254.3 (38). IR (KBr): ν = 3435, 3108, 2956, 2186, 1655 cm−1. Anal. calcd. for C34H40Fe2N2O2Se2: C, 52.47%; H, 5.18%; N, 3.60%; Found: C, 52.34%; H, 4.84%; N, 3.46%.General procedure for the addition of diethylzinc to aldehydes
A solution of 4 (20 mg, 0.025 mmol) in toluene (2 ml) is treated with diethylzinc (0.25 ml, 2.5 mmol) at room temperature. After stirring for 30 min the dark orange solution is cooled to 0°C and the aldehyde (1 mmol) is added neat in one portion. Stirring is continued at this temperature for 24 h, then the mixture is quenched by addition of a saturated aqueous solution of ammonium chloride (5 ml) and extracted with diethyl ether (3 × 10 ml). The collected organic layers are dried over MgSO4 and the solvent is evaporated. The crude product is purified by column chromatography (pentane–diethyl ether) and subjected to HPLC or GLC analysis.General procedure for the enantioselective phenyl transfer to aldehydes
In a glovebox a well-dried Schlenk flask is charged with diphenylzinc (36 mg, 0.16 mmol). The flask is sealed and removed from the glovebox. Freshly distilled toluene (3 ml) is added followed by diethylzinc (33 μl, 0.33 mmol). After stirring at room temperature for 30 min ferrocene 4 (10 mg, 0.013 mmol) is added, and then the resulting clear solution is cooled to 10°C. Stirring is continued for an additional 10 min at this temperature; the aldehyde (0.25 mmol) is then added neat in one portion. The Schlenk flask is sealed and the reaction mixture stirred at 10°C overnight. Quenching with water is followed by extraction of the mixture with diethyl ether. The combined organic layers are dried over MgSO4 and the solvent is evaporated. The product is purified by column chromatography using silica gel (pentane–diethyl ether).HPLC analysis: 1-phenyl-1-propanol [Chiralcel OD-H, heptane–PriOH = 96:4, 0.5 ml min−1, (R): 16.7, (S): 18.7 min]; 1-(4-chlorophenyl)-1-propanol [Chiralcel OD, heptane–PriOH = 97:3, 0.5 ml min−1, (S): 23.0, (R): 24.8 min]; α-(4-chlorophenyl)phenylmethanol [Chiralcel OB, heptane–PriOH = 80:20, 0.8 ml min−1, (R): 8.8, (S): 13.3 min]; α-(2-naphthyl)phenylmethanol [Chiralcel OD, heptane–PriOH = 90:10, 0.8 ml min−1, (S): 20.4, (R): 23.7 min]; α-(4-biphenyl)phenylmethanol [Chiralcel OD, heptane–PriOH = 98:2, 1.0 ml min−1, (R): 65.9, (S): 75.5 min]; α-(2-bromophenyl)phenylmethanol [Chiralcel OD, heptane–PriOH = 90:10, 0.9 ml min−1, (R): 11.6, (S): 14.9 min]; α-(4-tolyl)phenylmethanol [Chiralcel OB, heptane–PriOH = 98:2, 0.9 ml min−1, (S): 35.8, (R): 38.5 min]; 2,2-dimethyl-1-pheny lpropanol [Chiralcel OD, heptane–PriOH = 98: 2, 1.0 ml min−1, major: 11.0, minor: 16.7 min].
GLC analysis: 3-nonanol as trifluoroacetate derivative (Lipodex G; 50 m × 0.25 mm, 40–170°C, minor: 93.5, major: 95.1 min).
Acknowledgements
We are grateful to the Deutsche Forschungsgemeinschaft (DFG) within the Collaborative Research Center (SFB) 380 ‘ Asymmetric Synthesis by Chemical and Biological Methods ’ and the Fonds der Chemischen Industrie for financial support. M. K. acknowledges DFG for a predoctoral fellowship (Graduiertenkolleg), and we thank Witco and Degussa-Hüls for donations of chemicals. We also thank Dr. J. Blacker (Avecia) for pointing out reference 13 to us, and I. Schiffers for performing GLC analyses.References
-
(a)
C. Paulmier, Selenium Reagents
and Intermediates in Organic Synthesis, ed. J. E. Baldwin,
Pergamon, Oxford, UK, 1986 Search PubMed;
(b)
Organoselenium Chemistry: Modern De
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) elopments
in Organic Synthesis, ed. T. Wirth, Top. Curr. Chem.,
2000, 208, Springer, Berlin, Germany. Search PubMed.
elopments
in Organic Synthesis, ed. T. Wirth, Top. Curr. Chem.,
2000, 208, Springer, Berlin, Germany. Search PubMed. - For early contributions, see:
(a) J. L. Huguet, Ad
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) .
