A novel Suzuki reaction system based on a supported palladium catalyst
Egid B. Mubofu, James H. Clark* and Duncan J. Macquarrie
Clean Technology Centre, Department of Chemistry, University of York, York, UK YO10 5DD. E-mail: jhc1@york.ac.uk
First published on 18th January 2001
Abstract
A range of supported palladium complex-catalysed Suzuki reactions is described with notable features including fast and efficient reactions, excellent catalyst recyclability, and total catalyst stability under the reaction conditions. We have achieved turnover numbers of several thousand based on ten re-use experiments from batch reactions in air. Our system not only solves the basic problems of catalyst separation and recovery but also avoids the use of phosphine ligands.
Green ContextThese Suzuki reactions combine the efficiency and high turnover number typical of palladium catalysis, with the recoverability and reusability offered by heterogeneous systems, to give high yields in short times without the need for phosphine ligands.ST |
Introduction
The Suzuki reaction is proving to be an increasingly popular method for forming carbon–carbon bonds both in the laboratory and in commercial manufacturing; it has quickly become an integral part of modern organic synthesis.1,2 The reaction represents an attractive alternative over other methods using organometallics because organoboranes are air- and moisture-stable with relatively low toxicity.1 However, like many organic reactions using inorganic reagents and catalysts, the standard procedures suffer from wasted inorganics which are too difficult to recover and are lost in an aqueous work-up stage.3 For Suzuki reactions soluble complex palladium catalysts are normally employed alongside bases such as soluble amines; these are rarely recoverable without elaborate and wasteful procedures that are commercially unacceptable. Some progress has been made with the use of solid reagents, for example the combination of alumina-supported fluorides and palladium powder has been recently shown to be effective in the Suzuki coupling of phenyl boronic acids with iodobenzenes.4,5 However, the reaction is much less effective with bromobenzenes and there is no evidence for the recyclability of the palladium, an essential aspect of the process on environmental and economic grounds. The use of an efficient heterogeneously supported palladium catalyst (Pd/C) in Suzuki cross-coupling has been used to produce biphenylacetic acid.6 In other reported cases supported palladium catalysts have suffered on application from diffusion limitations and dissolution of the palladium complex in the reaction medium. Here we describe an entirely novel heterogeneous palladium-catalysed Suzuki reaction system that is very effective with the less expensive bromobenzenes, uses only hydrocarbon solvents, and requires very small amounts of the solid palladium catalysts which are entirely recoverable and reusable; there is no contribution to the reaction due to homogeneous catalysis from any leached metal complex. One salient feature of our catalyst is that it does not require addition of phosphines (commonly introduced into palladium-catalysed reactions) thus improving the atom economy of the reaction. While reducing process costs, it also eliminates side reactions that may occur between arylphosphines and arylboronic acid.7 The overall outcome of these improvements may lead to cost effective industrial processes and to the reduction of unwanted wastes.Results and discussion
The organically modified silica (Merck Kieselgel 100) and micelle templated silicas (MTS) materials used for preparing the catalysts were prepared by a one-pot method.8 They were studied for the presence and stability of bound organics by diffuse reflectance infrared spectroscopy (DRIFTS) and simultaneous thermal analysis (STA). The DRIFTS of 3-aminopropyl-MTS for example, displays the characteristic C–H2 stretching bands at 2938 and 2867 cm−1 and aliphatic CH2 deformation bands at 1471 and 1439 cm−1. The IR spectrum of the chemically modified 3-aminopropyl-MTS and 3-aminopropyl-silica prior to complexation with the metal had a peak at 1647 cm−1 due to the C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N stretching vibration of the imine. The peak disappears on
complexation with palladium, changing into bands at 1525 and 1593
cm−1 consistent with strong binding between the metal and
the ligand. Simultaneous thermal analysis shows a loss of residual solvent
at ca. 80 °C followed by a gradual minor weight loss of <
1% up to ca. 505 °C where a sudden major loss (ca.
