Highly selective molybdenum phosphate catalyst for the ammoxidation of 2-methylpyrazine to 2-cyanopyrazine†
K. Narasimha Rao, Rajesh Gopinath and P. S. Sai Prasad*
Catalysis and Physical Chemistry Division, Indian Institute of Chemical Technology, Hyderabad - 500 007, India.. E-mail: saiprasad@iict.ap.nic.in
First published on 18th January 2001
Abstract
A catalyst containing the ammonium salt of a 12-molybdophosphoric acid, prepared in situ, is found to be highly selective for the ammoxidation of 2-methylpyrazine to 2-cyanopyrazine.
Green ContextCatalytic ammoxidation of 2-methylpyrazine to 2-cyanopyrazine (2-CP) at low conversions using the in situ generated ammonium salt of 12-molybdophosphoric acid, offers the highest selectivity, reducing byproduct formation considerably.DJM |
Introduction
2-Amidopyrazine, often termed pyrazinamide, is an important anti-tubercular drug. It is conventionally prepared by a non-catalytic multi-step process starting from 1,2-phenylenediamine and glyoxol, in which substantial quantities of waste products are generated, which is economically as well as environmentally disadvantageous. Recently, ammoxidation of 2-methylpyrazine (MP) to 2-cyanopyrazine (CP) and its subsequent simple hydrolysis to amidopyrazine have been recognized as an alternative and highly competitive catalytic route.1 Ammoxidation of MP to CP is very crucial in this process, since the reaction carried out in the vapor phase is highly exothermic and the selectivity is affected by the facile formation of pyrazine as the main byproduct (due to oxidative dealkylation) as well as oxides of carbon (due to total combustion). Among the catalysts employed, the V–Sb oxide system proposed by Forni1 and Bondareva et al.2,3 is found to be very active for the ammoxidation. V–P–O based catalyst systems have been extensively studied for the ammoxidation of methylaromatics and heteroaromatics, particularly methylpyridines.4,5 Despite this, reports on the applicability of VPO catalysts for the ammoxidation of MP are scarce, although it may be inferred that vanadium based catalysts which operate at high reaction temperatures (close to 430 °C) could lead to thermal runaway since the highly exothermic oxidation of ammonia starts dominating under these conditions. Very recently, molybdenum based catalysts have also been adopted for this reaction. Bondareva et al6 have employed a V-containing 12-molybdophosphate heteropoly compound at lower reaction temperatures (380–390 °C) and achieved about a yield of CP of ca. 75%. This catalyst, however, produces 10–25% byproducts (pyrazine and others) depending on the process conditions. A recent American patent7 also proposes an Mo-based phosphate catalytic system for achieving higher selectivity. However, the structural details of the catalyst are not disclosed in this case. It appears from the open literature that attention has been focused on obtaining maximum activity rather than maximum selectivity. We now report the synthesis of a highly selective molybdenum phosphate based heteropolyacid catalyst, which offers maximum selectivity to CP so that the process could be environmentally acceptable.Results and discussion
During the ammoxidation of MP, apart from the main reaction [eqn. (1)], two byproduct reactions [eqns. (2) and (3)] also take place depending on the nature of the catalyst and the process conditions. Hence it is important to study the physico-chemical properties of the catalyst and to optimize the reaction conditions to reduce byproduct formation. | (1) |
 | (2) |
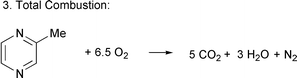 | (3) |
In the present investigation the performance of the in situ generated ammonium salt of 12-molybdophosphoric acid (referred to as salt catalyst) was examined during the ammoxidation of MP. A commercially available 12-molybdophosphoric acid (referred to as acid catalyst) was also used as a catalyst for comparison.