Chem. Ser., 1967, 76, 345 Search PubMed;
(b) D. N. Jones, D. Mundy and R. D. Whitehouse, Chem. Commun., 1970, 86 RSCFor a recent review, see:
(c) Y. Nishibayashi and S. Uemura, Top.
Curr. Chem., 2000, 208, 201 Search PubMed.
.
Chem. Ser., 1967, 76, 345 Search PubMed;
(b) D. N. Jones, D. Mundy and R. D. Whitehouse, Chem. Commun., 1970, 86 RSCFor a recent review, see:
(c) Y. Nishibayashi and S. Uemura, Top.
Curr. Chem., 2000, 208, 201 Search PubMed. - For recent reviews on chiral selenium compounds, see: (a) T. Wirth, Tetrahedron, 1999, 55, 1 CrossRef CASWith a special focus on ligands and catalysts: (b) Y. Nishibayashi and S. Uemura, Top. Curr. Chem., 2000, 208, 235 Search PubMed.
-
(a) Y. Nishibayashi, K. Segawa, J. D. Singh, S. Fukuzawa, K. Ohe and S. Uemura, Organometallics, 1996, 15, 370 CrossRef CAS;
(b) Y. Nishibayashi and S. Uemura, Re
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) . Heteroatom Chem., 1996, 14, 83 Search PubMed.
. Heteroatom Chem., 1996, 14, 83 Search PubMed. - D. Marquarding, H. Klusacek, G. Gokel, P. Hoffmann and I. Ugi, J. Am. Chem. Soc., 1970, 92, 5389 CrossRef CAS.
- Y. Nishibayashi, J. D. Singh and S. Uemura, Tetrahedron Lett., 1994, 35, 3115 CrossRef CAS.
- Y. Nishibayashi, J. D. Singh, S. Fukuzawa and S. Uemura, J. Org. Chem., 1995, 60, 4114 CrossRef CAS.
- H. Takada, Y. Nishibayashi and S. Uemura, J. Chem. Soc., Perkin Trans. 1, 1999, 1511 RSC.
-
(a) T. Wirth, Tetrahedron Lett, 1995, 36, 7849 CrossRef CAS;
(b) T. Wirth, K. J. Kulicke and G. Fragale, Hel
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) . Chim. Acta, 1996, 79, 1957 Search PubMed;
(c) C. Santi and T. Wirth, Tetrahedron: Asymmetry, 1999, 10, 1019 CrossRef CAS.
. Chim. Acta, 1996, 79, 1957 Search PubMed;
(c) C. Santi and T. Wirth, Tetrahedron: Asymmetry, 1999, 10, 1019 CrossRef CAS. - Reviews on diorganozinc additions
to aldehydes:
(a) K. Soai and S. Niwa, Chem. Re
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ., 1992, 92, 833 Search PubMed;
(b) R. Noyori and M. Kitamura, Angew. Chem., Int. Ed. Engl., 1991, 30, 49 CrossRef;
(c)
K. Soai and
T. Shibata, Comprehensi
., 1992, 92, 833 Search PubMed;
(b) R. Noyori and M. Kitamura, Angew. Chem., Int. Ed. Engl., 1991, 30, 49 CrossRef;
(c)
K. Soai and
T. Shibata, Comprehensi![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) e Asymmetric Catalysis, eds. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer,
Berlin, Germany, 1999, vol. 2, p. 911. Search PubMed.
e Asymmetric Catalysis, eds. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer,
Berlin, Germany, 1999, vol. 2, p. 911. Search PubMed. - (a) C. Bolm, K. Muñiz–Fernandez, A. Seger and G. Raabe, Synlett, 1997, 1051 CAS; (b) C. Bolm, K. Muñiz–Fernandez, A. Seger, G. Raabe and K. Günther, J. Org. Chem., 1998, 63, 7860 CrossRef CAS; (c) C. Bolm, K. Muñiz and J. P. Hildebrand, Org. Lett., 1999, 1, 491 CrossRef CAS.
