The investigation of an equilibrium dependent reaction for the formation of enamines in a microchemical system
Mike
Sands
a,
Stephen J.
Haswell
*a,
Stephen M.
Kelly
a,
Victoria
Skelton
a,
David O.
Morgan
b,
Peter
Styring
c and
Brian
Warrington
d
aDepartment of Chemistry, Faculty of Science and the Environment, University of Hull, Cottingham Road, Hull, UK HU6 7RX. E-mail: s.j.haswell@chem.hull.ac.uk; Fax: +44 (0) 1482 466416; Tel: +44 (0) 1482 465475
bGlaxoSmithkline, Old Powder Mills, Tonbridge, Nr Leigh, Kent, UK TN11 9AN
cDepartment of Chemical and Process Engineering, The University of Sheffield, Mappin Street, Sheffield, UK S1 3JD
dGlaxoSmithkline, New Frontiers Science Park, Third Avenue, Harlow, Essex, UK CM19 5AW
First published on 9th August 2001
Abstract
The paper describes the equilibrium dependant reaction for the formation of enamines in a microchemical system utilising electroosmotic flow (EOF) for fluid mobilisation. The authors have shown that the reaction can be carried out without the presence of a Lewis acid catalyst, in addition the enamine intermediate was synthesised at room temperature using mild solvent conditions. A 42% conversion of cyclohexanone into the enamine has been achieved to date.
Introduction
The paper describes the equilibrium dependent reaction for the formation of enamines in a microchemical system utilising electroosmotic flow (EOF) for fluid mobilisation. The authors have shown that the reaction can be carried out without the presence of a Lewis acid catalyst, in addition the enamine intermediate was synthesised at room temperature using mild solvent conditions. The largest conversion of cyclohexanone into the enamine achieved has been 42%.To date microreactors have been used to demonstrate a wide range of different chemistries. 1–3 The benefits of using microreactors for synthetic applications has been clearly demonstrated using the Wittig reaction4 where not only the reaction yields were increased but also stereoselective control was possible. In this paper we intend to evaluate the Stork-enamine reaction in a microreactor for the synthesis of enamines.
The enamine reaction was first proposed by Stork et al. in 19545 and allows the addition of an electrophile alpha to a carbonyl substituent. This carbon–carbon bond forming reaction can be used extensively over a wide range of chemistries. This presents an interesting challenge when performed in a microreactor due to the need for water removal from the reaction mixture to enable the enamine to be formed.
In order for equilibrium reactions to proceed, the removal of water can be achieved in one of two ways. Firstly, by the physical removal of water using molecular sieves, however this would introduce complications as molecular sieves may possibly disrupt EOF. In addition it would also cause problems with device design because of the need for immobilisation within the reaction channels. Alternatively chemical removal using a homogeneous drying agent can be achieved by a relatively simple operation to introduce it into a microreactor device. Thus the addition of 1,3-dicyclohexylcarbodiimide (DCC) to the reaction mixture to remove water has been investigated (see Scheme 1).
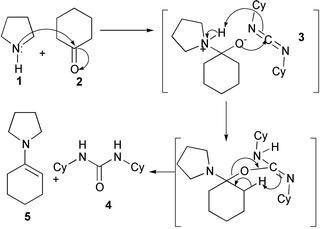 | ||
| Scheme 1 General reaction scheme for the enamine formation using DCC. | ||
Experimental
The reaction was carried out using a T-chip fabricated in borosilicate glass with channels produced by photolithography and wet etching.6A top plate of 17 mm borosilicate glass which included 3 mm pre-drilled holes was annealed at a temperature of 680 °C. Microporous silica structures were added into the annealed chip to reduce hydrodynamic effects.7 The final channel geometries were 200 µm wide at the top and 100 µm deep, with the overall outer dimensions of 20 mm × 20 mm and 25 mm in depth. EOF was generated and controlled via a computer/Labview interface developed by Kingfield electronics, Sheffield. An example of the chip used can be seen in Fig. 1.
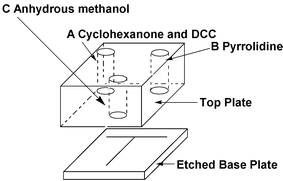 | ||
| Fig. 1 Schematic of the ‘T’ microreactor used for the synthesis of the enamine intermediate. Positive voltages were applied to platinum wire electrodes placed reservoirs A & B relative to a ground electrode being placed in reservoir C. | ||
All of the chemicals were purchased from Aldrich Fine Chemicals (Gillingham, Dorset). A solution of cyclohexanone (0.3 M, 50 µl) in anhydrous methanol with added DCC (approx 1 mg) was introduced into reservoir A. Pyrrolidine (0.3 M, 50 µl) in anhydrous methanol was added into reservoir B, whilst anhydrous methanol (30 µl) was added to reservoir C. Ranges and combinations of positive voltages were applied to the platinum wire electrodes placed in reservoirs A and B relative to the ground electrode situated in reservoir C. The variety of voltages applied was in the region from 300 to 1000 V.