The X-ray powder diffraction (XRD) pattern of the fresh salt catalyst is shown in Fig. 1A. Formation of the ammonium salt of the 12-molybdophosphoric acid [(NH4)3PO4- (MoO3)12·4H2O, ASTM File No. 9-412] is clearly seen along with traces of (NH4)2H2P2O7. The salt catalyst, after use at a reaction temperature of 420 °C, also displays the presence of the ammonium salt. However, the formation of basic molybdenum phosphate, MoPO4(OH)3, predominates. This could be due to exposure of the catalyst to higher reaction temperature for a reasonably longer period (>20 h). The XRD pattern (Fig. 1B) of the fresh acid catalyst corresponds to the 12-molybdophosphoric acid and that of the used catalyst indicates the formation of the ammonium salt of the 12-molybdophosphoric acid.
 | ||
| Fig. 1 XRD patterns of the fresh and used salt (A) and acid (B) catalysts: (▲)[(NH4)3PO4(MoO3) 12·4H2O, (●) (NH4)2H2P2O7, (○) MoPO4(OH)3, (■) H3PMo12O40·xH2 O. | ||
FTIR spectra of the fresh and used salt catalysts are shown in Fig. 2A with bands in the region 1500–600 cm−1 shown as an inset. The spectra reveal the presence of ammonium salt of the acid by a clear band appearing at 1410 cm−1 as reported by Albonetti et al.8 Characteristic bands of the fresh catalyst are observed at 1060, 960, 855, 775 and 722 cm−1 due to the Keggin ion these values being almost the same as those reported by Narasimha Rao et al.9 The spectrum of the used salt catalyst also demonstrates the presence of ammonium ions with a characteristic band at 1410 cm−1, though bands due to the Keggin ions are obscured by the dominance of bands corresponding to the phosphate ion.10 Well resolved bands due to the Keggin ion are observed in the FTIR spectrum (Fig. 2B) of the fresh acid catalyst at 1070, 965, 870 and 590 cm−1, in agreement with the values reported by Bielanski et al.10 Formation of the ammonium species could be observed from the band at 1410 cm−1 in the used acid catalyst, in addition to the peaks due to the Keggin structure. Bondareva et al.6 have also observed formation of a band corresponding to the ammonium ion in their used catalysts; their fresh catalysts, however, did not exhibit this band, possibly due to a different method of preparation. Berndt et al.11 have also reported formation of an ammonium salt of the form α-(NH4)2[(VO)3(P2O7 )2] during their studies on ammoxidation of toluene to benzonitrile using different vanadium phosphate precursors. The importance of the present investigation lies in the in situ preparation of the ammonium salt.
 | ||
| Fig. 2 FTIR spectra of fresh and used salt and acid catalysts. | ||
The effect of reaction temperature on the activity and selectivity of the salt as well as acid catalysts is shown in Fig. 3A and B, respectively. Both catalysts show a continuous increase in conversion with reaction temperature in the range 360–420 °C. A comparison of the activity and selectivity of the salt catalyst with that of the acid catalyst reveals some interesting observations. The acid catalyst is more active under the conditions of evaluation, however, it only demonstrates a maximum CP selectivity of ca. 80% and the corresponding selectivity towards pyrazine is as high as 20%. The salt catalyst, though less active, demonstrates a maximum selectivity towards CP of about 100%. Formation of a byproduct, pyrazine, is negligibly small; only above 400 °C does the selectivity to CP start to decrease and that of pyrazine increase.
 | ||
| Fig. 3 Influence of reaction temperature on the conversion of MP and the selectivities to CP and pyrazine. | ||
Almost all the studies on the ammoxidation of MP12 appear to concentrate on achieving high MP conversion despite the expense of considerable byproduct formation, perhaps owing to a high demand for the product. The present results indicate that at lower conversions the formation of the byproducts can be almost eliminated. This is also advantageous from the process point of view, in terms of better controllability of reaction temperature of an exothermic reaction. Besides, the reactant can be separated and recycled which could improve the overall yield. However, the economics of the process needs to be studied to estimate an acceptable conversion level.
It is interesting to understand why the salt catalyst shows higher selectivity towards CP. From the XRD and the FTIR results, it is known that this catalyst consists of the ammonium salt of the heteropolyacid. Hence, it appears that the high selectivity is related to the presence of the ammonium salt. Our observations indicate that by adopting the present method of preparation, the ammonium salt of the 12-molybdophosphoric acid can be prepared in situ, which could lead to higher selectivity. It has already been reported13 that the heteropolyacid H3PMoO12O40 in the solid state is a Brönsted acid which is stronger than conventional solid acids such as SiO2–Al2O3. The strength and the number of acid centres on the heteropolyacid can be controlled by its structure, composition, the extent of hydration, the type of support and the extent of thermal treatment.14 Though the exact mechanism is not yet understood, it might be expected that the formation of the ammonium salt substantially reduces the number of Brönsted acid sites on the heteropolyacid and thus formation of pyrazine on the acid sites by oxidative dealkylation is minimized. Pyrazine may also be formed by the after-oxidation of cyanopyrazine at high conversions of MP (>80%), as reported by Bondareva et al.6 In the present investigation the conversion of MP is restricted to a lower value, thus obviating this possibility as well.