- (a) C. Bolm and K. Muñiz, Chem. Commun., 1999, 1295 RSC; (b) C. Bolm, N. Hermanns, J. P. Hildebrand and K. Muñiz, Angew, Chem., in press. Search PubMed.
- Use of a combination of ZnPh2 and ZnMe2 in the addition to aldehydes has been described before. For example, aryl transfer to nicotinaldehyde catalysed by N,N-diethylnorephedrine results in phenyl transfer with 20:1 selectivity to give 3-phenylpyridylmethanol in 70% ee. J. Blacker, in Third International Conference on the Scale Up of Chemical Processes (Conference Proceedings), ed. T. Laird, Scientific Update, UK, 1998, p. 74. Search PubMed.
- For other catalytic systems for the asymmetric addition of diphenylzinc to aldehydes see: (a) E. I. Dosa, J. C. Ruble and G. C. Fu, J. Org. Chem., 1997, 62, 444 CrossRef CAS; (b) W.-S. Huang, Q.-S. Hu and L. Pu, J. Org. Chem., 1999, 64, 7940 CrossRef CAS; (c) W.-S. Huang and L. Pu, Tetrahedron Lett., 2000, 41, 145 CrossRef CAS.
- For reports of additions to aldehydes employing in situ-generated ZnPh2 from ZnCl2 and a Grignard precursor, see:
(a) enantioselective: K. Soai, Y. Kawase
and A. Oshio, J. Chem. Soc., Perkin Trans. 1, 1991,
1613 Search PubMed;
(b) diastereoselective: J. Hübscher
and R. Barner, Hel
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) . Chim. Acta, 1990, 73, 1068. It appears as if these catalyses do
not involve a defined ZnPh2 species [compare also discussion of this aspect in ref. 10(a)] Search PubMed.
. Chim. Acta, 1990, 73, 1068. It appears as if these catalyses do
not involve a defined ZnPh2 species [compare also discussion of this aspect in ref. 10(a)] Search PubMed. - Enantiomerically enriched diarylmethanols can also be synthesised by asymmetric reduction of unsymmetrical diaryl ketones. However, for achieving high enantioselectivities, either ortho-substitution of one of the aryl groups or electronically very different aryls are required. For leading references see: (a) E. J. Corey and C. J. Helal, Tetrahedron Lett., 1995, 36, 9153 CrossRef CAS; (b) E. J. Corey and C. J. Helal, Tetrahedron Lett., 1996, 37, 4837 CrossRef CAS; (c) E. J. Corey and C. J. Helal, Tetrahedron Lett., 1996, 37, 5675 CrossRef CAS; (d) T. Ohkuma, M. Koizumi, H. Ikehira, T. Yokozawa and R. Noyori, Org. Lett., 2000, 2, 659 CrossRef CAS.
- V. Snieckus, Chem. Re
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ., 1990, 90, 879 Search PubMed.
., 1990, 90, 879 Search PubMed. - For early studies on the diastereoselectivity of this deprotonation reaction, see: (a) C. J. Richards, T. Damalidis, D. E. Hibbs and M. B. Hursthouse, Synlett, 1995, 74 CrossRef CAS; (b) C. J. Richards and A. W. Mulvaney, Tetrahedron: Asymmetry, 1996, 7, 1419 CrossRef CAS; (c) Y. Nishibayashi and S. Uemura, Synlett, 1995, 79 CrossRef CAS; (d) Y. Nishibayashi, K. Segawa, Y. Arikawa, K. Ohe, M. Hidai and S. Uemura, J. Organomet. Chem., 1997, 545–546, 381 CrossRef CAS; (e) T. Sammakia, H. A. Latham and D. R. Schaad, J. Org. Chem., 1995, 60, 10 CrossRef CAS; (f) T. Sammakia and H. A. Latham, J. Org. Chem., 1995, 60, 6002 CrossRef CAS.
- Diselenide 4 was further tested in dimethylzinc additions to aldehydes. Unfortunately, the selectivities and yields were even lower than those in reactions with diethylzinc..
- For an investigation of this phenomenon see ref. 11(b) and K. Muñiz and C. Bolm, Chem. Eur. J., 2000, 6, 2309 CrossRef.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2001 |