10%) is observed. The major weight loss at such high temperatures in the
MTS material is characteristic of chemisorbed material and shows that the
3-aminopropyl is chemically bound. The thermal stability of the organically
modifed Kieselgel 100 is less, with decomposition occuring between 205 and
385 °C.
N stretching vibration of the imine. The peak disappears on
complexation with palladium, changing into bands at 1525 and 1593
cm−1 consistent with strong binding between the metal and
the ligand. Simultaneous thermal analysis shows a loss of residual solvent
at ca. 80 °C followed by a gradual minor weight loss of <
1% up to ca. 505 °C where a sudden major loss (ca.
10%) is observed. The major weight loss at such high temperatures in the
MTS material is characteristic of chemisorbed material and shows that the
3-aminopropyl is chemically bound. The thermal stability of the organically
modifed Kieselgel 100 is less, with decomposition occuring between 205 and
385 °C.The palladium catalysts bound on these materials have been tested in the Suzuki reaction between phenylboronic acid and bromobenzene in xylene in the presence of potassium carbonate as the base. Catalysts based on the solid carboxylic acids9 [TMS–(CH2)nCO2H leading to catalyst 1] are active but quickly lose activity (turnover number ArBr/Pd = ca. 100) although washing the catalyst after use (with dichloromethane and then water) restored some of the original activity, giving up to 76% yields (Table 1, cycle 3). Catalysts 2 and 3 are based on reaction of aminopropyl–TMS (leading to catalyst 2) and aminopropylsilica (leading to catalyst 3) with pyridinecarbaldehyde,10 followed by complexation of palladium acetate (Fig. 1) are more active and have excellent recyclability (Table 1). With these catalysts, we have achieved turnover numbers of several thousand based on ten filtrations and reuse experiments from batch reactions. The other advantage of our systems, compared to work up procedure,1a is that high yields of the biaryls are obtained in air at the lower temperature of 95 °C, at shorter times and without using phosphine ligands.
| Catalyst cycleb | Catalyst | t/min | GC Yieldb (%)c |
|---|---|---|---|
| a The reactions were carried out in xylene (13 ml) with 5 mmol of bromobenzene, 7.3 mmol of phenylboronic acid, 10 mmol of K2CO3 base and 0.2 g palladium catalyst (loading 0.3 mmol g−1) at 95 °C.b The used catalysts were thoroughly washed with dichloromethane then water before reuse.c GC yields obtained using n-dodecane as an internal standard and is based on the amount of haloarene employed in relation to authentic standard biphenyl. | |||
| 1 | 1 | 60 | 75 |
| 2 | 1 | 180 | 4.4 |
| 3 | 1 | 180 | 76 |
| 4 | 1 | 180 | 25 |
| 1 | 2 | 60 | 75 |
| 2 | 2 | 60 | 98 |
| 3 | 2 | 60 | 97 |
| 4 | 2 | 60 | 98 |
| 5 | 2 | 60 | 96 |
| 6 | 2 | 60 | 45 |
| 1 | 3 | 60 | 90 |
| 2 | 3 | 60 | 98 |
| 3 | 3 | 60 | 97 |
| 4 | 3 | 60 | 96 |
| 5 | 3 | 60 | 95 |
| 6 | 3 | 60 | 85 |
| 7 | 3 | 60 | 64 |
| 8 | 3 | 60 | 70 |
| 9 | 3 | 60 (120) | 36 (44) |
| 10 | 3 | 60 (180) | 17 (20) |
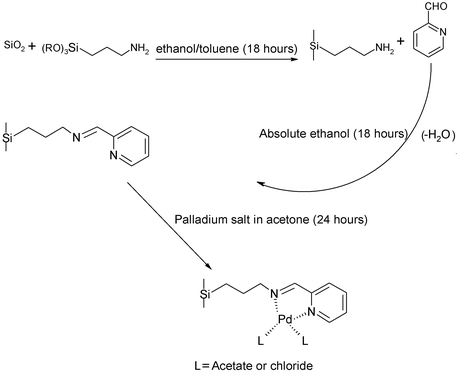 | ||
| Fig. 1 Preparation of the active and reusable supported palladium catalysts (catalysts 2 and 3) based on chemically modified aminopropyl mesoporous silicas. | ||