It may be concluded that under conditions of low conversion of MP and in the presence of the ammonium salt of a molybdophosphoric acid, maximum selectivity towards CP can be obtained.
Experimental
The salt catalyst was prepared by dissolving ammonium heptamolybdate [(NH4)6Mo7O24·4H2 O] and ammonium dihydrogen orthophosphate [NH4H2(PO4)], in a stoichiometric ratio 1∶1, in water. This solution was then evaporated in a water bath and dried first at 120 °C (6 h) and then at 180 °C (6 h). The catalyst mass was subsequently activated in air at 400 °C. Commercially available (Loba Chemie, GR) phosphomolybdic acid was used as catalyst for comparison studies. X-Ray diffraction (XRD) patterns of the catalysts were obtained on a Siemens D-5000 diffractometer using Cu-Kα radiation. FTIR spectra were recorded on a Biorad–175 C (USA) spectrometer using the KBr disc method.The reaction was studied taking 5 g (18/25 BSS mesh size) of catalyst in a tubular reactor and passing a mixture of MP, water, ammonia and air in the molar ratio 1∶13∶7∶38, respectively (at a liquid flow rate of 2 ml h−1), in the temperature range 360–420 °C. The liquid product collected, after the catalyst had attained steady state, was analyzed by gas chromatography. From the analysis of the non-condensable exit gas mixture it was confirmed that the presence of any organic species or oxides of carbon was negligible.
Acknowledgements
We are indebted to Dr K. V. Raghavan, Director, IICT, Hyderabad, for his encouragement and useful suggestions. Thanks are due to Dr I. Suryanarayana, Scientist, IICT, for his help in the FTIR analysis.References
- L. Forni, Appl. Catal., 1986, 20, 219 CAS.
- V. M. Bondareva, T. V. Andrushkevich and G. A. Zenkovets, Kinet. Catal., 1997, 38, 657 Search PubMed.
- V. M. Bondareva, T. V. Andrushkevich, L. M. Plyasova, E. B. Burgina, O. B. Lapina and A. A. Altynnikov, Kinet. Catal., 1997, 38, 662 Search PubMed.
- A. Martin, B. Lucke, H. Seeboth, G. Ladwig and E. Fischer, React. Kinet. Catal. Lett., 1989, 38, 33 Search PubMed.
- B. Lucke and A. Martin, Catalysis of Organic Reactions, Marcel Dekker, New York, 1995, p. 479. Search PubMed.
- V. M. Bondareva, T. V. Andrushkevich, L. G. Detusheva and G. S. Litvak, Catal. Lett., 1996, 42, 113 Search PubMed.
- K.-Y. Lee, C.-H. Shin, T.-S. Chang, D.-K. Lee and D.-H. Cho, US Pat., 5 786 478, Korean Institute of Chemical Technology, August, 1995..
- S. Albonetti, F. Cavani, F. Triffiro, M. Gazzano, M. Koutyrev, F. C. Aissi, A. Aboukais and M. Guelton, J. Catal., 1994, 146, 491 CrossRef CAS.
- K. L. Narasimha Rao, K. S. Sarma, C. Mathew, A. V. Jadav, J. P. Shukla, V. Natarajan, T. K. Seshagiri, S. K. Sali, K. I. Dhiwar, B. Pande and B. Venkataramani, J. Chem. Soc., Faraday Trans., 1998, 94, 1641 RSC.
- A. Bielanski and A. Malecka, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 2847 RSC.
- H. Berndt, K. Buker, A. Martin, S. Rabe, Y. Zhang and M. Meisel, Catal. Today, 1996, 32, 285 CrossRef CAS.
- L. Forni, C. Oliva and C. Rebuschini, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 2397 RSC.
- M. Furuta, K. Sakata, M. Misino and Y. Yoneda, Chem. Lett., 1979, 31 CAS.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 198 CrossRef.
Footnote |
| † IICT Communication number: 4576. |
| This journal is © The Royal Society of Chemistry 2001 |