The voltages were applied for a period of 40 min after which the content of reservoir C was analysed by gas chromatography coupled to a mass spectrometer (GC-MS). The GC-MS used was a Varian CP-3800 coupled to a Varian Saturn 2000 mass spectrometer. The column was a CP-Sil 8 (30 m capillary column). The following analysis conditions were employed: injector temperature 250 °C, helium flow rate 1ml min−1, oven temperature 50 °C held for 4 min, ramping to 250 °C over a period of 8 min and holding this temperature for 3.5 min. The use of GC-MS allowed the mass ion of m/z 151 to be identified at a retention time of 9.11 min. The retention time for the pure material had already been determined from batch reactions under similar conditions.
Results and discussion
Fig. 2 summarises the enamine yields obtained in the microreactor over a range of voltage combinations. Using an applied external voltage combination of 600 V at reservoir A (cyclohexanone) and 800 V at reservoir B (pyrrolidine) the largest yield of product obtained, based upon the percentage conversion of cyclohexanone to enamine, was 42% at room temperature, for which no catalyst was used. The yield obtained in the microreactor was comparable to that obtained using a batch Stork-enamine reaction performed using cyclohexanone, pyrrolidine and p-toluene sulfonic acid in methanol under Dean & Stark conditions.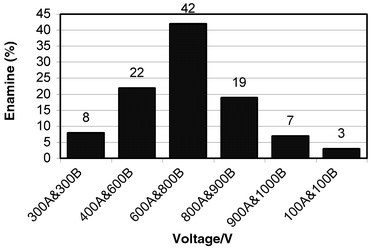 | ||
| Fig. 2 The percentage conversion of enamine for a range of voltages. The letter after the value denotes the reservoir in which the voltage was applied. | ||
The results presented represent preliminary findings, which demonstrates that enamine chemistry can be carried out in a mild solvent system at room temperature and in the absence of a Lewis acid catalyst in a microreactor. Product yields so far produced in the microreactor are comparable to those obtained by the bulk reaction, but the chip reaction negates the need for Dean and Stark conditions. It is also carried out in the absence of a Lewis acid catalyst utilising a different solvent system and work is continuing to fully optimise the reaction. Once the reaction conditions have been optimised, the next stage will be to investigate the reaction using a wide range of suitable electrophiles. The initial product will then be hydrolysed back into the ketone. Once this part of the reaction has been optimised, some asymmetric synthesis can be carried out to investigate enantioselectivity in microchemical devices.
Acknowledgements
We thank the EPSRC and GlaxoSmithkline for financial support to MS in the form of a CASE studentship.References
- R. D. Chambers and R. C. H. Spink, Chem. Commun., 1993, 883 Search PubMed.
- G. Veser, G. Fredrick, M. Freygan and R. Zengerle, Reaction Kinetics and the Development of Catalytic Processes, ed. G. F. Froment and K. C. Waugh, Elsevier Science, Amsterdam, 1999 Search PubMed.
- S. J. Haswell, R. J. Middleton, B. O’Sullivan, V. Skelton, P. Watts and P. Styring, Chem. Commun., 2001, 391 RSC .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This references the work, carried out to date, that has been performed using microreactors.
This references the work, carried out to date, that has been performed using microreactors. - V. Skelton, G. M. Greenway, S. J. Haswell, P. Styring, D. O. Morgan, B. H. Warrington and S. Y. F. Wong, Analyst, 2001, 126, 11 RSC .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This was the first paper to describe the use of microreactors to carry out specific solution based chemistries..
This was the first paper to describe the use of microreactors to carry out specific solution based chemistries.. - G. Stork, R. Terrell and J. Szmuszkovicz, J. Am. Chem. Soc., 1954, 76, 2029 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) The first paper where the Stork-enamine reaction was described.
The first paper where the Stork-enamine reaction was described. - T. McCreedy, Anal. Chim. Acta, 2001, 427, 39 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper describes
the fabrication and manufacture of devices that can support EOF.
This paper describes
the fabrication and manufacture of devices that can support EOF. - P. D. Christensen, S. W. P. Johnson, T. McCreedy, V. Skelton and N. G. Wilson, Anal. Commun., 1998, 35, 341 RSC .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper describes the fabrication and manufacture of devices that can support EOF.
This paper describes the fabrication and manufacture of devices that can support EOF.
| This journal is © The Royal Society of Chemistry 2001 |