The stability of the supported complex towards dissolution during the reaction is a problem of great concern for most anchored catalysts.11 In view of the leaching problems observed with palladium supported on polymer beads,12 quantitative analysis using atomic absorption spectroscopy (AAS) was employed to determine the amount of metal in the final reaction solution. We also performed the hot filtration test.13 No palladium could be detected in the liquid reaction mixtures by AAS and more significantly, hot filtration of the reaction mixture at an early stage of the reaction followed by taking the mixture back to reaction temperature completely stopped the reaction, effectively proving heterogeneous catalysis and no measurable contribution from homogeneous catalysis (Fig. 2).13 Very good to excellent yields of biphenyl in short reaction times for reactions run using catalysts 2 and 3 were achieved with the latter having the better lifetime. The study was then extended to substituted bromobenzenes involving electronically activating and deactivating groups. A favourable effect of electron-withdrawing substituents is normally observed in palladium catalysed reactions.14 With our catalysts however, electron withdrawing groups have relatively little effect on the reaction rate or selectivity (Table 2). Substituted chlorobenzenes are inert under the standard conditions giving a cross-coupling product (<2% yield) and a 4% yield of biphenyl from homocoupling of the phenylboronic acid. To further illustrate that chloroarenes are inert in the reaction system, p-chlorobiphenyl was selectivity produced in the reaction of phenylboronic acid with p-bromochlorobenzene (Table 2, entries 6, 14). The importance of the base is illustrated by the lack of desired cross-coupling product when the reaction is run in the absence of base.
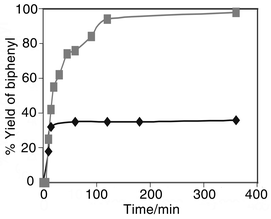 | ||
| Fig. 2 Effect of removing the supported palladium catalyst from the reaction of phenylboronic acid and bromobenzene (hot filtration test, see text). | ||

Experimental
Chemicals were obtained from Aldrich and Merck and were used as received. The detailed preparation schemes of the catalysts have already been published.9,10 In this instance however, the organically modified MTS was prepared by a one-pot synthesis method8 using a long-chain neutral templating amine (5.10 g n-dodecylamine in this case) in aqueous ethanol (46 ml ethanol–53 ml water). To this solution, 8.36 g (40 mmol) of tetraethoxysilane (TEOS) and 1.79 g (10 mmol) of 3-aminopropyl(trimethoxy)silane were separately but simultaneously added and stirred together at room temperature for 18 h. After this solution, the mixture is initially turbid, then turns milky after about 10 min and finally forms a thick white paste after 18 h. The thick white paste was filtered off and the template was extracted from the white solid by Soxhlet extraction with ethanol for 10 h. The template-extracted white 3-aminopropyl-MTS solid was dried in air at 90 °C overnight before further reactions appropriate for metal complex anchoring were carried out. The dried 3-aminopropyl-MTS (or silica) was reacted with an equivalent of 2-pyridinecarbaldehyde in ethanol at room temperature. The resulting modified material was dried overnight in air at 90 °C before reacting with a solution of palladium acetate in acetone. The mixture was magnetically strirred for 24 h, and the catalyst paste was filtered off and washed thoroughly with acetone until the washings were colourless. The catalyst was dried overnight in air at 90 °C to result into a brown palladium supported catalyst. The catalyst was conditioned for a total of 27 h by refluxing in ethanol, toluene and then acetonitrile before being tested for activity. The condition was done to remove any physisorbed palladium. The final conditioned catalyst was filtered off and dried overnight in air at 90 °C to be ready for use.The IR spectra of the final materials were measured on a Bruker Equinox 55 spectrometer fitted with an environmental chamber diffuse reflectance unit. Simultaneous thermal analysis (thermal analysis coupled with differential thermal analysis, STA) was carried out to determine the thermal stability of the bound organics. The pore size distribution and BET surface areas were determined by using Coulter SA2100 porosimeter with dinitrogen as an adsorbate.
A variety of silica supported palladium catalysts were evaluated in the Suzuki cross-coupling reactions. In a typical reaction, phenylboronic acid (914 mg, 7.3 mmol), bromobenzene (785 mg, 5 mmol), potassium carbonate (1381 mg, 10 mmol), dodecane (1 ml, internal standard) and the supported catalyst (0.2 g at 0.3 mmol Pd per gram) were stirred together in o-xylene (or m-xylene) (13 ml) at 95 °C. Samples were withdrawn periodically and analysed by GC/GC–MS. The isolated products were analysed by GC/GC–MS and NMR and compared with authentic samples.
Conclusion
These new supported palladium catalysts show remarkable versatility as they are capable of catalysing Suzuki and Heck reactions to high yields of coupling products at lower temperatures than conventional systems in air. Further catalyst reuse studies on other Suzuki cross-coupling reactions are being investigated.Acknowledgements
The financial support of the Norwegian Agency for International Development (NORAD) through the University of Dar es Salaam, Tanzania (to E. B. M.) is gratefully acknowledged. We also thank the Royal Academy of Engineering and EPSRC for a Clean Technology Fellowship (to J. H. C.) and the Royal Society for a University Research Fellowship (to D. J. M.).References
- (a) N. Miyaura, T. Yanagi and A. Suzuki, Synth. Commun., 1981, 11, 513 CrossRef CAS; (b) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS.
- J. H. Clark, Green Chem., 1999, 1, 1 RSC.
- G. W. Kabalka, R. M. Pagni and C. M. Hair, Org. Lett., 1999, 1, 1423 CrossRef CAS.
- G. W. Kabalka, R. M. Pagni, L. Wang, V. Namboodiri and C. M. Hair, Green. Chem., 2000, 2, 120 RSC.
- D. Gala, A. Stamford, J. Jenkins and M. Kugelman, Org. Proc. Res. Dev., 1997, 1, 163 Search PubMed.
- D. O’Keefe, M. Dannock and S. Marcuccio, Tetrahedron Lett., 1992, 33, 6679 CrossRef CAS; K.-C. Kong and C.-H. Cheng, J. Am. Chem. Soc., 1991, 113, 6313 CrossRef CAS.
- D. J. Macquarrie, Chem. Commun., 1996, 1961 RSC; D. J. Macquarrie and D. B. Jackson, Chem. Commun., 1997, 1781 RSC.
- A. Butterworth, J. H. Clark, P. H. Walton and S. J. Barlow, Chem. Commun., 1996, 1859 RSC; J. A. Elings, R. Ait-Meddour, J. H. Clark and D. J. Macquarrie, Chem. Commun., 1998, 2707 RSC.
- J. H. Clark, D. J. Macquarrie and E. B. Mubofu, Green Chem., 2000, 2, 53 RSC.
- W. M. H. Sachtler, Acc. Chem. Res., 1993, 26, 383 CrossRef CAS; D. Brunel, Coord. Chem. Rev., 1998, 178–180, 1085 CrossRef.
- M. S. Anson, M. P. Leese, L. Tonks and J. M. J. Williams, J. Chem. Soc., Dalton Trans., 1998, 3529 RSC.
- H. E. B. Lempers and R. A. Sheldon, J. Catal., 1998, 175, 62 CrossRef CAS.
- V. V. Grushin and H. Alper, Chem. Rev., 1994, 94, 1047 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
